Abstract
Due to the spread of a single CTX-M-type extended-spectrum β-lactamase (ESBL) clone of sequence type (ST) 131, community-onset bacteremia caused by ESBL-producing Escherichia coli has increased dramatically. We evaluated the risk factors and molecular features of ESBL-producing E. coli ST131 clones isolated from Korean patients with community-onset bacteremia. We collected a total of 124 ESBL-producing E. coli isolates from blood in patients with community-onset bacteremia over a 2 year-period. Among 124 patients, the number of community-associated bacteremia cases was 57 (46%). ST131 strains accounted for 49.1% (28/57) of community-associated bacteremia cases and 44.8% (30/67) of healthcare-associated community-onset bacteremia cases. Among 58 ST131 strains, nine isolates were shown to harbor O16-H41, and 61.1% (30/49) of O25 had H30Rx. In a multivariate analysis, independent risk factors for acquisition of ST131 isolates over non-ST131 isolates were underlying diabetes mellitus and absence of prior chemotherapy history. The most common ESBL genotype was CTX-M-15 (46.0%), followed by CTX-M-14 (37.1%). A considerable proportion of community-onset ESBL-producing E. coli bacteremia was observed. ST131 clones appear to be associated with the spread of community-associated bacteremia exhibiting high antimicrobial resistance and highly virulent H30Rx traits, which could become a major public health concern in Korea.
Introduction
Extended-spectrum β-lactamase (ESBL)-producing Escherichia coli has become widespread in hospitals around the world since the late 1980s1, but the sudden worldwide increase in communities is mainly due to CTX-M-type ESBLs (especially CTX-M-15)-producing sequence type (ST) 1312,3. The most common type of infection by ESBL-producing E. coli is urinary tract infection with good clinical outcomes4,5. Life-threatening infections such as bacteremia have not drawn public attention until now, but the emergence of community-onset bacteremia by ESBL-producing E. coli ST131 clones in Korea has been a concern in recent reports6–8. ST131 ESBL-producing E. coli from bacteremia cases possessed more virulence traits and showed more multidrug resistance patterns than non-ST131 isolates7. Recent molecular epidemiology showed that H30Rx subsets within ST131-O25-H30 subclones were associated specifically with fluoroquinolone resistance, and CTX-M-15 was widely detected in Korea (37% of total ST131 isolates)8. Risk factors of community-onset bacteremia by E. coli through a comparison of ESBL and non-ESBL groups have already been evaluated9, but the clinical impacts of ST131 have not been elucidated in community-onset bacteremia as far as we know. The potential spread of ESBL-producing E. coli causing blood stream infections is a challenge for the management of community-associated infections, so this study could be informative regarding current molecular epidemiologic shifts in community-onset bacteremia and could lead to better infection control strategies.
Methods
Design and Setting
We collected 124 non-duplicated (except initial isolate from each patient duplicate series) ESBL-producing E. coli blood culture isolates from consecutively encountered patients with community-onset bacteremia, as outpatients or within 48 hours of admission between 2013 and 2014 in Severance hospital, which is one one tertiary teaching hospital in Seoul, Korea. Community-associated bacteremia, risk factors, associated disease, and source of infection followed the definitions used in our previous study9. Briefly, healthcare-associated infections were classified in accordance to the definition by Friedman et al. with some modifications10. Any of the following criteria were considered as healthcare-associated infections: intravenous therapy, wound care, or nursing care received at home 30 days before bloodstream infection; attendance at a hospital or hemodialysis clinic, or receipt of intravenous chemotherapy 30 days before bloodstream infection; >48 hours of hospital admission or performance of invasive procedures such as urinary catheter, endoscopy, and naso-gastric tube 90 days before bloodstream infection; or residence at nursing home or long-term care facility. Thirty-day mortality was defined as death for any reason within 30 days after the onset of the bacteremia. Immunosuppression was defined as follows: therapy of prednisolone or an equivalent drug with a dosage of at least 10 mg/day for 15 days, and chemotherapy or radiotherapy within 6 months before the bacteremia11.
