Abstract
The term “sinonasal undifferentiated carcinoma (SNUC)” has been coined in 1986 for a highly aggressive sinonasal tract epithelial neoplasm showing distinctive morphology, but lacking any specific line of differentiation. Recent developments resulted in a dynamic splitting of new entities traditionally included in the spectrum of SNUC. Sinonasal NUT-midline carcinoma, adamantinoma-like Ewing family tumors and most recently, SMARCB1(INI1)-deficient sinonasal carcinoma are the main entities defined by specific genetic aberrations. To our knowledge, involvement of subunits of the SWItch/Sucrose Non-fermentable (SWI/SNF) chromatin remodeling complex other than SMARCB1 has not been implicated in the pathogenesis of SNUC-like neoplasms. We herein describe a 40-year-old woman who presented with a large infiltrative mass involving the right nasal cavity and the sinuses with extension into the skull base and periorbital tissue (cT4N2M0). Biopsies were interpreted initially as poorly differentiated neuroendocrine carcinoma followed by surgical resection and radiochemotherapy. No other extra-nasal tumor was detected on imaging. The patient was alive with disease at last follow-up (9 months from initial diagnosis). Histological evaluation showed poorly differentiated small round blue cell neoplasm with diffuse expression of pancytokeratin but not high molecular weight cytokeratin subsets, CK7, p63, S100, desmin or NUT. Neuroendocrine markers showed limited focal weak reactivity. SMARCB1, SMARCA2 and ARID1A were intact in the tumor cells but SMARCA4 was completely lost. This case highlights the rare occurrence of SMARCA4-deficiency in poorly differentiated sinonasal carcinomas and points to the importance of including other SWI/SNF complex subunits in the evaluation of SMARCB1-intact sinonasal malignancies.
Keywords: Sinonasal tract, SNUC, Small round cell tumor, NUT midline carcinoma, SMARCB1-deficient carcinoma, SMARCA4
Introduction
As a group, sinonasal tract malignancies represent no more than 1% of all malignant neoplasms and ≤5% of all head and neck cancers [1]. Their rarity and the fact that poorly differentiated sinonasal neoplasms comprise a group of histogenetically, genetically and biologically diverse, albeit morphologically and phenotypically highly overlapping neoplastic disease entities underline the need for exact classification [2, 3]. Accordingly, the diagnostic workup and exact subtyping of a given neoplasm at this site may pose a real diagnostic challenge in the daily practice of diagnostic surgical pathology. Thus, extended familiarity with and deep knowledge of their phenotypic diversity and their specific diagnostic criteria as well as access to certain adjunct tools are mandatory for diagnosis [4].
Recently, Agaimy et al. [5] and Bishop et al. [6] independently reported on the existence of undifferentiated sinonasal carcinoma characterized by loss of the tumor suppressor SMARCB1 (INI1) encoded by the SMARCB1 gene on chromosome 22q. SMARCB1 is a member of the SWItch/Sucrose Non-Fermentable (SWI/SNF) complex, a large complex of >20 functionally closely related proteins involved in the process of chromatin remodelling and hence in the regulation of gene expression, cell proliferation and differentiation [7]. This evolutionary highly preserved protein complex is composed of several core subunits (including SMARCB1) and two catalytic subunits: SMARCA2 (BRM) and SMARCA4 (BRG) [7]. Recent genome-wide investigations uncovered a surprisingly high rate of SWI/SNF subunit mutations in cancers of different histological subtypes occurring in different organs of the human body [8]. SWI/SNF-deficient neoplasms are generally unified by high frequency of undifferentiated anaplastic and/or rhabdoid cell phenotype and loss of the SWI/SNF subunit affected by the mutation making SWI/SNF immunohistochemistry (IHC) a highly valuable diagnostic tool in recognizing and properly classifying these aggressive neoplasms [9]. Among the SWI/SNF proteins, SMARCA4 loss has been recently identified as the main or sole alteration driving small cell carcinoma of the ovary, hypercalcemic type [10]. Other SMARCA4-driven neoplasms include a rare subset of highly aggressive thoracic malignancies [11] as well as a subset of non-small cell lung cancer (NSCLC) [12] and others. To our knowledge, SMARCA4 loss has not been demonstrated to date in sinonasal tract carcinoma. The aim of this case report is to alert to this rare occurrence and to encourage using other SWI/SNF markers in the immunohistochemical workup of poorly/undifferentiated sinonasal tract carcinoma, particularly if SMARCB1 is intact.
Case Report
A 40-year-old woman presented with a large infiltrative mass involving the right nasal cavity and the sinuses with extension into the skull base and periorbital tissue (cT4N2M0). Biopsies were obtained and a diagnosis of a poorly differentiated neuroendocrine carcinoma was rendered followed by surgical resection and radiochemotherapy. She was alive and well at last follow-up 9 months from initial diagnosis. No other tumor was detected on imaging.
