Abstract
Deletions of the Plasmodium falciparum hrp2 and hrp3 genes can affect the performance of HRP2-based malaria rapid diagnostic tests (RDTs). Such deletions have been reported from South America, India and Eritrea. Whether these parasites are widespread in East Africa is unknown. A total of 274 samples from asymptomatic children in Mbita, western Kenya, and 61 genomic data from Kilifi, eastern Kenya, were available for analysis. PCR-confirmed samples were investigated for the presence of pfhrp2 and pfhrp3 genes. In samples with evidence of deletion, parasite presence was confirmed by amplifying three independent genes. We failed to amplify pfhrp2 from 25 of 131 (19.1%) PCR-confirmed samples. Of these, only 8 (10%) samples were microscopic positive and were classified as pfhrp2-deleted. Eight microscopically-confirmed pfhrp2-deleted samples with intact pfhrp3 locus were positive by HRP2-based RDT. In addition, one PCR-confirmed infection showed a deletion at the pfhrp3 locus. One genomic sample lacked pfhrp2 and one lacked pfhrp3. No sample harbored parasites lacking both genes. Parasites lacking pfhrp2 are present in Kenya, but may be detectable by HRP-based RDT at higher parasitaemia, possibly due to the presence of intact pfhrp3. These findings warrant further systematic study to establish prevalence and diagnostic significance.
Introduction
Antigen-detecting RDTs play a key role in malaria control successes in many parts of the world, and the global availability and scale of use of RDTs has increased dramatically over the last 10 years1. Most tests currently in use detect Plasmodium falciparum histidine-rich protein 2 (HRP2) and/or plasmodium lactate dehydrogenase (pLDH) antigens; data indicate that at least some RDT products also detect HRP3 due to a shared antigenic epitope2,3. Compared with RDTs that detect pLDH, in general HRP2-detecting RDTs are more sensitive and are less susceptible to degradation from heat and humidity during transport and storage4. In sub-Saharan Africa, which bears 90% of the global malaria burden, RDTs accounted for 74% of diagnostic testing among suspected cases in 2015, and HRP2-based tests are the most widely used5.
Performance of HRP2-based RDTs can be affected by factors including antigenic variability of the target protein, antigen persistence in the bloodstream following elimination of parasites, and parasite density below the RDT threshold of detection3,6. In one study in western Kenya, HRP2-based RDT gave false-negative results in 5–10% and 25–30% of samples compared to microscopy and PCR respectively7. Historically such results have been ascribed to varying thresholds of detection for the different tests.
More recently, concerns have been raised that such false-negative results may reflect presence of parasites with pfhrp2 gene deletions or mutations. There has long been evidence of P. falciparum parasites lacking the pfhrp2 gene in regions of South America8, and public health authorities have always cautioned against using HRP2-based RDTs in the Amazon River basin. In the past few years, reports have emerged of pfhrp2 mutations or deletions in Africa and India9–12 leading to false-negative RDT results. More recently, in Eritrea, 80% of microscopically confirmed positive samples were negative by HRP2-based RDT, a finding attributed to deletion of the pfhrp2 gene13,14.
The existence of parasites lacking pfhrp2 would affect RDT accuracy in a broader range of malaria-endemic regions and would have significant implications for RDT implementation, clinical case management, and malaria control efforts15. Currently, there is little evidence to document the true extent of such mutations in Africa. Guidelines have recently been issued for study design and molecular assays to assess gene mutations16.
To extend current knowledge on the relationship between RDT failure and deletions of pfhrp2 in African populations, we investigated samples from a cross-sectional survey in Mbita (western Kenya) and from published P. falciparum genome sequence data from Kilifi (eastern Kenya).
Results
Detection of P. falciparum malaria
Of the 274 patient samples, 165 (60%) were positive by RDT. Of these, 114 (70%) were positive by HRP2 band only; 51 (31%) were positive by both HRP2 and pLDH bands. Microscopy detected parasites in 76/272 (28%) (Microscopy data were missing for two samples and of these, two (2.8%) were RDT negative. Of the 196 microscopic negative samples, 93 (52%) were RDT positives). The minimum parasite density reported by microscopy was 80 parasites/µL, suggesting that this was the threshold for detection by the field microscopist. Using 18SrDNA PCR and qPCR assays, 131 (47.8%) and 114 (41.6%), respectively, were positive for P. falciparum (Fig. 1).
Figure 1.
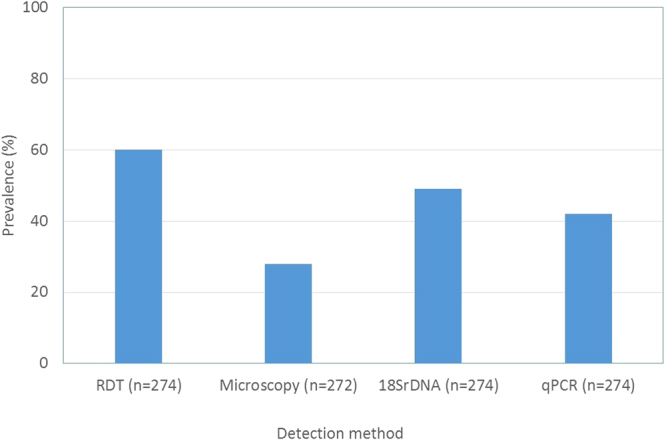
Proportion of different detection methods positive for malaria. Histidine-rich protein 2 (HRP2)-based rapid diagnostic tests (RDTs) generated more positive samples compared to other methods. PCR methods produced 13–20% more positive results compared to microscopy.