Microbiological Analysis
Identification, ESBL screening, and susceptibility testing were performed using the automated analyzer Vitek 2 system (bioMérieux, Marcy l′E’toile, France), and results of susceptibility were interpreted using the CLSI12. For detection of ST131, all isolates were screened via PCR for O16-ST131 and O25-ST13113. FimH type and H30Rx were determined using PCR and sequencing14,15. ESBL genotypes were determined via PCR and sequencing16. Sequence types (ST) were confirmed with full multilocus sequence typing (MLST) for representative isolates of community-associated bacteremia group17,18. Pulsed-field gel electrophoresis (PFGE) was performed as described in our previous study9. The patterns were analyzed using InfoQuest FP software (Bio-Rad) to generate a dendrogram based on the unweighted pair group method, with an arithmetic average (UPGMA) from the Dice coefficient with 1% band position tolerance and 0.5% optimization settings. A PCR-based replicon typing (PBRT) was schemed by targeting the replicons of major plasmid families occurring in Enterobacteriaceae (HI2, HI1, I1-γ, X, L/M, N, FIA, FIB, FIC, W, Y, P, A/C, T, K, B/O) for representative isolates19.
Statistical Analysis
Continuous variable, such as age, was analyzed by using the Mann-Whitney U test. The chi-squared test was used for the comparative analysis of categorical variables in order to determine independent risk factors. Odds ratio (OR) and 95% confidence interval (CI) values were calculated for binomial variables. Variables for which the P values were less than 0.1 in univariate analyses were included in a multivariate logistic regression analysis model to determine independent risk factors for acquisition of ST131 isolates. Statistical significance was defined as P < 0.05. SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for univariate analyses and multivariate analyses.
Data Availability
All data generated or analysed during this study are included in this published article.
Results
Clinical features of patients with community-onset ESBL-producing E. coli bacteremia
Of 124 total patients with ESBL-producing E. coli community-onset bacteremia, 57 (46%) had community-associated bacteremia and the others had healthcare-associated bacteremia. There were fewer patients with a Charlson comorbidity index score of 2 or above in the community-associated bacteremia group than in the healthcare-associated bacteremia group. The mortality rate in patients with community-associated bacteremia (5.3%) was lower than that in patients with healthcare-associated bacteremia (22.8%, Table 1). However, no statistical difference was found between mortality rates of ST131 and non-ST131 (Table 4). Also, there was no statistical difference between mortality rates of H30Rx subclone and non-ST131 (P = 0.090).
Table 1.
Clinical features of patients with community onset ESBL-producing E. coli bacteremia: Univariate Analysis.
| Clinical features | CA (n = 57) | HA (n = 67) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age in years, median (IQR) | 72.0 (61.0–77.0) | 68.0 (56.5–75.0) | 0.127 | |
| Male sex | 20 (35) | 32 (48) | 0.597 (0.289–1.232) | 0.163 |
| Associated disease | ||||
| Diabetes mellitus | 20 (35) | 22 (33) | 1.105 (0.525–2.330) | 0.792 |
| Heart failure | 3 (5) | 1 (1) | 2.846 (0.326–24.891) | 0.344 |
| Chronic pulmonary disease | 1 (2) | 1 (1) | 1.176 (0.072–19.239) | 0.909 |
