Abstract
Hyalinzing clear cell carcinoma is a rare tumor of the oral cavity and is even more rarely located in the tonsil. Here, we present a case report of a pedunculated tonsillar mass in a nearly asymptomatic patient that was present for 2 years prior to removal. Complete surgical excision was achieved and no adjuvant therapy was recommended. We include a discussion of this rare pathology and its typical clinical presentation and course.
Keywords: Hyalinizing clear cell carcinoma, Tonsil cancer, Salivary gland cancer
Introduction
Hyalinizing clear cell carcinoma (HCCC) was first described by Milchgrub in 1994 as a separate entity from epithelial-myoepithelial salivary gland carcinoma (EMEC) and is currently classified as clear cell carcinoma, not otherwise specified by the World Health Organization Classification of Tumours [1, 2]. It is histologically distinct based on its round cells with clear cytoplasm arranged in nests or cords with broad bands of hyalinized stroma [1, 2]. Molecular analysis shows the presence of EWSR1-ATF1 gene fusion, distinguishing HCCC from EMEC [3, 4].
This is an exceedingly rare tumor making up <1% of salivary gland tumors, which themselves make up only 3–5% of head and neck tumors [5]. In a recent review of the English literature by Albergotti et al., there were only 130 reported cases in total. Clinically, patients with HCCC are predominantly female and typically present in the fifth to sixth decade [6]. Patients will have a firm nontender mass usually without any signs of local or distant metastasis [7]. These tumors are known to take an indolent course and wide local excision is the primary treatment with radiotherapy reserved for patients with aggressive features, positive margins or local metastasis [8].
The majority of tumors are found in the base of tongue and oral cavity, but there have been a few reports in the literature of masses found in the tonsils, making up <5% of known cases [9]. Here, we present a case of HCCC of the tonsil.
Case Report
A 52-year old African-American woman presented to our clinic with a history of right tonsillar swelling. She first noted the mass two years prior to her presentation when it was associated with a globus sensation. She denied any significant throat pain, odynophagia, otalgia, dysphagia, dyspnea, dysphonia, weight loss, fevers, chills or fatigue. She was uncertain whether the tonsil mass had grown in the recent past. Her past medical history was significant for a treated positive PPD test and hypertension. Physical examination demonstrated a well-developed and well-nourished woman in no distress. Her oropharynx had an asymmetric, enlarged, non-obstructive, exophytic right tonsil with erythema but no exudate (Fig. 1). There was no palpable cervical lymphadenopathy. Laboratory evaluations including complete blood count, electrolytes and basic metabolic profile were normal. A contrast enhanced computed tomography (CT) scan of the neck revealed a rounded, pedunculated mass likely arising from the right palatine tonsil which was predominantly cystic but with an enhancing irregular rim that measured 2.1 × 2.2 × 2.3 cm (Fig. 2). No pathologic lymphadenopathy was noted radiologically. Given the cystic nature of the mass without cervical lymphadenopathy, the decision was made to take the patient to the operating room for endoscopy and removal. Under general anesthesia with the patient’s mouth suspended with the Crowe-Davis mouth retractor, the mass again appeared cystic and exophytic with its origin at the inferior anterior tonsillar pillar. As the attachment of the mass to the tonsil was very narrow, the decision was made to perform a tonsillectomy to remove the entire mass rather than perform a biopsy. The contralateral tonsil was also removed at the time (Fig. 3).
Fig. 1.
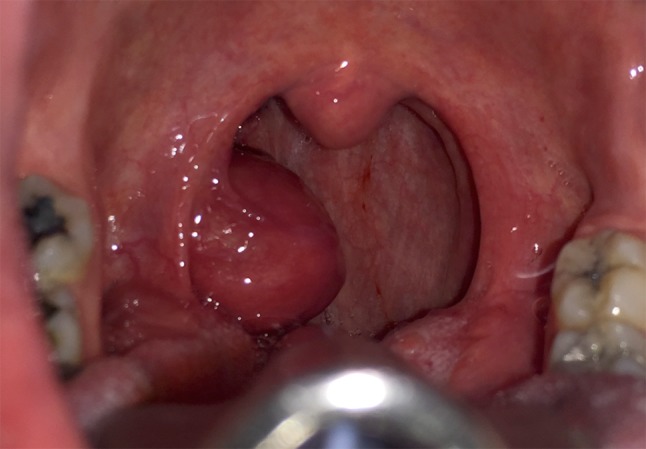
Intraoperative photograph of the HCCC tonsillar cancer
Fig. 2.

A representative axial cut from a CT neck with IV contrast. The 2.1 × 2.2 × 2.3 cm cystic mass can be seen centered on the right tonsillar fossa
Fig. 3.

