Abstract
Objective
We assessed the trends in lung cancer incidence over a 25-year period by socioeconomic groups for men in New South Wales (NSW), Australia.
Methods
Men diagnosed with lung cancer between 1987 and 2011 were divided into five quintiles according to an Index of Education and Occupation (IEO). We assessed relative socioeconomic differences over time by calculating age-standardized incidence ratios (SIRs) by 5-year period of diagnosis, and estimated absolute differences by comparing the observed and expected numbers of cases using the highest IEO quintile as the reference.
Results
Lung cancer incidence for men decreased from 1987 to 2011 for all IEO quintiles, with a greater rate of decline for men living in the highest IEO areas. Thus, the relative disparity increased significantly over the 25-year period (P=0.0006). For example, the SIR for the lowest IEO quintile increased from 1.28 during 1987–1991 to 1.74 during 2007–2011. Absolute differences also increased with the proportion of " potentially preventable” cases doubling from 14.5% in 1987–1991 to 30.2% in 2007–2011.
Conclusions
Despite the overall decline in lung cancer incidence among men in NSW over the past 25 years, there was a significant increase in disparity across socioeconomic areas in both relative and absolute terms.
Keywords: Australia, lung cancer, socioeconomic inequality, temporal trends, tobacco control, tobacco smoking
Introduction
Lung cancer remains one of the most commonly diagnosed cancers and the leading cause of cancer death amongst Australian men (1), despite steady declines in incidence and death rates since the early 1980s (1). These reductions in incidence and death rates are mirrored by the decrease in smoking prevalence (2), resulting from a range of effective tobacco control policies in the country (3). However, whether this decline is uniform across socioeconomic groups is currently unknown.
A gradient in smoking prevalence exists across socioeconomic groups in Australia, with a higher prevalence occurring in lower socioeconomic areas (2). Given that the majority (83.5%) of lung cancer cases among Australian men are attributable to tobacco smoking (4), it is expected that these areas will have a higher incidence rate of lung cancer. While previous research has reported disparities in lung cancer incidence between socioeconomic groups in Australia in 1987–1991 (5) and 2008–2012 (1), these studies provided no information on temporal trends, and used only a relative measure, comparing the rates between lower and higher socioeconomic groups. To provide a clear picture of health disparity, multiple outcome measures may be needed (6), with the most commonly used being absolute and relative differences, which complement each other. While the relative difference is preferred in epidemiology as it provides an estimate of the magnitude of the effect, the absolute difference indicates the magnitude of the problem and quantifies significance from a public health perspective (7).
The aim of this study was to investigate temporal trends in lung cancer incidence rates from 1987 to 2011 among men in New South Wales (NSW) across areas in quintiles of socioeconomic status (SES) using data from the long-standing NSW Cancer Registry, covering nearly one-third of the total Australian population. We used two measures of disparity, one relative and another absolute, to examine temporal trends in lung cancer incidence by SES.
Materials and methods
Data
Incidence data for first primary lung cancer (ICD-O3: C33–C34) diagnosed between 1972 and 2011 were available from the NSW Cancer Registry (NSWCR) database. Notification of cancer has been a statutory requirement for all NSW public and private hospitals, radiotherapy departments and nursing homes since 1972, and for pathology departments since 1985 (8). The NSWCR has high standards of data completeness and quality, with a low proportion of death certificate only cases (1.0% for 1993–1997 and 0.9% for 2004–2008) and a high proportion of histologically verified diagnoses (9,10). Over the period 1987–2011, 76% of male lung cancer cases registered in the NSWCR were histologically confirmed, with this proportion being 88%, 70%, 78%, 79% and 79% for each 5-year period from 1987–1991 to 2007–2011 respectively. We chose to use data from 1987 onwards because the data on histology type were more complete after reporting by pathology laboratories became compulsory in 1985 (8). We also restricted the analyses to men aged ≥25 years because diagnoses of lung cancer are rare among men younger than 25 years.
Study variable
Individuals’ socioeconomic characteristics are not routinely collected by the NSWCR, so instead we used an area-based measure of SES, based on an individual’s residential address at diagnosis. This measure is the “Index of Education and Occupation” (IEO) score (11), which is one of the four indexes derived from the 2001 Australian Bureau of Statistics’ Census of Population and Housing. This index was used because education is the socioeconomic indicator most strongly related to lung cancer risk (12). A higher score on this index indicates that the area has a larger proportion of residents with higher levels of education and employed in higher skilled occupations than an area with a lower score. Cases were grouped into five quintiles based on Local Government Areas (LGA) of their residential address at the time of diagnosis.