PCR detection of pfhrp2 and pfhrp3
In this report, the term pfhrp2- or pfhrp3-negative is used for isolates that were negative by PCR for pfhrp2 and/or pfhrp3 in duplicate. The term pfhrp2- or pfhrp3-deleted is used for isolates that met the following four criteria: microscopy positive, three independent control genes amplified (18SrDNA, msp1 and msp2), pfhrp2- or pfhrp3-negative in duplicate, and parasite density ≥5 p/μL. Pfhrp2 genotyping of samples positive by 18SrDNA produced 51% (67/131) positive and 10.6% (14/131) negative results. The remaining 38.2% samples (50/131) either had parasitaemia ≤5 p/μL by qPCR or were negative by msp1/msp2 PCR and were excluded from further pfhrp2 analysis. Therefore, only 14 of the pfhrp2-negative samples were included in further gene deletion call (Fig. 2). No significant difference in multiplicity of infection was observed between pfhrp2-deleted samples (n = 8, mean = 4.77, range 3–7) and pfhrp2-positive samples (n = 25, mean = 4.8, range 3–7).
Figure 2.
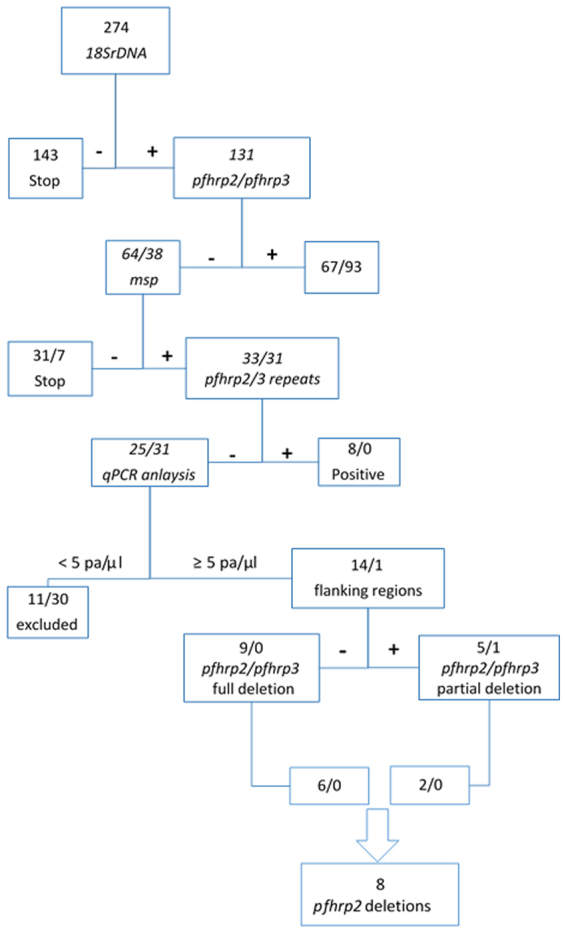
Flow diagram showing the strategy for determining deletion of pfhrp2 and pfhrp3 genes. Total of 274 Plasmodium falciparum samples were amplified using 18SrDNA PCR. All the positives (131 samples) were then subjected to pfhrp2 and pfhrp3 PCR amplifications. Pfhrp2 and pfhrp3 negative samples (64 and 38 respectively) were then further subjected to PCR amplification using msp1 and msp2 primers to confirm the presence of parasites. Pfhrp2 and pfhrp3 PCR amplification were repeated if the samples were msp1/msp2 and 18SrDNA positive but pfhrp2/pfhrp3 negative (33 and 31 respectively). A qPCR analysis was then performed on those samples negative by pfhrp2 and pfhrp3 but positive by other PCR methods (25 and 31 respectively). Samples with parasitaemia less than 5 parasites per microliter were excluded (11 and 30 respectively). The upstream and downstream flanking regions of pfhrp2 and pfhrp3 for the remaining samples were then amplified using specific primers (14 and 1 respectively). To reduce the risk of false pfhrp2-negative due to low parasite density, only microscopy positive samples were called pfhrp2 and pfhrp3 deletions (8 samples for pfhrp2 only).
Pfhrp3 genotyping of samples positive by 18SrDNA PCR gave a positive result for 71% (96/131). A single sample that was positive by all other criteria, including for pfhrp2, was negative for pfhrp3. Conversely, all 14 of the pfhrp2 negative samples were pfhrp3 positive.
Association between parasitaemia and pfhrp2 PCR results
To test whether parasitaemia affected the pfhrp2 and pfhrp3 PCR outcome, we compared the mean parasitaemia for samples that were positive by 18SrDNA and qPCR, excluding samples with parasite density ≤5 p/μL by qPCR. Mean qPCR parasitaemia for pfhrp2 positive and negative samples, respectively, was 688 ± 923 (n = 73, mean + SD) and 611 ± 1444 (n = 18, mean + SD) p/μL, a statistically significant difference (Kruskall-Wallis test, P = 0.01; Table 1). However, if only microscopy-positive samples are included, the difference is not significant (n = 65, p = 0.48). The same analysis was not done for pfhrp3 as the negative sample size was only one.
Table 1.
Correlation between pfhrp2 PCR result and Plasmodium falciparum parasitaemia.
| 18SrDNA + ve N = 131 | 18SrDNA + ve > 5 parasite/μl N = 91 | 18SrDNA/msp1/msp2 PCR + ve > 5 parasite/ul N = 15 | Mean parasite per µL (range) by qPCR1 | Mean parasite/µL by microscopy1 | P-value2 | |
|---|---|---|---|---|---|---|
| pfhrp2 + RDT − | 1 (0.8%) | 1 (1.1%) | 1 | 167 | 0 | 0.012a 0.482b |
| pfhrp2 + RDT + | 74 (56.4%) | 72 (79.1%) | NA | 689 (8.2–3923) | 1317 (0–7840) | |
| pfhrp2− RDT+ | 34(26%) | 10 (11%) | 8 | 1088 (8.5–6097) | 1540 (240–3560) | |
| pfhrp2− RDT− | 22 (16.8%) | 8(8.8%) | 6 | 10 (1.2–28.1) | 0 |
1For samples that were positive by both 18SrRNA and qPCR, with qPCR parasitaemia ≥5 parasites per µL.2 Kruskal-Wallis rank test for2a pfhrp2+ and pfhrp2− of all samples, and2b for samples with microscopically detected parasitaemia (i.e. excluding samples with submicroscopic parasitaemia). S18rDNA, 18 ribosomal RNA subunit gene; pfhrp2 and pfhrp3, Plasmodium falciparum histidine-rich protein 2 and 3 respectively; RDT, rapid diagnostic test.