| Chronic renal insufficiency | 6 (11) | 12 (18) | 0.561 (0.197–1.595) | 0.278 |
| Liver cirrhosis | 1 (2) | 2 (3) | 0.696 (0.065–7.403) | 0.764 |
| Chemotherapy | 8 (14) | 22 (33) | 0.347 (0.141–0.853) | 0.021 |
| Vascular disease | 8 (14) | 9 (13) | 1.057 (0.379–2.948) | 0.915 |
| Transplantation | 3 (5) | 3 (4) | 1.183 (0.229–6.105) | 0.841 |
| Immunosuppression | 3 (5) | 3 (4) | 1.183 (0.229–6.105) | 0.841 |
| Major surgery 30 d before infection | 16 (28) | 20 (30) | 0.921 (0.423–2.008) | 0.837 |
| Charlson comorbidity index ≥ 2 | 24 (42) | 48 (72) | 0.294 (0.139–0.620) | 0.001 |
| Device | ||||
| Urinary catheter | 0 (0) | 10 (15) | 0.048 (0.002–0.955) | 0.047 |
| Tracheostomy/intubation | 0 (0) | 2 (3) | 0.228 (0.005–9.549) | 0.438 |
| Nasogastric tube | 0 (0) | 3 (4) | 0.16 (0.005–4.998) | 0.297 |
| Any device | 0 (0) | 11 (16) | 0.043 (0.002–0.841) | 0.038 |
| Previous antibiotics within last month | ||||
| Penicillin | 1 (2) | 6 (9) | 0.251 (0.036–1.734) | 0.161 |
| Cephalosporin | ||||
| First generation | 0 (0) | 2 (3) | 0.228 (0.005–9.549) | 0.438 |
| Second generation | 1 (2) | 4 (6) | 0.375 (0.048–2.929) | 0.350 |
| Third generation | 5 (9) | 10 (15) | 0.574 (0.185–1.776) | 0.335 |
| Carbapenem | 1 (2) | 8 (12) | 0.186 (0.029–1.201) | 0.077 |
| Fluoroquinolone | 1 (2) | 9 (13) | 0.164 (0.026–1.033) | 0.054 |
| Any antibiotics | 9 (16) | 35 (52) | 0.179 (0.077–0.420) | <0.001 |
| Source of infection | ||||
| Primary | 11 (19) | 21 (31) | 0.535 (0.232–1.232) | 0.142 |
| Urinary | 39 (68) | 37 (55) | 1.737 (0.831–3.628) | 0.142 |
| Hepatobiliary | 5 (9) | 5 (7) | 1.191 (0.327–4.339) | 0.791 |
| Gastrointestinal | 2 (4) | 0 (0) | 6.074 (0.145–254.296) | 0.344 |
| Respiratory | 0 (0) | 5 (7) | 0.099 (0.004–2.404) | 0.155 |
| Polymicrobial infection | 4 (7) | 8 (12) | 0.589 (0.169–2.046) | 0.405 |
| ST131 clone | 28 (49) | 30 (45) | 1.188 (0.585–2.411) | 0.634 |
| Septic shock/severe sepsis | 11 (19) | 21 (31) | 0.535 (0.232–1.232) | 0.142 |
| Pitt bacteremia score ≥ 2 | 16 (28) | 18 (27) | 1.064 (0.482–2.346) | 0.878 |
| 30-day mortality | 3 (5) | 13 (19) | 0.259 (0.073–0.923) | 0.037 |
Data are no. (%) of patients. CA, community-associated; HA, healthcare-associated; OR, odds ratio; CI, confidence interval.
Table 4.
Risk factors of acquisition of ST131 in community-onset ESBL-producing E. coli bacteremia: Univariate Analysis.
| Risk factors | ST131 (n = 58) | Non-ST131 (n = 66) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age in years, median (IQR) | 73.5 (61.5–80.0) | 68.5 (59.0–74.0) | 0.057 | |
| Male sex | 24 (41) | 28 (42) | 0.959 (0.469–1.961) | 0.909 |
| Associated disease | ||||
| Diabetes mellitus | 26 (45) | 16 (24) | 2.495 (1.162–5.356) | 0.019 |
| Heart failure | 0 (0) | 4 (6) | 0.119 (0.004–3.174) | 0.204 |
| Chronic pulmonary disease | 1 (2) | 1 (2) | 1.139 (0.070–18.621) | 0.928 |
| Chronic renal insufficiency | 13 (22) | 5 (8) | 3.317 (1.116–9.856) | 0.031 |
| Liver cirrhosis | 1 (2) | 2 (3) | 0.673 (0.063–7.163) | 0.743 |
| Chemotherapy | 8 (14) | 22 (33) | 0.333 (0.136–0.818) | 0.016 |
| Vascular disease | 10 (17) | 7 (11) | 1.718 (0.609–4.844) | 0.306 |
| Transplantation | 1 (2) | 5 (8) | 0.292 (0.040–2.119) | 0.223 |
| Immunosuppression | 3 (5) | 3 (5) | 1.145 (0.222–5.906) | 0.872 |
| Major surgery 30 d before infection | 11 (19) | 25 (38) | 0.394 (0.173–0.895) | 0.026 |
