Abstract
Meningiomas are benign extraaxial tumors of the central nervous system (CNS). Extracranial meningiomas are extremely rare (2%) and can develop as a direct extension from a primary intracranial meningioma or as true primary extracranial meningioma originating from ectopic arachnoid cells. Only eight cases of primary meningioma in the jaw have been reported to date. Extracranial meningiomas are frequently misdiagnosed, resulting in inappropriate clinical management. The aim of this article was to describe the case of a man with an asymptomatic swelling in the right retromolar area over a period of 2 months. Cone beam computed tomography was performed to determine the extension and involvement of the adjacent structures. Histopathological findings and immunohistochemical analysis aided in the diagnosis of primary extracranial meningioma in the mandible and several aspects of this unusual neoplasm are reviewed. The treatment of choice was a partial resection of the mandible and reconstruction with autogenous iliac tricortical bone. Five years after surgery, the patient remains free of disease.
Keywords: Meningioma, Extracranial meningioma, Perineurioma, Oral neoplasm
Introduction
Meningiomas are benign neoplasms of meningothelial cells and represent the most common neoplasms that develop in the central nervous system. This type of tumor originates from the cellular elements of the meninges, including the dura, the cap cell layer of the arachnoid, the arachnoidal granulations, the subarachnoid blood vessels and fibroblasts, and the pia. Most meningiomas are attached to the dura; however, they may invade the bone or originate within an extracranial bone [1]. Although meningiomas represent the most common extra-axial neoplasm; they are nevertheless extremely rare and may develop as a direct extension of a primary intracranial meningioma or as a true primary extracranial meningioma originating from ectopic arachnoid cells [2]. Stimulation of these ectopic cells or of multipotential mesenchymal cells may occur during the extraction of teeth, or due to chronic apical inflammation that promotes the proliferation of these cells, causing tumor formation [3].
Extracranial meningiomas are frequently misdiagnosed, resulting in inappropriate clinical management. The most frequent lesions to be considered in differential diagnoses include schwannoma, neurofibroma, paraganglioma and perineurioma [4, 5].
Perineurioma is considered a rare lesion that exhibits many similarities with meningioma. In fact, perineurial cells are considered the peripheral counterpart of meningeal cells, thus accounting for many shared morphologic features. Although there are also different histological aspects the literature has often discussed the diagnostic process. It is important to distinguish these lesions by considering various clinical aspects; for instance, meningioma has a wider biological potential to infiltrate anatomical structures and has a higher morbidity index. Furthermore, a meningioma is more likely to recur than a perineurioma and requires more radical surgery and potentially even radiation therapy in infiltrative cases [4, 5]. In general, the diagnosis of a meningioma is established using ultrastructural examination, but immunohistochemistry are useful in supporting the diagnosis [4].
To date, only eight cases of primary meningioma in the jaw have been reported in the English literature, including seven in the mandible and two in the maxilla. The aim of this study was to describe an unusual case of primary extracranial meningioma in the mandible and discuss the diagnosis process.
Case Report
A 35-year-old man was referred to Aráujo Jorge Hospital complaining of swelling in the right retromolar area for approximately 2 months. In his dental history, he had received endodontic treatment of the right second molar. According to the patient, his periapical pathology was diagnosed by his dental surgeon. The post-treatment endodontic periapical radiograph revealed a radiolucent lesion in the posterior region of the right second molar. Panoramic radiography was obtained to better visualization of the lesion’s limits and demonstrated the presence of a poorly circumscribed, multilocular osteolytic lesion compromising the body and ramus of the right mandible (Fig. 1). Although the features of the image were representative of an aggressive lesion, the initial diagnosis was a benign ameloblastoma-like lesion.
Fig. 1.