Confirmation of pfhrp2 and pfhrp3 deletions
QPCR parasite density was used to further investigate presence or absence of pfhrp2 and pfhrp3 genes in microscopy-negative samples. Of the total 15 samples included in the pfhrp2 and pfhrp3 deletion call, one was pfhrp2-positive and pfhrp3-negative with a parasite density of 167 p/μL and negative RDT result. The remaining 14 samples were pfhrp2-negative and pfhrp3-positive (pfhrp2−/pfhrp3+); of these, 42.8% (6/14) were RDT-negative. All six of these RDT-negative samples were also negative by microscopy suggesting that the parasitaemia was sub-microscopic. Fifty-seven per cent (8/14) of the pfhrp2−/pfhrp3+ and RDT-positive (either HRP only, or both HRP and pLDH) samples were also positive by microscopy with a mean density of 1540 ± 1123 p/μL and mean relative parasite density by qPCR of 1358 ± 1680. Therefore, 9% (8/89) of samples with adequate parasitaemia as measured by both microscopy and qPCR were pfhrp2-deleted (Table 2). In addition, 6.7% (6/89) and 1.1% (1/89) of qPCR-confirmed samples were pfhrp2 and pfhrp3-negative, respectively. All the pfhrp2−/pfhrp3+ samples were positive by three separate PCR assays, two of which targeted single-copy genes (18sDNA, msp1 and msp2).
Table 2.
Plasmodium falciparum samples with pfhrp2 or pfhrp3 deletion. Nine samples positive by three independent PCR assays, two of which target single-copy genes, were negative by PCR for pfhrp2 or pfhrp3. PCR outcome of the corresponding flanking regions are also shown. *Positive by qPCR with parasitaemia of 167 parasites per µL. Pfhrp2 and pfhrp3, Plasmodium falciparum histidine-rich protein 2 and 3 respectively; RDT, rapid diagnostic test; PF3D7_0831900 and PF3D7_0831700, upstream and downstream flanking regions of pfhrp2, PF3D7_1372100 and PF3D7_1372400, upstream and downstream flanking regions of pfhrp3.
| Isolate | RDT | Parasitaemia | PF3D7_0831900 | pfhrp2 | PF3D7_0831700 | PF3D7_1372100 | pfhrp3 | PF3D7_1372400 |
|---|---|---|---|---|---|---|---|---|
| K165 | + | 3560 | − | − | + | + | + | + |
| K027 | + | 240 | − | − | − | + | + | + |
| K065 | + | 1720 | − | − | − | + | + | + |
| K031 | + | 2400 | − | − | − | + | + | + |
| K063 | + | 2120 | − | − | − | + | + | + |
| K233 | + | 840 | − | − | − | + | + | + |
| K263 | + | 480 | − | − | − | + | + | + |
| K320 | + | 960 | − | − | + | + | + | + |
| K182 | − | 0* | + | + | + | − | − | + |
Relationship between RDT and parasitaemia of pfhrp2−/pfhrp3+ parasites
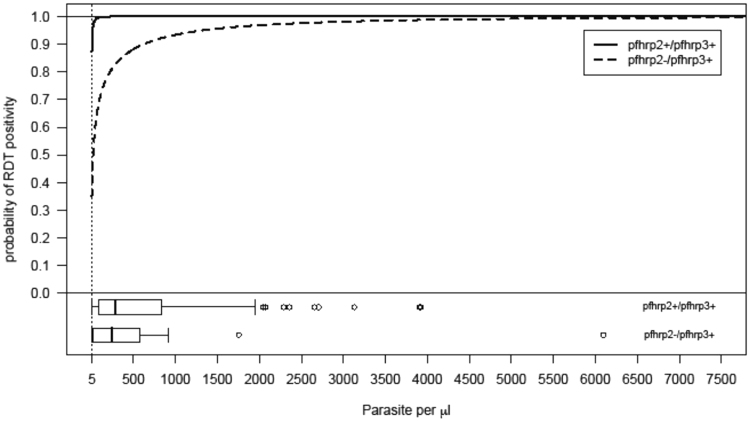
To explore the possibility that differences in parasitaemia of the pfhrp2−/pfhrp3+ samples affect RDT results, samples were categorized by RDT result. Those pfhrp2−/pfhrp3+ samples that were RDT-positive had significantly higher parasite density (mean + SD, 1358 ± 1980 p/µL) compared to the RDT-negative samples (mean + SD, 17 ± 9, p = 0.0014). However, with available data it is not possible to determine whether these RDT were negative due to absence of pfhrp2 or due to low HRP2 antigenemia resulting from low parasitaemia. To extend this analysis, generalized linear models including parasitaemia and pfhrp2/pfhrp3 status as covariates were fit to the data from field samples with parasitaemia ≥5 μl (See Supplementary Table 1). The best model for the data was based on complementary log-log regression and it would appear to describe the data well (Hosmer-Lemeshow goodness-of-fit test, p = 0.90) and a good prediction power (area under the receiver operating characteristic curve = 0.96; 95% CI = (0.92–1)). According to this model for the data, the probability of RDT positivity for pfhrp2−/pfhrp3+ samples increased with parasitaemia and was close to the one for pfhrp2+/pfhrp3+ samples for parasitaemia levels >1000 μl (Fig. 3). Such proximity of the two probabilities together with the reduced number of pfhrp2−/pfhrp3+ samples implied that they were not statistically different as function of parasitaemia (effect of HRP3 = 1.09, SE = 0.73, p = 0.14; Supplementary Table 1).