| Charlson comorbidity index ≥ 2 | 30 (52) | 42 (64) | 0.617 (0.301–1.266) | 0.188 |
| Device | ||||
| Urinary catheter | 5 (9) | 5 (8) | 1.150 (0.316–4.189) | 0.833 |
| Tracheostomy/intubation | 1 (2) | 1 (2) | 1.139 (0.070–18.621) | 0.928 |
| Nasogastric tube | 0 (0) | 3 (5) | 0.155 (0.005–4.836) | 0.288 |
| Any device | 5 (9) | 6 (9) | 0.957 (0.276–3.315) | 0.945 |
| Previous antibiotics within last month | ||||
| Penicillin | 4 (7) | 3 (5) | 1.498 (0.322–6.965) | 0.606 |
| Cephalosporin | ||||
| First generation | 1 (2) | 1 (2) | 1.139 (0.070–18.621) | 0.928 |
| Second generation | 1 (2) | 4 (6) | 0.363 (0.046–2.832) | 0.335 |
| Third generation | 8 (14) | 7 (11) | 1.335 (0.453–3.940) | 0.600 |
| Carbapenem | 3 (5) | 6 (9) | 0.587 (0.142–2.422) | 0.461 |
| Fluoroquinolone | 2 (3) | 8 (12) | 0.305 (0.066–1.398) | 0.126 |
| Any antibiotics | 18 (31) | 26 (39) | 0.698 (0.332–1.468) | 0.344 |
| Source of infection | ||||
| Primary | 12 (21) | 20 (30) | 0.610 (0.268–1.389) | 0.239 |
| Urinary | 40 (69) | 36 (55) | 1.829 (0.875–3.823) | 0.108 |
| Hepatobiliary | 6 (10) | 4 (6) | 1.720 (0.463–6.398) | 0.418 |
| Gastrointestinal | 0 (0) | 2 (3) | 0.221 (0.005–9.242) | 0.428 |
| Respiratory | 0 (0) | 4 (6) | 0.119 (0.004–3.174) | 0.204 |
| Polymicrobial infection | 5 (9) | 7 (11) | 0.815 (0.245–2.718) | 0.740 |
| CA | 28 (48) | 29 (44) | 1.188 (0.585–2.411) | 0.634 |
| Septic shock/severe sepsis | 16 (28) | 16 (24) | 1.188 (0.531–2.658) | 0.675 |
| Pitt bacteremia score ≥ 2 | 12 (21) | 22 (33) | 0.532 (0.236–1.200) | 0.128 |
| 30-day mortality | 4 (7) | 12 (18) | 0.360 (0.111–1.163) | 0.088 |
Data are no. (%) of patients. CA, community-associated; OR, odds ratio; CI, confidence interval.
Microbiological Analysis
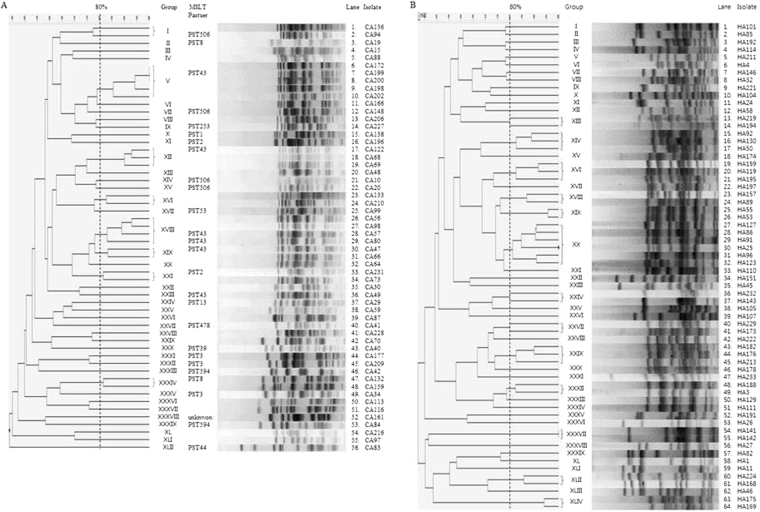
The antimicrobial susceptibilities of community-onset ESBL–producing E. coli were similar between the community-associated and healthcare-associated bacteremia groups, except for the results of ceftazidime and aztreonam, which were associated with more resistance in the healthcare-associated bacteremia group (Table 2). Globally, epidemic ST131 strains accounted for 49.1% (28/57) of community-associated bacteremia cases and 44.8% (30/67) of healthcare-associated bacteremia cases. Of 58 total ST131 strains, nine O16-H41 strains were detected, and 61.1% (30/49) of O25-H30 strains had H30Rx (Table 3). The most common ESBL genotype was CTX-M-15 (46.0%, 57/124), followed by CTX-M-14 (37.1%, 46/124). PFGE patterns did not show a dominant clonality in community-associated or community-onset healthcare-associated bacteremia (Fig. 1). Eighteen “Pasteur” sequence type (PST) isolates other than ST131 from community-associated bacteremia (based on representative PFGE patterns) were PST3 (n = 3), PST8 (n = 3), PST2 (n = 2), PST594 (n = 2), and one each for PST13, PST39, PST44, PST53, PST253, PST478, PST666, and PST unknown by the Pasteur MLST scheme. A PBRT was conducted for 16 representative isolates of community-onset ESBL–producing E. coli. Among these 16 isolates, only nine succeeded in conjugation. Of the nine isolates, five (two of CA group and three of HA group) were positive for IncFIA replicon, two showed positive for IncI1-Iγ, and the rest were negative for all tested replicons. The two types of replicons almost equally belonged to CA group (n = 3) and HA group (n = 4).