The excised mass (right) and attached tonsil (left)
Histologically, the tumor was comprised of nests and cords of bland appearing epithelial cells within the submucosa of the tonsil, with prominent cystic changes present (Figs. 4, 5). Additionally, the tumor cells were separated by broad bands of hyalinization. They showed round nuclei, variably prominent nucleoli and abundant voluminous clear cytoplasm. There was no perineural or lymphovascular invasion and the tumor was confined to the tonsil and extended to within 1 mm of the closest margin. Differential diagnosis on H&E included tumors with clear cell morphology including salivary gland type neoplasms (HCCC, mucoepidermoid carcinoma, myoepithelioma/myoepithelial carcinoma) and metastatic clear cell renal cell carcinoma. Tumor cells were diffusely positive for keratin AE1/AE3, keratin 8/18, cytokeratin 5/6 and p63 (Figs. 6, 7). Tumor cells were negative for S-100, SMA, GATA-3 and PAX-8. Tumor cells were also negative for mucicarmine stain. Absence of myoepithelial differentiation by immunostains and demonstrable mucin, supported the diagnosis of HCCC, salivary gland type. FISH studies were sent and confirmed the presence of gene rearrangements in both EWSR1 and ATF1, in keeping with an EWSR-ATF1 fusion, confirming HCCC.
Fig. 4.

Sections show a well circumscribed, subepithelial tumor within the tonsillar submucosa. Note prominent bands of hyalinization within the tumor (arrows). The lymphoid follicles of the tonsillar tissue surround the tumor
Fig. 5.
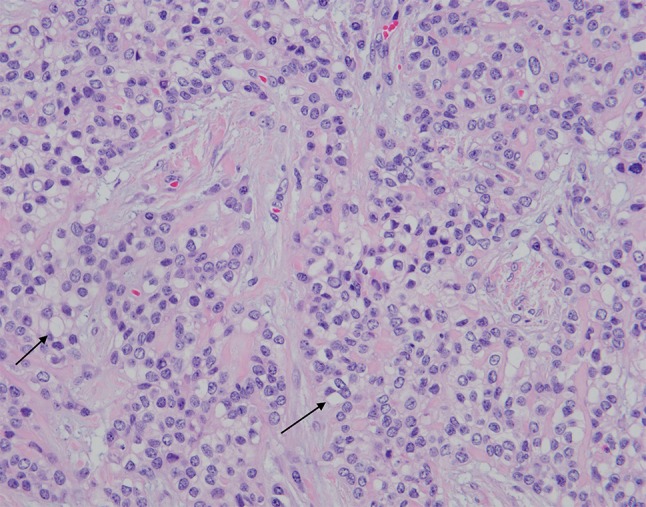
Higher magnification (200×) show bland tumor cells with round nuclei, abundant clear cytoplasm (arrows) and bands of hyalinization. Tumor necrosis is absent
Fig. 6.
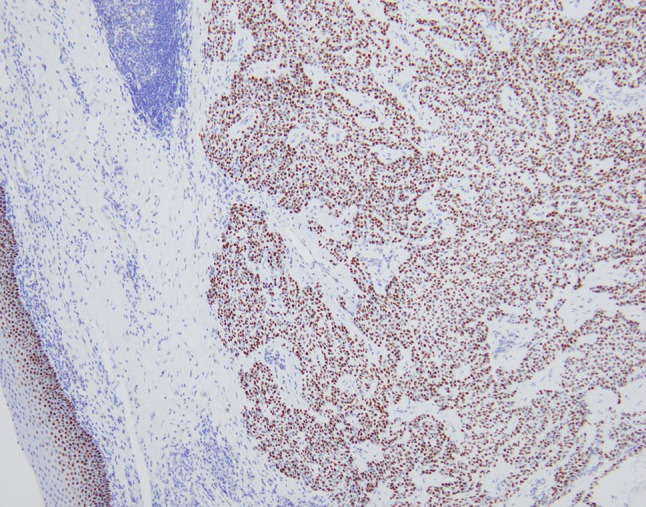
Immunohistochemical stain for p63 shows diffuse, strong, nuclear staining in the tumor nuclei. The surface squamous epithelium serves as an in-built positive control
Fig. 7.

Immunohistochemical stain for cytokeratin5/6 shows diffuse, strong cytoplasmic staining in the tumor cells. The surface squamous epithelium serves as an in-built positive control
The patient was followed with observation with no adjuvant therapy and showed no signs of recurrence four months postoperatively.