Figure 3.
Complementary log-log regression model for the probability of RDT positivity as function of parasitaemia and pfhrp2/pfhrp3 status in field samples from western Kenya with parasitaemia ≥5 parasite per μl (n = 91): The model shows the probability of RDT positivity in samples where both pfhrp2 and pfhrp3 genes are present (pfhrp2+/pfhrp3+, solid line) and in samples with pfhrp2 deletion (pfhrp2−/pfhrp3+, dashed line) across a range of parasitaemia (pfhrp2−/pfhrp3- not included in the model as there were no such profile in the samples). The corresponding boxplots of parasitaemia as function of pfhrp2/pfhrp3 status are also shown at the bottom of the plot.
Analysis of pfhrp2 and pfhrp3 genes using genomic data from Kilifi
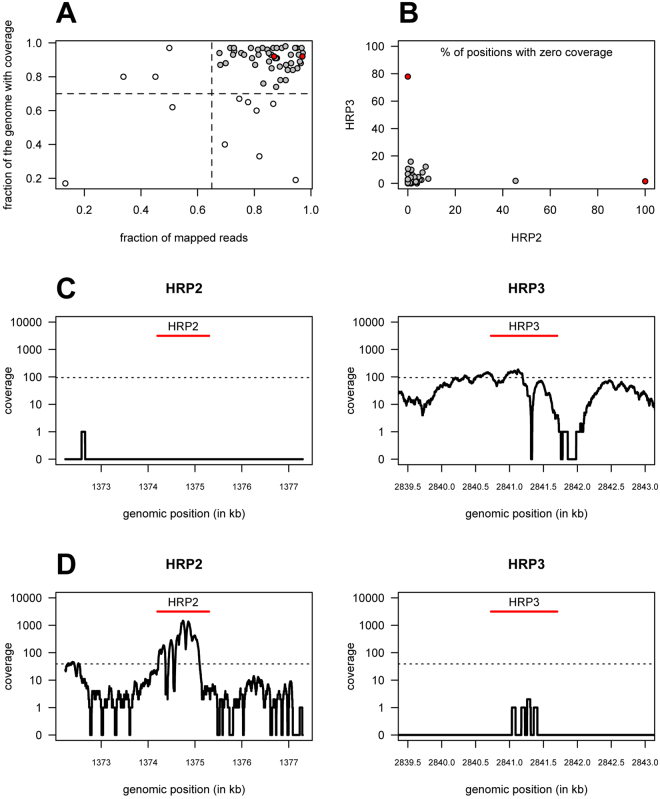
According to the cutoff values for coverage in Fig. 4 (0.7 and 0.65 cutoff values for genome with coverage and mapped reads respectively), 12 of 61 genomes could not be mapped onto the 3D7 reference genome (Fig. 4A), and thus they were discarded from further analysis.
Figure 4.
Coverage analysis of pfhrp2 and pfhrp3 genes using whole genome sequencing data from Kilifi, eastern Kenya. (A) Scatterplot of the percentage of genome with coverage (i.e., percentage of positions with at least one read mapped) versus percentage of reads that could be mapped on the 3D7 reference genome before quality checks (n = 61). Vertical and horizontal lines indicate the quality control cut-off used in the analysis while grey and white dots represent the genomes selected and not selected for the subsequent coverage analysis. (B) Scatterplot of the percentage of positions of pfhrp2 and pfhrp3 genes with no coverage after quality checks (n = 49). Red dots in A and B represent samples with possible evidence for a deletion in either gene. (C and D) Coverage profiles of the pfhrp2 and pfhrp3 genes and their respective 2 kb flanking regions to the either side for two genomes with possible evidence for a deletion in either gene, where dotted lines represent the average coverage of the whole genome in the respective samples.
Among the 49 remaining genomes, only two showed a high percentage (>50%) of genomic positions with zero coverage in pfhrp2 and/or pfhrp3 genes (Fig. 4B). Data from one of these genomes revealed no coverage for the entire pfhrp2 locus with the exception of coverage provided by a single read mapped onto the left flanking region, providing evidence for a possible deletion of this gene (Fig. 4C). In contrast, the pfhrp3 locus shows some variations in coverage but mostly in a good agreement with the average coverage over the whole genome. This result suggests that the lack of coverage observed in the pfhrp2 locus is unlikely to have resulted from problems in mapping these genes. Data from the other genome showed a possible deletion of the pfhrp3 gene with only three reads mapped onto its extended locus (Fig. 4D). Given the good agreement between the coverage observed in the pfhrp2 coding regions and the overall average coverage, the existence of three mapped reads onto the pfhrp3 gene could have simply resulted from a mapping artifact due to sequence homology between the two genes. Finally, there is no evidence for the presence of genomes with possible deletions in both genes (Pearson-Clopper 95% confidence interval for the respective proportion: 0–7.3%).
Discussion
This study provides evidence of P. falciparum lacking the pfhrp2 gene in two areas of Kenya. Eight of 89 (9%) samples analyzed from western Kenya were pfhrp2-deleted; and genomic data from eastern Kenya identified a genome with no coverage for the pfhrp2 gene or flanking regions. In addition, we have confirmed that parasite density is a factor both for PCR detection of pfhrp2 and for determining RDT outcome in samples lacking pfhrp2, particularly in samples with submicroscopic parasitaemia. Of note, the microscopically-confirmed pfhrp2-deleted samples were positive by HRP2-based RDTs. This is surprising as previously reported parasites lacking pfhrp2 were generally negative by HRP2-based RDTs regardless of pfhrp3 status2,8,10,17 though some reported positive RDT results8,18.