Materials and Methods
The tumor specimen was fixed in buffered formalin and embedded for routine histological examination. Immunohistochemistry was performed on 3-µm sections cut from paraffin blocks using a fully automated system (“Benchmark XT System”, Ventana Medical Systems Inc, 1910 Innovation Park Drive, Tucson, Arizona, USA) and the following antibodies: pancytokeratin (clone AE1/AE3, 1:40, Zytomed, Berlin, Germany), CK7 (OV-TL, 1:1000, Biogenex), p63 (4A4, 1:100, Zytomed), CK5 (clone XM26, 1: 50, Zytomed), chromogranin A (clone LK2H10, 1:500, Beckman-Coulter GmbH), synaptophysin (clone SY38, 1:50, Dako), CD56 (clone MRQ-42, 1:100, CELL MARQUE), desmin (clone D33, 1:250, Dako), protein S100 (polyclonal, 1:2500, Dako), CD117 (polyclonal rabbit antibody, 1:100; Dako), anti-NUT (clone C52B1, 1:45, Cell Signaling), SMARCB1 (INI1) (MRQ-27, 1:50, Zytomed), SMARCA2 (polyclonal antibody, 1:100, Atlas Antibodies AB, Stockholm, Sweden), SMARCA4 (anti-BRG1 antibody, clone EPNCIR111A, 1:100, Abcam; Cambridge, UK) and ARID1A (rabbit polyclonal antibody, ab97995, 1:100; Abcam). Assessment of the staining results of the SWI/SNF components was done as recently described [13], i.e. only the nuclei of viable tumor tissue (away from necrotic areas) were assessed. The presence of a homogeneous strong nuclear staining in the background normal cells was a prerequisite for assessable staining.
Results
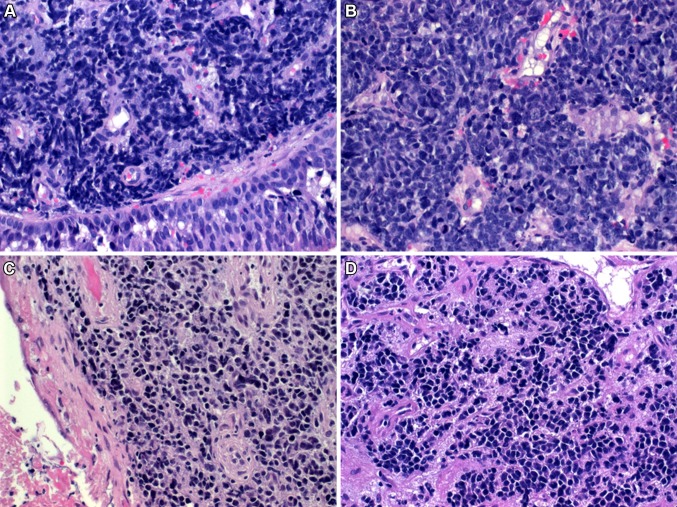
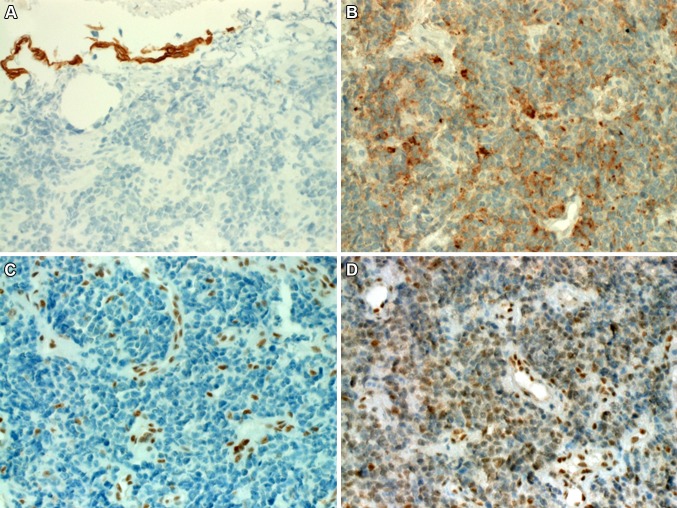
Histological examination showed a diffuse infiltration of the sinonasal lamina propria by monomorphic relatively small to medium-sized rounded hyperchromatic “blue” undifferentiated neoplastic cells having condensed chromatin and a narrow rim of pale eosinophilic cytoplasm (Fig. 1a, b). The neoplastic cells were arranged in a diffuse pattern superficially mimicking small cell carcinoma with focal areas of necrosis and brisk mitotic as well as apoptotic activity. In other areas the tumor cells were loosely arranged and less blue-looking (Fig. 1c). Focally, subtle plasmacytoid features with eccentric rim of condensed eosinophilic cytoplasm were seen in a few scattered cells in these loosely arranged areas but no frankly rhabdoid or large cells were evident (Fig. 1d). There were no glands, pseudorosettes or surface epithelial dysplasia nor were there remnants of Schneiderian papilloma or any other differentiated component. Immunohistochemically, the tumor cells showed strong diffuse cytoplasmic staining for pancytokeratin AE1/AE3, but were negative with CK5 (Fig. 2a), p63, CK7, CD117, desmin, protein S100 and NUT antibody. Variable weak expression of CD56 and synaptophysin (Fig. 2b) was seen in 10–30% of the tumor cells. Chromogranin A was negative. As this neoplasm was initially identified as possible SMARCB1-deficient carcinoma on histological grounds, SMARCB1 was stained and showed intact reactivity in the neoplastic cells (not shown). Accordingly, other members of the SWI/SNF complex were investigated as well which showed complete loss of SMARCA4 (Fig. 2c), reduced SMARCA2 (Fig. 2d) and intact expression of ARID1A.