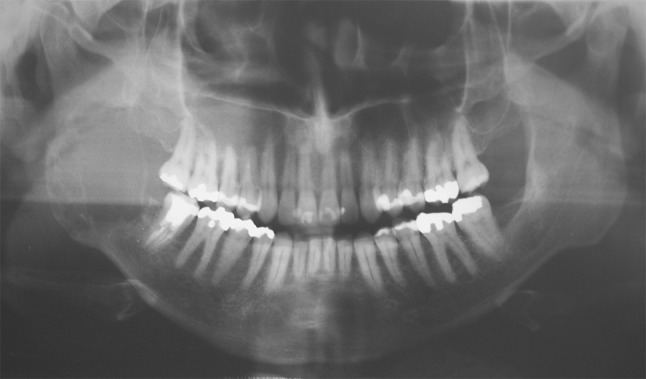
Panoramic radiography showing presence of osteolytic lesion, multilocular with imprecise limits compromising body and ramus mandibular of the right side
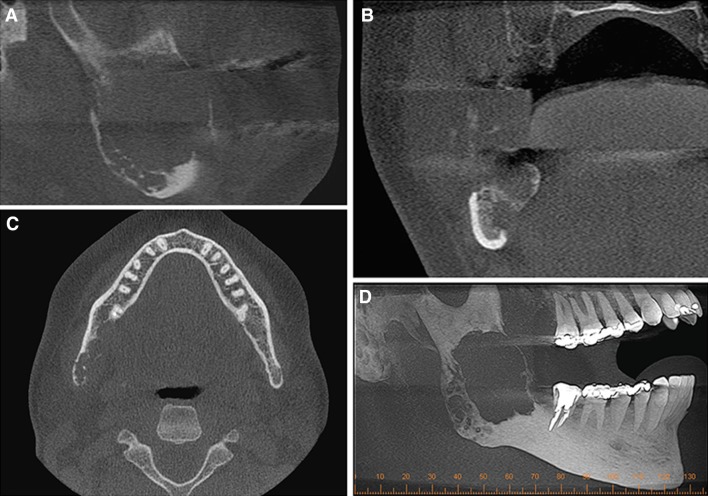
Cone beam computed tomography (CBCT) aided in determining the extension and involvement of the adjacent structures. A hypodense and osteolytic lesion with imprecise limits and rupture of the lower cortical bone was observed. The mandibular canal was found to be involved by the tumor mass (Fig. 2a–d).
Fig. 2.
Cone Beam Compute Tomography—Sagittal (a), coronal (b) and axial (c) section showing hypodense area with destruction of higher cortical, lower, buccal and lingual. (d) Maximum intensity projection (MIP) showing multilocularity of the lesion
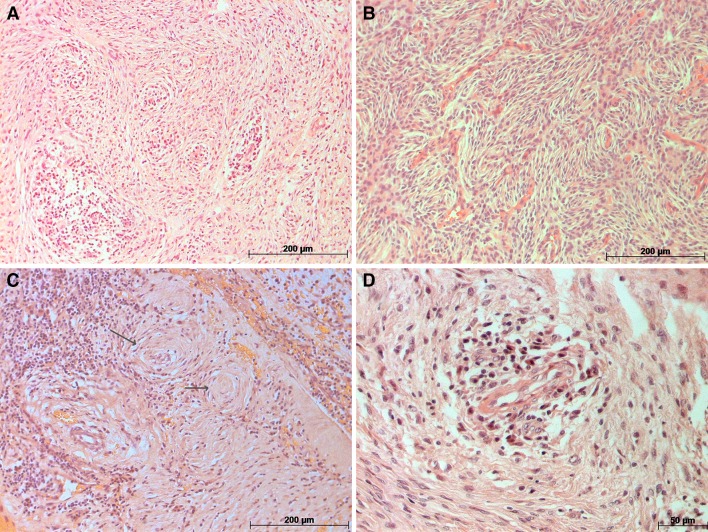
An incisional biopsy was performed and the specimen was submitted for histological examination. Microscopic findings identified a spindle cell neoplasm composed of sheets of small polygonal cells with prominent nucleoli separated by fine collagen bundles. With the exception of prominent nucleoli, there were no other features of atypia such as high cellularity, high nuclear to cytoplasmic ratio, necrosis or increased mitotic activity. Of note, pseudo-onionbulb or whorl formations were seen, reminiscent of perineurioma or meningioma (Fig. 3a–d). Accordingly, a panel of immunohistochemical stains was performed including S-100, epithelial membrane antigen (EMA), smooth muscle actin (SMA), vimentin, claudin-1, CD34, and glucose transporter-1 (glut-1).
Fig. 3.