The pfhrp2-negative samples included in this deletion call had microscopy-confirmed P. falciparum density of at least 250 p/μL. The integrity of the DNA in the samples and exclusion of PCR inhibitors was verified by successful amplification using three separate PCR assays, two of which targeted single-copy genes. One other possible cause for pfhrp2- or pfhrp3-negative is sequence variability of the region where the primers bind. However, the upstream and downstream segments of pfhrp2 also gave negative results for at least six samples. Possible conclusions are that either these HRP2-based RDT results were false-positive, or the HRP signal was generated by protein expressed from pfhrp3 or other genes; the latter explanation seems more plausible as explained below.
These results are in agreement with an in vitro study showing that the D10 parasite clone, which lacks pfhrp2 19, gave positive RDT results on two HRP2-based RDT at parasitaemia ≥1000 p/μL8. Six of the eight RDT-positive Kenyan samples with pfhrp2 deletion had parasitaemia ≥890 p/μL (mean, 1358), which supports the in vitro finding. Sequence analysis of HRP2 and HRP3 revealed that they share similar alanine- and histidine-rich amino acid repeats and that four of the eight repeats found in HRP3 are identical to those of HRP22. It is possible that antibodies raised against HRP2 cross-react with epitopes of HRP3 to generate an RDT signal, as previously reported20. However, the level of HRP3 contribution to the signal is unknown. It is also not clear whether the HRP3 expressed is enough to generate signal in the absence of HRP2 expression or if other proteins are involved, as Baker et al. observed no compensatory increase of pfhrp3 transcription in the absence of pfhrp2 21. However, a study using yeast knockout collections reported that deletion of individual genes in a genome results in a genomic disturbance capable of driving the selection for mutations elsewhere in the genome22. The loss of pfhrp2 may drive selection for mutations in pfhrp3 or other similar genes particularly in the alanine- and histidine-rich amino acid repeats. This could be established by sequencing pfhrp3 in parasites with and without pfhrp2 and determining whether there is any sequence or repeat pattern difference. Overall, the genotypic data presented here together with the in vitro study elsewhere8 show that parasites with the pfhrp2−/pfhrp3+ genetic profile can produce a positive result on at least some brands of HRP2-based RDTs.
Genomic data from eastern Kenya samples also showed evidence for parasites with possible deletions in pfhrp2 or pfhrp3 genes. If the detected deletions are true, it is difficult to infer whether they existed at the time of original infection, or resulted from a subsequent laboratory adaptation. Illumina short read sequencing used to generate the genomic data also poses a limitation of this study because of its propensity to generate uneven coverage profiles across the genome (e.g., high coverage at certain loci and little to no coverage at other loci) thus possibly influencing the probability of correctly detecting possible gene deletions. The origin of such coverage heterogeneity can be due simply to library preparation and sequencing bias of smaller fragments. The highly AT-enriched P. falciparum genome also is known to generate biases in the coverage distribution23. In theory, the use of alternative sequencing platforms that are able to generate longer reads (i.e., pacbio or minION) could produce higher quality coverage data either by mapping onto the 3D7 reference genome or by de novo assembly of the sampled genomes. In practice, this study used publicly available genomic data generated elsewhere and, therefore, it was not possible to perform alternative genomic approaches. However, the exclusion of genomic samples with sufficiently large proportions of regions with zero coverage minimized the chance of inferring deletions in the pfhrp2 and pfhrp3 loci when there are in fact none. A limitation of our analysis is the possibility of underestimating the true number of deletions due to challenges in mapping these genes. Pfhrp2 and pfhrp3 genes share high sequence homology; therefore mapping algorithms such as the bwa-mem, used in the pre-processing of the samples, usually allocate reads randomly to different homologous sequences present in the genome. If this is the case, however, the existence of a deleted pfhrp2 gene in presence of fully functional pfhrp3, although limiting the power of genomic analysis, is unlikely to have strong consequences in the RDT testing as shown by our model (Fig. 3).
These data also confirm the role of parasitaemia in generating RDT signal by the pfhrp2−/pfhrp3+ parasites. The model shows that parasites lacking pfhrp2 produce positive HRP test bands at similar parasitaemia to those with the intact gene particularly at higher parasitaemia, supporting a previous report of the absence of compensatory increase of the HRP3 protein21. This was evident on the six pfhrp2-negative samples, which were also negative by RDT and microscopy but positive for P. falciparum by qPCR at very low parasitaemia. We did not identify these parasites as lacking pfhrp2 since the negative result was more likely due to low parasitaemia, and a criterion for establishing presence of deletion is the confirmation of parasites by microscopy16. We identified the only sample with pfhrp3-negative result as deletion, as the sample had adequate parasitaemia by qPCR, and microscopy might have been false negative as the slide was only read once. Further study of samples with adequate levels of parasitaemia will help to confirm these findings.
The sensitivity and clinical performance of HRP2-based RDTs in Kenya are not expected to be affected by the findings of this study. In this study, HRP2-based RDT results were positive in the presence of P. falciparum infection regardless of pfhrp2 gene status, particularly at higher parasitaemia (above approximately 1360 p/µL), presumably due to antigen-antibody cross-reactivity with HRP3 or other proteins. In addition, parasitaemia tends to be higher in symptomatic patients and therefore the effect of pfhrp2 deletion on patient diagnosis is expected to be even lower. Reassuringly, a recent study that evaluated eight different HRP2-based RDTs among 500 symptomatic and asymptomatic individuals in western Kenya, and reported sensitivity compared with microscopy of 90–95%7.