Population denominators were derived from the Australian Bureau of Statistics’ estimated mid-year resident male population for each LGA, and aggregated into IEO quintiles and 5-year age groups for 1987–2011.
Lung cancer histological types were classified into four groups as in our recently published analysis of lung cancer trends for Australian women (13). These groups are small cell carcinoma, adenocarcinoma, squamous cell carcinoma, and other specified or unspecified carcinoma.
Statistical analysis
A detailed description of the analyses can be found in our recently published analysis of lung cancer trends for Australian women (13). In brief, trends in lung cancer incidence for all men and by IEO quintile were examined by calculating annual age-standardized incidence rates (per 100,000). Then, rates were compared between IEO quintiles over time by calculating the age-standardized incidence ratio (SIR) for IEO quintiles 2–5 using the highest IEO quintile as the reference population over five time periods (1987–1991, 1992–1996, 1997–2001, 2002–2006 and 2007–2011). The 95% confidence intervals (95% CI) for the SIRs were obtained using the exact method with a beta distribution (14). To test whether socioeconomic disparity changed over time, a Poisson regression model was fitted with age group, IEO quintile (one linear term) and period of diagnosis (one linear term). Significance of the association was tested by adding to the model an interaction term of IEO quintile with time period, and then performing a likelihood ratio test between the nested models.
To further illustrate the impact of socioeconomic disparities in lung cancer incidence, the number of “potentially preventable” lung cancer diagnoses was estimated by calculating the difference between the observed and expected numbers of cases for IEO quintiles 2–5, with the expected numbers being calculated using the rates for IEO quintile 1. Estimates were calculated for both NSW and the whole of Australia [based on published data (http://www.aihw.gov.au/acim-books/)]. A chi-squared test was then used to determine if the proportions of “potentially preventable” lung cancer diagnoses changed significantly over time.
It is possible that changes in the patterns of lung cancer histology types over time may affect the association between socioeconomic groups and lung cancer incidence, thus, we examined the changes in histology types over time by dividing the number of cases for each histology type by the total number of cases over five 5-year periods, and performed a sensitivity analysis with adjustment for histological type.
All analyses were performed using STATA (Version 13.1; StataCorp LLC, TX, USA) and P<0.01 indicated statistical significance.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study involves analysis of routinely collected data and the records were de-identified (name, address, date of birth had been removed) before being provided to the research team. For this type of study, formal consent is not required. The NSW Population and Health Services Research Ethics Committee approved the use of the data from the NSW Cancer Registry (reference number: 2009/03/139).
Results
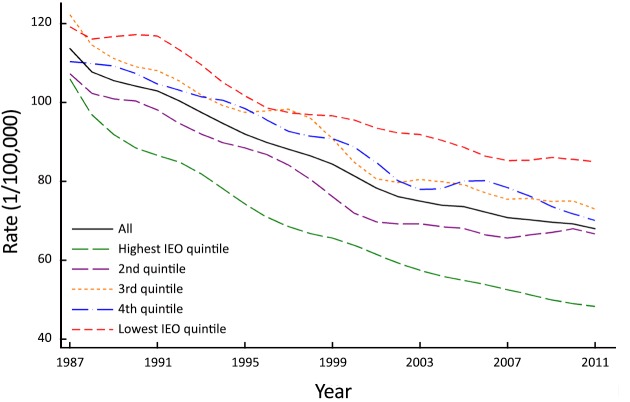
Between 1987 and 2011 there were 40,758 men aged 25 years and over diagnosed with first primary lung cancer in NSW, Australia. Overall incidence rates decreased steadily over the study period from 115.1/100,000 in 1987 to 67.6/100,000 in 2011, although the rate of decline differed across the IEO quintiles, with consistently greater declines observed for areas in the highest IEO quintile (Figure 1).
1.
Annual age-standardized incidence rate† of lung cancer for men by Index of Education and Occupation (IEO)‡ quintile in NSW, Australia 1987–2011. †, age-standardized to 2001 Australian standard population; ‡, derived from the 2001 Australian Bureau of Statistic’s Census of Population and Housing.