Fig. 1.
a SMARCA4-deficient sinonasal carcinoma growing beneath surface epithelium lacking dysplasia. b Higher magnification showing monotonous small round cell neoplasm. c Loosely arranged small neoplastic cells with less basaloid appearance were seen focally. d Same area showed scattered cells with subtle plasmacytoid features
Fig. 2.
a The neoplastic cells were immunonegative with CK5 (surface epithelium was positive). b In this area of the tumor, weak to moderate punctate synaptophysin expression is seen. c Complete loss of SMARCA4 was seen in the neoplastic cells (normal endothelia as a control in the background). d SMARCA2 showed significantly reduced, albeit still recognizable nuclear staining in the tumor cells
Discussion
A significant proportion of sinonasal tract malignancies display a poorly/undifferentiated overlapping morphology despite being of diverse histogenesis and having significantly varying response rate to multimodal radiochemotherapy [2, 3]. Accordingly, the differential diagnostic workup of these poorly differentiated sinonasal neoplasms aims at exclusion of non-epithelial mimickers (small round cell sarcomas and hematolymphoid malignancies) on one side and trying to work out an exact subtype of poorly differentiated carcinomas on the other side. Since first description of SNUC as highly aggressive site-specific sinonasal carcinoma type in 1986 [14], it became evident that the morphological spectrum of neoplasms included in the SNUC category is heterogeneous and might represent a final common pathway of dedifferentiation for different entities making SNUC a diagnosis of exclusion [15]. Thus, SNUC has undergone dynamic splitting of several molecular subtypes resulting in establishment of genetically defined sinonasal carcinoma categories such as NUT-rearranged midline carcinoma [16], the recently described SMARCB1-deficient sinonasal carcinoma [5, 6, 17] and adamantinoma-like Ewing family tumors [18].
To our knowledge, SMARCA4 deficiency has not been reported in a primary sinonasal tract carcinoma before. However, during revision of this manuscript, a single case of SMARCA4-mutated sinonasal carcinoma was included in a cohort of 11 SNUC cases investigated by next generation sequencing and was confirmed as SMARCA4-deficient by IHC [19]. Absence of IDH2 mutations (detected in 6 of 11 SNUC as potential genetic driver in that study) in the SMARCA4-mutant tumor suggests SMARCA4-deficiency as likely molecular driver of the neoplasm. The illustrations of that case and our case are very similar, both cases being small round cell undifferentiated malignancies. Thus, the small cell morphology seen in the current case and in the case reported by Jo et al. [19] is analogous to the classical variant of ovarian small cell carcinoma, hypercalcemic type [20]. Unfortunately, no serological data were available to see if the patients have had pre-therapeutic paraneoplastic hypercalcemia or not. However, similar to the ovarian small cell carcinoma and to SMARCA4-deficient carcinomas of other organs [13, 21], histological variation of SMARCA4-deficient sinonasal carcinoma may be expected as well including large cell and rhabdoid cell variants. Thus, one should not restrict using SMARCA4 immunostaining for those neoplasms with the prototypical basaloid small cell morphology until the full spectrum of SMARCA4-deficient sinonasal carcinoma has been well delineated.
Taken together, we believe the current case does not fit any of the WHO-defined sinonasal carcinoma categories other than undifferentiated carcinomas (SNUC) as a diagnosis of exclusion. Furthermore, the appearance of the current SMARCA4-deficient sinonasal carcinoma is essentially not distinguishable from the recently described SMARCB1-deficient sinonasal carcinoma which displayed a stereotypic “blue cell” basaloid pattern in the majority of cases [5, 6, 17]. Variable expression of neuroendocrine markers has been reported in SNUC and may suggest neuroendocrine carcinoma [19, 22]. However, expression of the specific neuroendocrine markers was generally limited and weaker than in bone fide neuroendocrine carcinomas [23]. Notably, variable neuroendocrine traits have been recently reported in SMARCB1-deficient sinonasal carcinomas (CD56 in 28%, synaptophysin in 18% and chromogranin A in 10% of cases) with some cases coexpressing 2 markers [17]. This fact should be taken in mind when approaching poorly differentiated sinonasal neoplasms with small cell basaloid morphology to avoid misinterpretation as genuine neuroendocrine carcinomas [23].