Table 2.
Comparison of antimicrobial resistance between CA and HA in the community-onset ESBL–producing E. coli: Univariate Analysis.
| Antimicrobial agent | CA (n = 57) | HA (n = 67) | OR (95% CI) | P value |
|---|---|---|---|---|
| Ampicillin-sulbactam | 46 (81) | 54 (81) | 1.002 (0.410–2.449) | 0.997 |
| Piperacillin-tazobactam | 0 (0) | 3 (4) | 0.160 (0.005–4.998) | 0.297 |
| Cefotaxime | 55 (96) | 67 (100) | 0.165 (0.004–6.892) | 0.344 |
| Ceftazidime | 38 (67) | 57 (85) | 0.361 (0.152–0.857) | 0.021 |
| Cefepime | 35 (61) | 49 (73) | 0.590 (0.276–1.260) | 0.173 |
| Meropenem | 0 (0) | 0 (0) | — | — |
| Ertapenem | 0 (0) | 0 (0) | — | — |
| Levofloxacin | 42 (74) | 53 (79) | 0.743 (0.323–1.710) | 0.486 |
| Aztreonam | 43 (75) | 60 (90) | 0.372 (0.139–0.994) | 0.049 |
| Amikacin | 0 (0) | 0 (0) | — | — |
| Gentamycin | 24 (42) | 34 (51) | 0.710 (0.349–1.446) | 0.346 |
| Trimethoprim-sulfamethoxazole | 30 (53) | 37 (55) | 0.902 (0.444–1.832) | 0.776 |
Data are no. (%) of resistant isolates. CA, community-associated; HA, healthcare-associated; OR, odds ratio; CI, confidence interval.
Table 3.
Genetic subgroup and result of susceptibility test to fluoroquinolone of the community-onset ESBL–producing E. coli.
| CA (n = 57) | HA (n = 67) | |||
|---|---|---|---|---|
| O25b-ST131 (n = 22) | O16-ST131 (n = 6) | O25b-ST131 (n = 27) | O16-ST131 (n = 3) | |
| FimH type | ||||
| Null | 0 | 1 | 0 | 0 |
| H30 | 22 | 0 | 27 | 0 |
| H41 | 0 | 5 | 0 | 3 |
| CTX-M type | ||||
| CTX-M-14 | 6 | 3 | 5 | 2 |
| CTX-M-15 | 15 | 2 | 15 | 1 |
| CTX-M-27 | 1 | 1 | 6 | 0 |
| Other CTX-M | 0 | 0 | 1 | 0 |
| Susceptibility test to fluoroquinolone | ||||
| Resistance | 22 | 2 | 27 | 2 |
| Susceptibility | 0 | 4 | 0 | 1 |
Data are no. of resistant isolates. CA, community-associated; HA, healthcare-associated.
Figure 1.
Pulsed-field gel electrophoresis (PFGE) of XbaI-restricted DNA of 120 community-onset ESBL-producing E. coli: (A) 56 community-associated ESBL-producing E. coli; (B) 64 healthcare-associated ESBL-producing E. coli.