Discussion
HCCC is a very rare tumor and a relatively new entity, having been described just over 20 years ago [1]. As such, there are few case reports; fewer of which are primary tumors arising in the tonsil. Our case report identifies a representative patient, a female in her fifties, in an unusual location. As there have been so few case reports of this disease, there is no true standard of treatment. This cancer is mainly indolent in its behavior and patients present anywhere from months to decades after first noticing a mass [10]. However, there have been case reports of aggressive tumors causing early metastasis and even death [9]. Recurrence has been described in 10–20% of the cases and can happen many years after initial diagnosis [7].
Even with the paucity of data, surgical excision does seem to be the consensus treatment for these tumors. Regional control with neck dissection only seems necessary when there is clinical or radiologic evidence of disease. Albergotti et al. showed that when neck dissection was performed for clinically N0 disease, there was no regional metastasis found [9]. For patients with locally aggressive features, close margins, unresectable primary tumors or local metastasis, radiotherapy appears to be a reasonable primary of adjunctive treatment [9]. Even with dual modality therapy, however, some of these patients still recurred. Close observation of HCCC patients even a decade after initial presentation still seems necessary [8].
Depending on the location of the mass, CT scan and possibly magnetic resonance imaging (MRI) along with a chest X-ray seem to be an appropriately extensive work up, with MRI reserved for nasopharyngeal or base of tongue tumors for further characterization. Biopsy with or without molecular testing is a key portion of the work up to ascertain the diagnosis.
In this particular patient, the location on the tonsil and pedunculated nature of the tumor made upfront excision a preferred option to biopsy, and although a simple mucocele was high on the preoperative differential, a complete excision was ensured as a minor salivary gland malignancy was also considered a possibility. Adjunctive radiation was discussed in multidisciplinary tumor board, however given the pedunculated nature of the mass and its complete excision close follow up only was recommended.
What makes this case report so unusual is the location of the HCCC tumor. Only six cases of the total of 136 reviewed by Albergotti et al. were tonsillar. Of those six, only two papers described the clinical course of the tonsillar HCCC patients in detail. The first had an indolent and asymptomatic course with an incidentally noted mass coming off of the inferior tonsil, similar to our patient [10]. The other was different from most cases of HCCC, in that he had been treated for a tonsil carcinoma of unclear etiology with radiotherapy 17 years prior to presentation [9].
Conclusion
HCCC is a rare tumor typically of the oral cavity and base of tongue, however it can be found in other areas of the upper aerodigestive tract, such as this case of a tonsillar tumor, and the parotid gland. Although this carcinoma is considered a low grade salivary gland tumor and typically takes an indolent course, there are case reports of aggressive disease and of late recurrences. Tumors require full surgical excision if possible and radiotherapy should be considered for any patients with incomplete resection or locally or regionally aggressive disease.
Compliance with Ethical Standards
Conflict of interest
There is no conflicts of interest for this case report.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Milchgrub S, Gnepp DR, Vuitch F, et al. Hyalinizing clear cell carcinoma of the salivary gland. Am J Surg Pathol. 1994;18(1):74–82. doi: 10.1097/00000478-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Barnes L, Eveson JW, Reichart P, et al. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. Clear cell carcinoma not otherwise specified; pp. 227–228. [Google Scholar]
- 3.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 4.Nakano T, Yamamoto H, Nishijima T, et al. Hyalinizing clear cell carcinoma with EWSR1-ATF1 fusion gene: report of three cases with molecular analyses. Virchows Arch. 2015;466(1):37–43. doi: 10.1007/s00428-014-1676-5. [DOI] [PubMed] [Google Scholar]
- 5.Kauzman A, Tabet JC, Stiharu TI. Hyalinizing clear cell carcinoma: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):e26–e34. doi: 10.1016/j.tripleo.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan-Mejia ED, Massey HD, Faquin WC, et al. Hyalinizing clear cell carcinoma: report of eight cases and a review of literature. Head Neck Pathol. 2009;3(3):179–185. doi: 10.1007/s12105-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solar AA, Schmidt BL, Jordan RC, et al. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115(1):75–83. doi: 10.1002/cncr.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su HK, Wang BY, Mannan AA, et al. Very delayed cervical lymph node metastases from hyalinizing clear cell carcinoma: report of 2 cases. Head Neck. 2015;37(2):E19–E21. doi: 10.1002/hed.23764. [DOI] [PubMed] [Google Scholar]
- 9.Albergotti WG, Bilodeau EA, Byrd JK, et al. Hyalinizing clear cell carcinoma of the head and neck: case series and update. Head Neck. 2016;38(3):426–433. doi: 10.1002/hed.23902. [DOI] [PubMed] [Google Scholar]
- 10.Hijjawi SB, Abdullah SE, Abdelhadi K, et al. Hyalinizing clear cell carcinoma of the tonsil and its treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):e32–e36. doi: 10.1016/j.oooo.2012.02.003. [DOI] [PubMed] [Google Scholar]