Measure of relative disparity
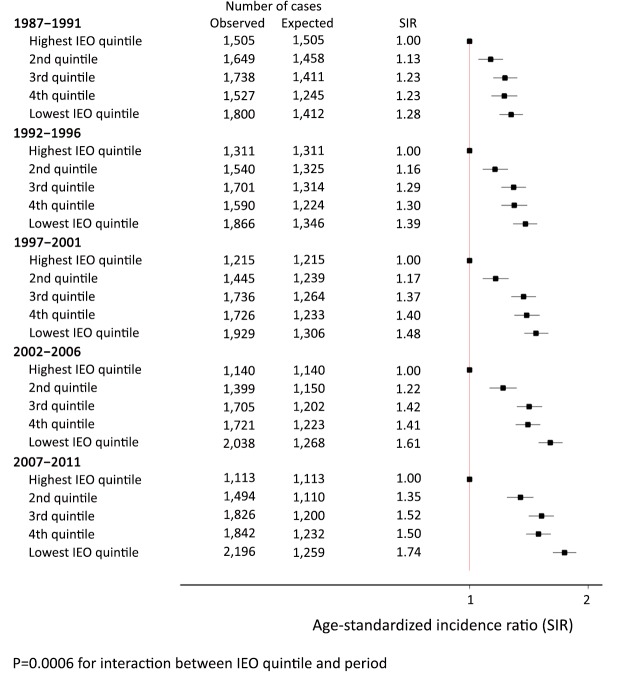
Figure 2 shows the relative differences in lung cancer incidence by IEO quintile within each 5-year time period. There was a significant socioeconomic gradient for each of the five periods, with men in areas in the lower quintiles having a higher rate of lung cancer than men in areas in the highest quintile. The SIR for the lowest quintile increased from 1.28 (95% CI, 1.19–1.37) during 1987–1991 to 1.74 (95% CI, 1.63–1.87) during 2007–2011. Thus, this relative disparity increased significantly over time (P=0.0006).
2.
Age-standardized incidence ratio (SIR) for lung cancer by Index of Education and Occupation (IEO)‡ quintile, with the reference group being those in the highest IEO quintile for NSW men 1987–2011. ‡, derived from the 2001 Australian Bureau of Statistic’s Census of Population and Housing.
Variations in histological type were observed over time (chi-squire test P<0.0001) with the proportion of cases diagnosed with squamous cell carcinoma decreasing over time and the proportion of those with adenocarcinoma increasing over the same time period. However, sensitivity analysis with adjustment for histological type yielded similar patterns by socioeconomic groups, and the variation in incidence across IEO quintiles remained statistically significant.
Measure of absolute disparity
Table 1 shows the numbers of “potentially preventable” lung cancers (if incidence rates across the whole population were equivalent to that of the highest IEO quintile, which represents 20% of the NSW male population) diagnosed over five time periods (1987–2011) in NSW, and the whole of Australia. Although the incidence rates for all socioeconomic areas decreased steadily over the study period, the estimated proportion prevented as a fraction of total incident cases increased significantly: from 14.5% in 1987–1991 to 30.2% in 2007–2011 (P<0.001). When extrapolated to the national lung cancer incidence, 9,357 lung cancers were “potentially preventable” in Australian men during 2007–2011, equivalent to 1,870 men per year.
1.