Risk factors of acquisition of ST131 in community-onset ESBL-producing E. coli bacteremia
According to a univariate analysis, independent risk factors for acquisition of ST131 isolates over non-ST131 isolates were diabetes mellitus (OR, 2.495; 95% CI, 1.162–5.356; P = 0.019), chronic renal insufficiency (OR, 3.317; 95% CI, 1.116–9.856; P = 0.031), absence of active cancer (OR, 2.740; 95% CI, 1.314–5.682; P = 0.007), absence of history of prior chemotherapy (OR, 3.003; 95% CI, 1.222–7.353; P = 0.016), and absence of history of surgery (OR, 3.003; 95% CI, 1.222–7.353; P = 0.016) (Table 4). A multivariate analysis showed that diabetes mellitus (OR, 2.347; 95% CI, 1.074–5.128; P = 0.032) and absence of prior chemotherapy history (OR, 2.882; 95% CI, 1.147–7.246; P = 0.024) were significant risk factors for acquisition of ST131 isolates. Antimicrobial susceptibility of the community-onset ESBL–producing E. coli appeared similar between the ST131 and non-ST131 bacteremia groups, except for the results of levofloxacin, which were associated with more resistance in the ST131 bacteremia group (Table 5).
Table 5.
Comparison of antimicrobial resistance between ST131 and non-ST131 in the community-onset ESBL–producing E. coli: Univariate Analysis.
| Antimicrobial agent | ST131 (n = 58) | Non-ST131 (n = 66) | OR (95% CI) | P value |
|---|---|---|---|---|
| Ampicillin-sulbactam | 50 (86) | 50 (76) | 1.941 (0.765–4.925) | 0.163 |
| Piperacillin-tazobactam | 2 (3) | 1 (2) | 1.933 (0.182–20.565) | 0.585 |
| Cefotaxime | 58 (100) | 64 (97) | 4.531 (0.108–189.767) | 0.428 |
| Ceftazidime | 46 (79) | 49 (74) | 1.315 (0.567–3.048) | 0.523 |
| Cefepime | 41 (71) | 43 (65) | 1.281 (0.600–2.735) | 0.522 |
| Meropenem | 0 (0) | 0 (0) | — | — |
| Ertapenem | 0 (0) | 0 (0) | — | — |
| Levofloxacin | 53 (91) | 42 (64) | 5.608 (2.014–15.616) | 0.001 |
| Aztreonam | 49 (84) | 54 (82) | 1.195 (0.464–3.077) | 0.712 |
| Amikacin | 0 (0) | 0 (0) | — | — |
| Gentamycin | 29 (50) | 29 (44) | 1.271 (0.626–2.580) | 0.507 |
| Trimethoprim-sulfamethoxazole | 28 (48) | 39 (59) | 0.651 (0.319–1.325) | 0.236 |
Data are no. (%) of resistant isolates. OR, odds ratio; CI, confidence interval.
Risk factors of acquisition of H30Rx subclone in community-onset ESBL-producing E. coli bacteremia
Based on univariate analysis, independent risk factors for acquisition of H30Rx subclone over non-ST131 isolates were diabetes mellitus (OR, 4.33; 95% CI, 1.77–10.99; P = 0.002), chronic renal insufficiency (OR, 4.99; 95% CI, 1.55–17.80; P = 0.009), absence of active cancer (OR, 3.33; 95% CI, 1.37–8.33; P = 0.010), and absence of surgical history (OR, 4.17; 95% CI, 1.41–14.29; P = 0.017). Multivariate analysis indicated no significant risk factors for acquisition of H30Rx subclone.
Discussion
We have already reported that independent risk factors of community-onset ESBL-producing E. coli bacteremia are healthcare-associated infection, malignancy, urinary tract infection, hepatobiliary tract infection, third generation cephalosporin usage during the preceding three months, and severe sepsis/septic shock9. The most common types of ESBL causing community-onset bacteremia were CTX-M-15 and CTX-M-14, and the most commonly defined sequence type (ST) was ST131 (11/60, 18.3%) during the study period (from 2005 to 2009)9.
In this study, recent epidemiology (observed between 2013 and 2014) changed with a remarkable increase of ST131 in community-onset bacteremia (from 18.3 to 46.8%). A recent dramatic increase of ST131 has been reported worldwide, causing serious concern20,21. This epidemiologic shift explains the recent increase of community-onset bacteremia in that the ST131-O25-H30 subclone is associated with persistent infections and later adverse outcomes, which are independent of multidrug resistance and the association with compromised hosts20. Although this earlier study differed in its study population compared to our study (community-onset urinary tract infection in the majority vs. community-onset bacteremia only), it supports the hypothesis that H30 has distinctive properties that allow it to evade host defenses and cause delayed complications20.