These data illustrate the difficulty of amplifying pfhrp2 and pfhrp3 genes in samples with submicroscopic parasite density, and support the guidance to rule out low parasitaemia as the cause of negative PCR results before reporting pfhrp2 and pfhrp3 deletions16. These results also highlight that to determine the true prevalence of pfhrp2 deletions in the population, the surveys should include HRP2 RDT-positive, as well as HRP2-RDT negative samples. Furthermore, investigations should include PCR testing for both pfhrp2 and pfhrp3, because this may be the most accurate way to estimate the risk of false negative RDT samples due to pfhrp2 deletions in clinical settings. A limitation of this study is lack of HRP ELISA data to quantify HRP antigenemia and further PCR findings. The difference between pfhrp2+/pfhrp3+ and pfhrp2−/pfhrp3+ samples particularly at lower parasitaemia could be due to difference in the amount of antigens generated and in relative affinity of HRP2 and HRP3 for the RDT antibodies, which we could not measure and which our model (Fig. 3) did not take into account. This study is also limited in that the genomic and genotype data are not from the same samples or from the same area in Kenya, although malaria endemicity in both regions is similar24.
In summary, results of this study demonstrate that P. falciparum isolates lacking pfhrp2 genes are present in Kenya, they infect humans, and cause symptomatic disease but they are also still detectable by at least some brands of HRP2-based RDTs in the presence of an intact pfhrp3 expressing antigen levels found at parasitaemia ≥1000p/µL However, these findings warrant further systematic study to understand factors driving pfhrp2 deletions; to establish the true prevalence and distribution of pfhrp2 and pfhrp3 deletions and to monitor for false negative HRP2 based RDTs and the potential implications for diagnostic testing strategies.
Materials and Methods
Study populations
A total of 274 blood samples were collected between January and July 2014 from asymptomatic children aged 5 to 12 years attending schools near Mbita, western Kenya, as part of a study on mosquito behavior and the odor profile of malaria infected individuals. The study received approval from the Kenya Medical Research Institute Ethical Review Committee (KEMRI/RES/7/3/1) and the London School of Hygiene and Tropical Medicine Ethics Committee (reference 8510). Written informed consent was obtained from a parent or guardian of each participating child. All experiments were performed in accordance with relevant guidelines and regulations.
Data from 61 P. falciparum samples from Kilifi District, eastern Kenya, were obtained from the MalariaGEN Plasmodium falciparum Community Project as described in Genomic epidemiology of artemisinin resistant malaria25. These genomic data were generated from parasite samples which were obtained from pediatric patients enrolled in a clinical trial as described elsewhere, and then cultured in the lab until full adaptation26.
Blood sample preparation of the Mbita samples
Finger-prick blood from each participant was analyzed by light microscopy for the presence of parasites; applied to an RDT (SD Bioline Malaria Ag P.f/Pan test, HRP2/pan-pLDH, catalog number 05FK60, Standard Diagnostics, Hong Dong, Korea); and used to prepare dried blood spots (DBS) which were stored at room temperature until they were shipped to London School of Hygiene & Tropical Medicine (LSHTM). Slides were read by a study team microscopist trained at the Walter Reed Project in Kisumu, Kenya, and a subset (10%) of slides were re-read independently by a second microscopist at the LSHTM Malaria Reference Laboratory, London, UK. For the double-read subset, correlation between the two readers was moderate (r = 0.76, P < 0.001). RDTs were prepared and interpreted by a study team member according to the manufacturer’s instructions.
The RDTs and DBS were sent to LSHTM for molecular analysis. RDTs were available for all 274 samples, while DBS were available for 141. (Investigators for the primary entomology study modified their protocol to include DBS only midway through the study). Both template types were available for 141 samples, which were used as a comparison to validate the extraction of DNA from RDTs and subsequent quantification of parasitaemia by qPCR, with no significant difference observed (A Robinson, in preparation).
DNA was extracted using a robotic extraction system (QIAsymphony) using DSP DNA mini kit according to the manufacturer’s protocol (QIAGEN, Germany). In order to reduce costs, we validated the DSP DNA mini kit instead of using the manufacturer-recommended investigator kit for DNA extraction from DBS and RDTs with no significant difference (A Robinson, in preparation). DBS were pre-treated according to the manufacturer’s manual before DNA extraction. Briefly, 1 × 3 mm diameter punch from the DBS or three cuts of the nitrocellulose strip of the RDTs27 were placed in the deep-well plate. Buffer ATL (180 µL) and proteinase K (20 µL) were added to each well and mixed by thermomixer at 900 rpm at 56 °C for 15 min. The deep-well plate was then placed directly into the sample compartment of the QIAsymphony for DNA extraction. The extracted DNA samples were stored at −20 °C for further molecular biology investigation.
Amplification of pfhrp2 and pfhrp3
The presence of parasites was detected by standard PCR targeting the 18S ribosomal RNA gene of P. falciparum (18SrDNA), which has a limit of detection of 0.1 parasite per µL (0.000002% parasitaemia)28,29. All samples positive by 18SrDNA assay were then subjected to pfhrp2 and pfhrp3 genotyping using previously published PCR conditions and primers as described by Baker et al.2,8. The elongation temperature was modified to 60 °C and annealing temperature to 50 °C in the repeated experiments of pfhrp2 and pfhrp3 (Fig. 2)11.
Confirmation of pfhrp2 and pfhrp3 deletion
To verify the presence of parasites in the pfhrp2-negative samples, PCR assays targeting msp1 and msp2 genes were amplified using previously reported methods30. Microscopy-positive samples with positive results for 18SrDNA, msp1 and msp2 assays but negative results for pfhrp2 /pfhrp3 were considered pfhrp2 /pfhrp3-deleted after excluding low parasitaemia in order to avoid incorrect deletion calls (See Fig. 2 for algorithm).