Microscopic analysis revealed a uniform spindle cells arranged in vague fascicles b–c spindle cell neoplasm, without evidence of atypia, whorls suggesting meningothelial origin (black arrow) d Blood vessel with inflammatory cells surrounding it
The staining with antibodies against EMA, vimentin and CD34 was positive, while the others exhibited no reactivity (Table 1). Considering the microscopic findings and immunostaining features together, the histopathological diagnosis was a primary extracranial meningioma.
Table 1.
Immunohistochemical results
| Antibody | Result |
|---|---|
| EMA | Positive |
| Smooth muscle actin | Negative |
| VIM | Positive |
| S-100 protein | Negative |
| Glut-1 | Negative |
| CD34 | Positive |
| Claudin | Negative |
The treatment of choice was a partial resection of the mandible and reconstruction with autogenous iliac tricortical bone, as well as fixation with a 2.4 mm lockable system board. The use of a prototype obtained from DICOM tomography files allowed prior customization of the universal reconstruction plate. After 21 days, a panoramic radiograph was obtained; adequate position of the graft was observed with the use of orthodontic mini-implants and a maxillomandibular lock with an elastic.
Five years after surgery, the patient remains free of disease and radiographic examinations have indicated bone graft repair (Fig. 4).
Fig. 4.
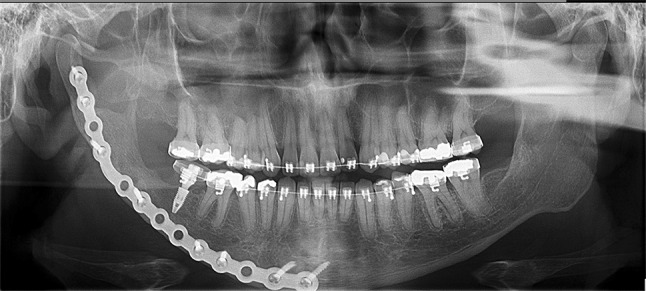
Panoramic radiography after 5 years of surgery indicating bone graft repair
Discussion
Primary extracranial meningioma is an extremely unusual neoplasm, especially in the maxillomandibular complex. In our specific case, the diagnosis was a challenge. Firstly, extracranial meningioma was not our first diagnostic impression a multilocular ameloblastoma or an odontogenic keratocyst tumor was our initial differential diagnosis. Therefore, the incisional biopsy and histopathological findings were fundamental to advancing the diagnostic process and sufficient to exclude the hypothesis of an odontogenic tumor and to confirm the diagnosis of a possible neural lesion. Spindle cell proliferation was prominent in the throughout the entire specimen and in some fields, spindle-shaped cells were arranged in whorls. The diagnostic considerations encompassed schwannoma, a common neural lesion; however, neither the histological features nor the immunohistochemical profile (S100 negative, EMA positive) favored schwannoma [6, 7]. The remaining diagnostic options included perineurioma and meningioma. Although the histological features are similar in both entities, Jones and Freedman [8] reported that whorls, rosettes, pseudonuclear inclusions and psammoma bodies are more likely to be found in meningiomas [8].
Due to the histological similarities of perineurioma and meningioma, immunohistochemical analysis played an important role in establishing the final diagnosis. Of the eight cases reported in the literature, five cases were supported by immunohistochemistry techniques to establish the diagnosis. Kubota et al. [9] observed that the tumor cells stained positive for monoclonal antibodies against EMA, human vimentin, and desmoplakin, with no immunoreactivity for S-100 protein. Other reports [8, 10, 11] used vimentin, EMA, and S-100 and found that the tumor cells were positive for vimentin and EMA, and negative for S-100. Other markers were also negative, such as epithelial cytokeratins, actin, desmin [10], AE1/AE3 cytokeratin, CK18, CD34 [10], smooth muscle actin, carcinoembryonic antigen, and antimelanoma [8]. In agreement with the literature, our case was also positive for vimentin and EMA and negative for S-100 protein and smooth muscle actin. Several reports indicate that a low Ki-67 proliferation index (less than 5%) is associated with an indolent clinical course [11, 12].