In this study, the H30Rx subclone was prevalent as ST131-O25-H30 (61.1%, 30/49), and O16-H41 strains were not negligible (15.5% of the total ST131 strains). Serotype O16 was assigned to ST131 by the Achtman MLST scheme, but was distinct from the classic ST131-O25 that has resistance to ampicillin, gentamicin, and trimethoprim-sulfamethoxazole, and showed susceptibility to fluoroquinolones and extended-spectrum cephalosporin13. This study suggests that ST131 isolates show more multidrug resistance patterns than non-ST131 isolates. A recent multicenter surveillance study of Korea reported the similar result, although the resistance rate to piperacillin-tazobactam was much higher than that seen in our results7. The single, rapidly expanding ST131 subclone H30-Rx, which is strongly associated with fluoroquinolone resistance and CTX-M-15 ESBL, is the most resistant ST131 strain14. The spread of the O16 and H30Rx clones in the Korean community could be important for transmission prevention-based control strategies because of their resistance to effective antibiotics.
PFGE patterns did not show a dominant clone for community-associated or healthcare-associated bacteremia, and PSTs were also varied by the Pasteur MLST scheme. IncFIA and IncI1-Iγ replicons were distributed evenly in community-associated and healthcare-associated bacteremia. This is suggestive of multiple evolutionary processes in the extraintestinal E. coli community in the course of the emergence of dominant ST131 clones.
Many studies on risk factors for ST131 have been reported worldwide since 201322–25. However, despite the high incidence of E. coli ST131 ESBLs in Korea, the characteristics have rarely been investigated. We investigated risk factors for acquisition of ST131 in patients with community-onset ESBL-producing E. coli causing bloodstream infections, and we adjusted confounding variables such as severity of underlying disease, and co-morbidities. Although diabetes mellitus and absence of prior chemotherapy history were significantly associated with acquisition of ST131 clones, in the present study, other underlying disease and co-morbidities were similar between the ST131 group and the non-ST131 group. These results suggest that E. coli ST131 strains producing ESBLs have disseminated in both the community and in hospitals in Korea. Previous studies investigated the risk factors for colonization or infection caused by isolates of ST131 E. coli 22–26. According to such studies, there were many risk factors for acquisition of ST131 E. coli such as recent surgery, unknown source of bacteremia, old age, long-term residency at care facility, urinary tract infection within the previous 30 days, complex infection, previous receipt of extended-spectrum cephalosporins and macrolides or fluoroquinolones, female gender, diabetes mellitus, bedridden status, secondary bacteremia, and nonuse of urinary catheter. Risk factors were different in each study, including the current study. Our interpretation is that the markedly different population in each study caused the difference in risk factors for acquisition of ST131 E. coli. There are some limitations in this study in that it was conducted at a single university hospital; therefore the results cannot be generalized to community hospitals and other university hospitals with different settings. Although community-onset healthcare-associated bacteremia group included patients with records of previous hospitalization (within the last 3–6 months) and residency at long-term care facilities, we did not analyze them separately.
In conclusion, a considerable proportion of community-onset ESBL-producing E. coli bacteremia was observed. ST131 clones appear to be associated with the spread of community-associated bacteremia exhibiting high antimicrobial resistance and highly virulent H30Rx traits, which could become a major public health concern in Korea. The potential spread of ESBL-producing E. coli causing blood stream infections is a challenge for the management of community-associated infections, so this study could be informative regarding current molecular epidemiologic shifts in community-onset bacteremia and could lead to better infection control strategies.
Acknowledgements
The study was approved by the institutional review board of our hospital (4–2015–1137).
Author Contributions
Y.A.K. and K.L. conceived the experiment(s), K.L. conducted the experiment(s), H.K., Y.A.K. and Y.S.P. analysed the results. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Young Ah Kim and Kyungwon Lee contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young Ah Kim, Email: yakim@nhimc.or.kr.
Kyungwon Lee, Email: leekcp@yuhs.ac.