Parasitaemia estimation by qPCR
To explore the role of low parasitaemia in apparently negative pfhrp2 /pfhrp3 genotyping results, the parasitaemia of each sample was quantified using a previously published PgMET qPCR assay, which has a limit of detection of 5 parasites per µL31,32. All samples, including samples that were negative by 18SrDNA assay, were included in order to capture any false negatives. International Standard was used as a positive control (calibrator) with the original sample (9.6% parasitaemia) diluted to 0.0096% (~500 parasite per µL)33. The accuracy of this quantification was then compared to parasitaemia generated by microscopic examination of blood films, when available.
Statistical analysis of field samples
The main outcomes for this study were results of HRP-based RDT, results of pfhrp2 and pfhrp3 PCR, and parasitaemia as determined by microscopy and qPCR. Parasite density measured by qPCR for each sample was calculated using International Standard (IS) as a calibrator32,33, which was used as factor to calculate the parasitaemia of each sample. Parasitaemia was compared between groups (e.g. RDT-positive vs RDT-negative samples, pfhrp2-positive vs pfhrp2-negative) using Kruskal-Wallis rank test. Samples below the qPCR limit of detection (≤5 parasites per µL [p/μL]) were excluded from further pfhrp2 and pfhrp3 deletion call. Generalized linear models for binomial responses were used to study the probability of RDT positive with log parasitaemia, pfhrp2/pfhrp3 status, as described in S1 Table. The best model was selected according to the Akaike’s and Bayesian Information Criteria. The statistical validation of this model was made via the Hosmer-Lemeshow goodness-of-fit test using 5 bins to calculate the quantiles. The area under the curve (AUC) of the receiver operating characteristic curve predicted by the model was also estimated in order to assess the predictive power of the model. For the Hosmer-Lemeshow test, a p-value > 0.05 indicated a good fit of the model while an estimate of the AUC close to 1 provided evidence for a very good prediction of the observed RDT positivity data by the model.
Data analysis was performed using either STATA (version 14, USA), or R (version 3.3.2, http//www.r-project.org). In the R software, the packages ResourceSelection and pROC were specifically used to perform the Hosmer-Lemeshow test and to estimate AUC, respectively.
Analysis of pfhrp2 and pfhrp3 genes using whole-genome sequencing data
The 61 P. falciparum samples from Kilifi underwent whole-genome sequencing and were processed as described in detail elsewhere34. Briefly, for each sample, the raw sequence reads were aligned onto the 3D7 reference genome (version 3.0) using the bwa-mem short read alignment algorithm. For each sample, the total number of mapped reads per position (coverage) was calculated for the whole genome. The genomic analysis of pfhrp2 and pfhrp3 genes (PF3D7_0831800 and PF3D7_1372200) was restricted to position 1,372,236-1,377,299 of chromosome 8 (pfhrp2 locus), and to position 2,838,727-2,843,703 of chromosome 13 (pfhrp3 locus). These two genomic regions included the coding regions of each gene and the respective 2 kb flanking regions (to either side).The corresponding coverage data was then analyzed to identify samples with possible deletions of these genes, using a statistical description of the coverage data. The application of existing genomic tests for detecting deletions using coverage data were outside the scope of this paper as they typically require integration of information from the entire genome23,35.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Supplementary Table S1. Data generated using RDT, pfhrp2, pfhrp3 and parasitaemia (>5 parasites per microliter, n=91).
Acknowledgements
We thank the primary study participants and their parents, and village and district authorities, for their assistance obtaining samples in Mbita; Geoffrey Omondi Olweru and David John Odoyo for their contributions to the Mbita field work; Angela Hunt Cooke (MRL, LSHTM) for microscopic slide reading and Ernest Diez-Benavente (LSHTM) for helping with the access to the genomic data. We are also grateful to Qin Cheng (Australian Army Malaria Institute) for valuable advice on laboratory methods during preparatory discussions for this work. We also thank ACT Consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the London School of Hygiene & Tropical Medicine, for sponsoring the laboratory work. The collection of samples in Mbita was supported by the Netherlands Organization for Scientific Research, division Medical Science (grant number 91211038). Fundação para Ciência e Tecnologia for partially funding N.S (grant UID/MAT/00006/2013).
Author Contributions
K.B.B., C.J.S., J.C. and H.H. contributed to study design. A.R., A.O.B., J.G.B. coordinated and conducted the field study. K.B.B., J.B., A.R. and J.M. did laboratory work. K.B.B., N.S. and A.R. analyzed the data. K.B.B., N.S., C.J.S., J.C. and H.H. wrote the manuscript. All authors contributed to and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15031-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Information note on recommended selection criteria for procurement of malaria rapid diagnostic tests (RDTs). Geneva, Switzerland: World Health Organization (2016).
- 2.Baker J, et al. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis. 2005;192:870–877. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- 3.Lee N, et al. Effect of sequence variation in Plasmodium falciparum histidine- rich protein 2 on binding of specific monoclonal antibodies: Implications for rapid diagnostic tests for malaria. J Clin Microbiol. 2006;44:2773–2778. doi: 10.1128/JCM.02557-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 6 (2014–2015). Geneva: World Health Organization (2015).
- 5.WHO. World Malaria Report 2016. Geneva: World Health Organization (2016).