Koutlas, Scheithauer and Folpe [5] and Agaimy et al. [4] described cases of intraoral perineurioma and stained for EMA, glut-1, claudin-1, type IV collagen, as well as SSTR (somatostatin receptor 2) and PR (progesterone receptor) for perineuriomas. The staining revealed uniform expression of EMA in the majority of meningiomas and perineuriomas, while claudin-1 was strongly positive in perineuriomas. Glut-1 expression is associated with the malignant potential and invasiveness of a tumor [4, 5]. In cases of meningioma described by Smith et al. [13] and Koutlas, Scheithauer and Folpe [5], glut-1 focal expression was observed; however, Yamaguchi et al. [14] reported that glut-1 was positive only in perineuriomas. Other immunohistochemical markers, including EMA, S-100, CD34 and SMA have been used for the diagnosis of perineurioma, exhibiting EMA positivity but with negative for the other markers [15]. In our case, the tumor cells were positive for EMA and negative for glut-1 and claudin-1, supporting the diagnosis of a meningioma. Other antibodies, such as SSTR and PR have been used in other studies to discern between these lesions and were found to be positive in meningioma cases [4] (Table 2).
Table 2.
Summary of Immunohistochemical patterns based on literature of meningioma versus perineurioma
| Meningioma | Perineurioma | |
|---|---|---|
| EMA [3–6, 8–12, 14, 15] | + | + |
| Vimentin [1, 3, 6, 8–11, 14] | + | + |
| S-100 [1, 3, 6, 8–12, 14, 15] | − | − |
| Smooth muscle actin [8, 10, 12, 15] | − | − (50% cases +) |
| GLUT-1 [4, 5] | − (some cases+) | + |
| Desmoplakin [9] | + | − |
| Claudin-1 [4, 5] | − | + |
| Desmin [10, 14] | − | − |
| AE1/AE3 [6, 11] | − | − |
| CK 18 [11] | − | − |
| CD 34 [11, 12, 14, 15] | − | − |
| Ki67 [6, 11, 12] | − | − |
| Anticytokeratin [3, 10, 12, 14] | − | − |
| Type IV collagen [5, 14] | − | + |
| PR (progesterone receptor) [4] | + | − |
| SSTR2 (somatostatin receptor subtype 2) [4] | + | − |
There are reports of pain accompanying the development of this neoplasm in extracranial bones [6]; however, our patient did not report any symptoms. Despite the large size of the tumor and its location near the mandibular nerve, our patient surprisingly did not present with any signs or symptoms. In the maxillomandibular lesions, the most frequent symptoms consisted of pain, paresthesia, and anesthesia [3, 8–10]. The presence of symptoms is frequently associated with the anatomic site of the tumor and the involvement of adjacent anatomic structures [6].
The diagnosis of an extracranial meningioma is challenging, and when misdiagnosed can result in inappropriate clinical and surgical management, especially when localized in the maxillomandibular complex [4, 5]. When osteolytic or mixed radiolucent-radiopaque masses are observed in the mandible, primary tumors from central nervous system are not considered, because they are rare and the presence of neural cells in this region [6–10]. Simpson and Sneddon (1987) [1] and Reddi et al. (1999) [2] described three cases of extracranial meningioma characterized by mixed radiolucent with displacement, thinning, and the eventual disruption of cortical bone [1, 2]. Moreover, in mandibular lesions, the mandibular nerve is frequently involved [3, 6–10]. The lesion in our patient was osteolytic and well-defined, causing displacement, thinning and cortical disruption. Interruption of the cortical margins was identified in the buccal, lingual, and upper cortical bone, indicating aggressive behavior.
Surgical treatment with a partial excision of the mandible and reconstruction using autologous iliac tricortical bone was the treatment of choice for our patient. The follow-up period of five years has revealed no evidence of recurrence. To prevent recurrence, complete removal of the lesion must be performed. Close follow-up is recommended, particularly, if there is histological evidence of atypia. In our case, these aspects were not observed. Rushing et al. [6] revealed that while some patients die of the disease soon after discovery, the majority of patients exhibit long-term survival and die from other causes. There are no related cases of patients that have died from an extracranial primary meningioma of the maxillomandibular complex [6].