References
- 1.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin. Infect. Dis. 2010;51:286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 3.Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 2011;66:1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 4.Doi Y, et al. Community-acquired extended-spectrum β-lactamase producers, United States. Emerg. Infect. Dis. 2007;13:1121–1123. doi: 10.3201/eid1307.070094. [DOI] [PubMed] [Google Scholar]
- 5.Doi Y, et al. Community-Associated extended-spectrum β-lactamase–producing Escherichia coli infection in the United States. Clin. Infect. Dis. 2013;56:641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang CI, et al. Clinical features and outcome of community-onset bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:85–88. doi: 10.1007/s10096-007-0401-6. [DOI] [PubMed] [Google Scholar]
- 7.Cha MK, et al. Comparison of the microbiological characteristics and virulence factors of ST131 and non-ST131 clones among extended-spectrum β-lactamase–producing Escherichia coli causing bacteremia. Diagn. Microbiol. Infect. Dis. 2016;84:102–104. doi: 10.1016/j.diagmicrobio.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, et al. Prevalence and characteristics of Escherichia coli sequence type 131 and its H30 and H30Rx subclones: a multicenter study from Korea. Diagn. Microbiol. Infect. Dis. 2016;84:97–101. doi: 10.1016/j.diagmicrobio.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Park YS, et al. Risk Factors and Molecular Epidemiology of Community-Onset Extended-Spectrum β-Lactamase-Producing Escherichia coli Bacteremia. Yonsei Med. J. 2014;55:467–475. doi: 10.3349/ymj.2014.55.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman ND, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, et al. Risk Factors for Occurrence and 30-Day Mortality for Carbapenem-Resistant Acinetobacter baumannii Bacteremia in an Intensive Care Unit. J. Korean Med. Sci. 2012;27:939–947. doi: 10.3346/jkms.2012.27.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA (2015).
- 13.Johnson JR, et al. Rapid and specific detection, molecular epidemiology, and experimental virulence of the O16 subgroup within Escherichia coli sequence type 131. J. Clin. Microbiol. 2014;52:1358–1365. doi: 10.1128/JCM.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman SJ, et al. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 2012;78:1353–1360. doi: 10.1128/AEM.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price LB, et al. The Epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. MBio. 2013;4:e00377–13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidjabat HE, et al. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob. Agents Chemother. 2009;53:4733–4739. doi: 10.1128/AAC.00533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute Pasteur. Institute Pasteur MLST and whole genome MLST databases. http://bigsdb.web.pasteur.fr/ (2017).
- 18.University of Warwick. Escherichia coli MLST database. http://mlst.ucc.ie/mlst/dbs/Ecoli (2017).
- 19.Carattoli A, et al. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods. 2005;63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JR, et al. The pandemic H30 subclone of Escherichia coli sequence type 131 is associated with persistent infections and adverse outcomes independent from its multidrug resistance and associations with compromised hosts. Clin. Infect. Dis. 2016;62:1529–1536. doi: 10.1093/cid/ciw193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karfunkel D, Carmeli Y, Chmelnitsky I, Kotlovsky T, Navon-Venezia S. The emergence and dissemination of CTX-M-producing Escherichia coli sequence type 131 causing community-onset bacteremia in Israel. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:513–521. doi: 10.1007/s10096-012-1765-9. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee R, et al. Escherichia coli type 131 is a dominant, antimicrobial resistant clonal group associated with healthcare and elderly hosts. Infect. Control Hosp. Epidemiol. 2013;34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Cerero L, et al. Escherichia coli belonging to the worldwide emerging epidemic clonal group O25b/ST131: risk factors and clinical implications. J. Antimicrob. Chemother. 2014;69:809–814. doi: 10.1093/jac/dkt405. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Barroso I, et al. Bacteremia due to non-ESBL-producing Escherichia coli O25b:H4 sequence type 131: insights into risk factors, clinical features and outcomes. Int. J. Antimicrob. Agents. 2017;49:498–502. doi: 10.1016/j.ijantimicag.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Cho SY, et al. Clinical features and treatment outcomes of bloodstream infections caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type 131. Microb. Drug Resist. 2015;21:463–469. doi: 10.1089/mdr.2014.0261. [DOI] [PubMed] [Google Scholar]
- 26.Chung HC, et al. Bacteremia caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob. Agents Chemother. 2012;56:618–622. doi: 10.1128/AAC.05753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.