- 6.Houze S, Boly MD, Le Bras J, Deloron P, Faucher JF. PfHRP2 and PfLDH antigen detection for monitoring the efficacy of artemisinin-based combination therapy (ACT) in the treatment of uncomplicated falciparum malaria. Malar J. 2009;8:211. doi: 10.1186/1475-2875-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanja EW, et al. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar J. 2016;15:456. doi: 10.1186/s12936-016-1508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamboa D, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharti PK, et al. Prevalence of pfhrp2 and/or pfhrp3 Gene Deletion in Plasmodium falciparum Population in Eight Highly Endemic States in India. PLoS One. 2016;11:e0157949. doi: 10.1371/journal.pone.0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koita OA, et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg. 2012;86:194–198. doi: 10.4269/ajtmh.2012.10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parr JB, et al. Pfhrp2-Deleted Plasmodium falciparum Parasites in the Democratic Republic of the Congo: A National Cross-sectional Survey. J Infect Dis. 2017;216:36–44. doi: 10.1093/infdis/jix347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozycki CT, et al. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J. 2017;16:123. doi: 10.1186/s12936-017-1768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berhane A, et al. Rapid diagnostic tests failing to detect Plasmodium falciparum infections in Eritrea: an investigation of reported false negative RDT results. Malar J. 2017;16:105. doi: 10.1186/s12936-017-1752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berhane A, M. S., Mohammed, S., Embaye G, H. F., Zehaie, A. & Chinorumba A, C. J. Pfhrp2 detecting malaria rdts: Alarming false negative results in eritrea,. In: Program and Absracts of the 65th Conference on American Soceity for Tropical Medicine and Hygiene, Atlanta. 879 (2016).
- 15.Gatton, M. L. et al. Use of PfHRP2-only RDTs rapidly select for PfHRP2-negative parasites with serious implications for malaria case management and control. J Infect Dis, 10.1093/infdis/jix094 (2017).
- 16.Cheng Q, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J. 2014;13:283. doi: 10.1186/1475-2875-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamau E, et al. K13-Propeller Polymorphisms in Plasmodium falciparum Parasites From Sub-Saharan Africa. J Infect Dis. 2014 doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amoah LE, Abankwa J, Oppong A. Plasmodium falciparum histidine rich protein-2 diversity and the implications for PfHRP 2: based malaria rapid diagnostic tests in Ghana. Malar J. 2016;15:101. doi: 10.1186/s12936-016-1159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp DJ, Thompson JK, Walliker D, Corcoran LM. Molecular karyotype of Plasmodium falciparum: conserved linkage groups and expendable histidine-rich protein genes. Proc Natl Acad Sci USA. 1987;84:7672–7676. doi: 10.1073/pnas.84.21.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock EP, et al. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology. 1987;95(Pt 2):209–227. doi: 10.1017/S0031182000057681. [DOI] [PubMed] [Google Scholar]
- 21.Baker J, et al. Transcription and expression of Plasmodium falciparum histidine-rich proteins in different stages and strains: implications for rapid diagnostic tests. PLoS One. 2011;6:e22593. doi: 10.1371/journal.pone.0022593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng X, et al. Genome-wide consequences of deleting any single gene. Mol Cell. 2013;52:485–494. doi: 10.1016/j.molcel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sepulveda N, et al. A Poisson hierarchical modelling approach to detecting copy number variation in sequence coverage data. BMC Genomics. 2013;14:128. doi: 10.1186/1471-2164-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Githinji S, et al. A national health facility survey of malaria infection among febrile patients in Kenya, 2014. Malar J. 2016;15:591. doi: 10.1186/s12936-016-1638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malaria, G. E. N. P. f. C. P. Genomic epidemiology of artemisinin resistant malaria. eLife5, 10.7554/eLife.08714 (2016). [DOI] [PMC free article] [PubMed]
- 26.Borrmann S, et al. Genome-wide screen identifies new candidate genes associated with artemisinin susceptibility in Plasmodium falciparum in Kenya. Scientific reports. 2013;3:3318. doi: 10.1038/srep03318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cnops L, Boderie M, Gillet P, Van Esbroeck M, Jacobs J. Rapid diagnostic tests as a source of DNA for Plasmodium species-specific real-time PCR. Malar J. 2011;10:67. doi: 10.1186/1475-2875-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Schalkwyk DA, et al. Culture-adapted Plasmodium falciparum isolates from UK travellers: in vitro drug sensitivity, clonality and drug resistance markers. Malar J. 2013;12:320. doi: 10.1186/1475-2875-12-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods in molecular medicine. 2002;72:189–203. doi: 10.1385/1-59259-271-6:189. [DOI] [PubMed] [Google Scholar]
- 30.Snounou, G. & Beck, H. P. The use of PCR genotyping in the assessment of recrudescence or reinfection after antimalarial drug treatment. Parasitol Today 14, 462–467, doi:S0169-4758(98)01340-4 [pii] (1998). [DOI] [PubMed]
- 31.Beshir KB, et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017–2024. doi: 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beshir KB, et al. Measuring the efficacy of anti-malarial drugs in vivo: quantitative PCR measurement of parasite clearance. Malar J. 2010;9:312. doi: 10.1186/1475-2875-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padley, D. J., Heath, A. B., Sutherland, C., Chiodini, P. L. & Baylis, S. A. Establishment of the 1st World Health Organization International Standard for Plasmodium falciparum DNA for nucleic acid amplification technique (NAT)-based assays. Malar J7, 139, [pii] 10.1186/1475-2875-7-139 (2008). [DOI] [PMC free article] [PubMed]
- 34.Campino S, et al. Genomic variation in two gametocyte non-producing Plasmodium falciparum clonal lines. Malar J. 2016;15:229. doi: 10.1186/s12936-016-1254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boeva V, et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics. 2012;28:423–425. doi: 10.1093/bioinformatics/btr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Data generated using RDT, pfhrp2, pfhrp3 and parasitaemia (>5 parasites per microliter, n=91).
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.