In conclusion, meningioma is a benign neoplasm that rarely affects the mandible. Immunohistochemical markers are an important tool in establishing the final diagnosis, especially for the differentiation from histological mimics such as perineurioma. Vimentin, S-100, claudin-1, glut-1, and EMA are important markers to discern meningioma from other lesions.
Funding
This study was not funded.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research Involving Human Participants and/or Animals
Not applicable.
References
- 1.Simpson MT, Sneddon KJ. Extracranial meningioma of the oral cavity. Br J Oral Maxillofac Surg. 1987;25:520–525. doi: 10.1016/0266-4356(87)90146-X. [DOI] [PubMed] [Google Scholar]
- 2.Reddi SP, Strauss SI, Strauss JE, Blanchaert RH., Jr Anterior maxillary lesion. J Oral Maxillofac Surg. 1999;57:1234–1238. doi: 10.1016/S0278-2391(99)90494-9. [DOI] [PubMed] [Google Scholar]
- 3.Landini G, Kitano M. Meningioma of the mandible. Cancer. 1992;69:2917–2920. doi: 10.1002/1097-0142(19920615)69:12<2917::AID-CNCR2820691209>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Agaimy A, Buslei R, Coras R, Rubin BP, Mentzel T. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60–70. doi: 10.1111/his.12366. [DOI] [PubMed] [Google Scholar]
- 5.Koutlas IG, Scheithauer BW, Folpe AL. Intraoral perineurioma, soft tissue type: report of five cases, including 3 intraosseous examples, and review of the literature. Head Neck Pahtol. 2010;4:113–120. doi: 10.1007/s12105-010-0177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rushing EJ, Bouffard JP, McCall S, Olsen C, Mena H, et al. Primary extracranial menigiomas: an analysis of 146 cases. Head Neck Pathol. 2009;3:116–130. doi: 10.1007/s12105-009-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger PC, Scheithauer BW. Atlas of Tumor Pathology, Series 3, Fascicle 10. Washington DC: Armed Forces Institute of Pathology; 1994. pp. 259–286. [Google Scholar]
- 8.Jones AC, Freedman PD. Primary extracranial meningioma of the mandible: a report of 2 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:338–341. doi: 10.1067/moe.2001.112947. [DOI] [PubMed] [Google Scholar]
- 9.Kubota Y, Yamashiro T, Kobayashi I, Kawazu T, Shirasuna K. Primary meningioma of the mandible. Oral Oncol Extra. 2005;41:18–21. doi: 10.1016/j.ooe.2004.10.004. [DOI] [Google Scholar]
- 10.Lell M, Tudor C, Aigner T, Kessler P. Primary intraosseous meningioma of the mandible: CT and MR imaging features. AJNR Am J Neuroradiol. 2007;28:129–131. doi: 10.3174/ajnr.A0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosqueda-Taylor A, Dominguez-Malagon H, Cano-Valdez AM, Montiel-Hernandez AM. Primary extracranial meningioma of the mandible. Med Oral Patol Oral Cir Bucal. 2009;14(4):E167–E170. [PubMed] [Google Scholar]
- 12.Possanzini P, Pipolo C, Romagnoli S, Falleni M, Moneghini L, et al. Primary extra-cranial meningioma of head and neck: clinical, histopahtological and immunohistochemical study of three cases. Acta Otorhinolaryngol Ital. 2012;32:336–338. [PMC free article] [PubMed] [Google Scholar]
- 13.Smith ME, Awasthi R, O’Shaughnessy S, Fisher C. Evaluation of perineurial differentiation in epithelioid sarcoma. Histopathology. 2005;47:575–581. doi: 10.1111/j.1365-2559.2005.02298.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi U, Hasegawa T, Hirose T, Fugo K, Mitsuhashi T, et al. Sclerosing perineurioma: a clinicopathological study of five cases and diagnostic utility of immunohistochemical staining for. GLUT1. Virchows Arch. 2003;443:159–163. doi: 10.1007/s00428-003-0849-4. [DOI] [PubMed] [Google Scholar]
- 15.Xiao WL, Xue LF, Xu YX. Soft tissue perineurioma of the tongue: report of a case and review of the literature. World J Surg Oncol. 2014;12:11. doi: 10.1186/1477-7819-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]