In summary, we described herein a novel case of undifferentiated sinonasal carcinoma demonstrating SMARCA4 deficiency pointing to the involvement of SWI/SNF proteins other than SMARCB1 in rare variants of sinonasal carcinomas in the spectrum of undifferentiated sinonasal neoplasms. Inclusion of other SWI/SNF immunomarkers in the differential diagnostic workup of poorly differentiated sinonasal carcinomas not fitting well-known subtypes is thus recommended. The full morphological and clinicopathological spectrum of this rare variant remains to be delineated in future reports on more cases.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Ethical approval
This study is covered by the ethical vota for retrospective translational research studies of the FAU, Erlangen, Germany.
References
- 1.Sanghvi S, Khan MN, Patel NR, et al. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124:76–83. doi: 10.1002/lary.24264. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JA. Newly described tumor entities in sinonasal tract pathology. Head Neck Pathol. 2016;10:23–31. doi: 10.1007/s12105-016-0688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons SA, Bridge JA, Leon ME. Sinonasal small round blue cell tumors: an approach to diagnosis. Semin Diagn Pathol. 2016;33:91–103. doi: 10.1053/j.semdp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Mills SE, Fechner RE. “Undifferentiated” neoplasms of the sinonasal region: differential diagnosis based on clinical, light microscopic, immunohistochemical, and ultrastructural features. Semin Diagn Pathol. 1989;6:316–328. [PubMed] [Google Scholar]
- 5.Agaimy A, Koch M, Lell M, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–1281. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masliah-Planchon J, Bièche I, Guinebretière JM, et al. SWI/SNF chromatin remodeling and human malignancies. Annu Rev Pathol. 2015;10:145–171. doi: 10.1146/annurev-pathol-012414-040445. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Haswell JR, Roberts CW. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer–mechanisms and potential therapeutic insights. Clin Cancer Res. 2014;20:21–27. doi: 10.1158/1078-0432.CCR-13-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agaimy A. The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol. 2014;21:394–410. doi: 10.1097/PAP.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 10.Jelinic P, Mueller JJ, Olvera N, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;46:424–426. doi: 10.1038/ng.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet. 2015;47:1200–1205. doi: 10.1038/ng.3399. [DOI] [PubMed] [Google Scholar]
- 12.Herpel E, Rieker RJ, Dienemann H, et al. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol. 2017;26:47–51. doi: 10.1016/j.anndiagpath.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Agaimy A, Daum O, Märkl B, et al. SWI/SNF complex-deficient undifferentiated/ rhabdoid carcinomas of the gastrointestinal tract. A series of 13 cases highlighting mutually exclusive loss of SMARCA4 and SMARCA2 and frequent co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016;40:544–553. doi: 10.1097/PAS.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 14.Frierson HF, Jr, Mills SE, Fechner RE, et al. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10:771–779. doi: 10.1097/00000478-198611000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Wenig BM. Undifferentiated malignant neoplasms of the sinonasal tract. Arch Pathol Lab Med. 2009;133:699–712. doi: 10.5858/133.5.699. [DOI] [PubMed] [Google Scholar]
- 16.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 17.Agaimy A, Hartmann A, Antonescu CR, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma: a series of 39 cases expanding the morphological and clinicopathological spectrum of a recently described entity. Am J Surg Pathol 2016, accepted in press. [DOI] [PMC free article] [PubMed]
- 18.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267–1274. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol. 2017 doi: 10.1038/modpathol.2016.239. [DOI] [PubMed] [Google Scholar]
- 20.Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol. 1994;18:1102–1116. doi: 10.1097/00000478-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Agaimy A, Cheng L, Egevad L, et al. Rhabdoid and Undifferentiated Phenotype in Renal Cell Carcinoma: Analysis of 32 Cases Indicating a Distinctive Common Pathway of Dedifferentiation Frequently Associated with SWI/SNF Complex Deficiency. Am J Surg Pathol. 2017;41:253–262. doi: 10.1097/PAS.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 22.Barnes L, Eveson JW, Reichart P, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. p. 19. [Google Scholar]
- 23.Thompson ED, Stelow EB, Mills SE, Westra WH, Bishop JA. Large cell neuroendocrine carcinoma of the head and neck: a clinicopathologic series of 10 cases with an emphasis on hpv status. Am J Surg Pathol. 2016;40:471–478. doi: 10.1097/PAS.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]