Numbers of potentially preventable* lung cancer cases diagnosed during 1987–2011 for men in NSW and Australia
| Time period | NSW # diagnosed | NSW # preventable | Proportion preventable (%) | National # diagnosed& | National # preventable |
| *, If rates for all population groups were equivalent to those for the highest socioeconomic quintile (20% of the whole population); &, Data source: Australian Cancer Incidence and Mortality (ACIM) books from http://www.aihw.gov.au/acim-books/ accessed 31 Jan 2017; †, P value for χ2 test of no change in the proportion preventable (%) over time. | |||||
| 1987–1991 | 8,219 | 1,188 | 14.5 | 25,623 | 3,705 |
| 1992–1996 | 8,008 | 1,487 | 18.6 | 26,283 | 4,880 |
| 1997–2001 | 8,051 | 1,793 | 22.3 | 27,231 | 6,065 |
| 2002–2006 | 8,003 | 2,020 | 25.2 | 28,839 | 7,278 |
| 2007–2011 | 8,471 | 2,558 | 30.2 | 30,987 | 9,357 |
| P† | <0.001 | ||||
Discussion
We found that over the 25 year period from 1987 to 2011, the incidence of lung cancer decreased among men in all socioeconomic areas in NSW, Australia. However, we also found that the magnitude of the decrease in lung cancer incidence was significantly greater for men living in areas in the highest IEO quintile compared with those living in areas in the lower quintiles, leading to increasing disparity between socioeconomic groups in both relative and absolute terms. Moreover, an increased risk of lung cancer was apparent across the socioeconomic continuum for areas in the highest to lowest IEO quintiles, strengthening the inverse association between SES and lung cancer incidence. We also found that the number of “potentially preventable” lung cancers, assuming no socioeconomic gradient, more than doubled over time. The impact of these findings is substantial, as over 30% of lung cancers in men during the most recent period were “potentially preventable” were it possible for men in areas falling in the lower IEO quintiles to have the same risk as those areas in the highest quintile. Extrapolating this proportion to the national lung cancer incidence meant that as many as 9,357 lung cancer cases in Australian men could potentially have been prevented over the last 5 years. These results highlight the huge impact of socioeconomic inequality in lung cancer incidence in Australia. Our sensitivity analysis with adjustment for histological type did not change the main findings.
Our findings are generally consistent with the few previous studies that have examined the risk of lung cancer by socioeconomic groups in Australia (1,5). The association between lung cancer and SES was recognised in the mid-nineties, when Smith et al. (5) reported significantly higher rates of lung cancer among men living in lower socioeconomic areas of urban NSW, with an odds ratio of 1.7 for the lowest socioeconomic quintile compared to the highest. This finding was also supported by more recent research, with the Australian Institute of Health and Welfare reporting that people who lived in areas in the lowest socioeconomic quintile were 1.7 times more likely to be diagnosed with lung cancer than those who lived in areas in the highest socioeconomic quintile between 2008 and 2012 (1). A clear gradient was observed in both studies, with lung cancer incidence decreasing with increasing SES. This study extends the previous work by examining trends over the last 25 years, using both relative and absolute measures, to show that disparities in lung cancer incidence across socioeconomic quintiles are increasing among Australian men. The social inequalities observed in this study have also been found in many other developed countries, regardless of whether the socioeconomic measure is based on education, occupation or income (12).
The most likely reason for the patterns of lung cancer incidence and SES that we observed is that there is a correlation between SES and smoking behaviours. There is a robust body of evidence that tobacco smoking causes up to 90% of lung cancers among men (2), and disadvantaged populations are generally more likely to take up and continue smoking in Australia and internationally (15). A recent review reported that lower socioeconomic groups not only have a higher smoking prevalence than higher socioeconomic groups, but also start at a younger age, smoke more cigarettes per day, and are less likely to quit (16). Furthermore, smokers with lower SES may smoke each cigarette more intensively than those with higher SES. These characteristics are each independently associated with an increased risk of lung cancer. Long-term smoking prevalence data in Australia showed consistently higher rates for those living in areas of lower SES and socioeconomic disparity increased between 1980 and 2001 (17). These trends appear to be continuing, with more recent data on smoking prevalence showing a similar pattern of higher education level and lower likelihood of smoking between 1998 and 2013 (18). These recent findings of persistent socioeconomic differences in smoking (18) are concerning, as they suggest that without a dramatic reversal of this pattern there may be even greater inequality in lung cancer incidence across socioeconomic groups in the foreseeable future.
Possible reasons why men living in lower socioeconomic areas have higher prevalence of smoking, despite the monetary and health costs, include increased stress, family, peer and community influences, and lack of education, resulting in reduced access to the successful public health interventions (19). In contrast, men living in areas with a higher proportion of well-educated people are more likely to have greater health literacy and interaction with the health system (20), thus they may be more responsive to public health campaigns and be more likely to use effective resources for quitting smoking. The finding that the disparity in lung cancer incidence between areas across the IEO quintiles is increasing has important implications for tobacco control in the future. Australia has had successful tobacco control policies since the 1970s, including tobacco advertising bans, tax increases, anti-smoking media campaigns, smoke-free legislation and policy, plain packaging, and behavioural interventions, which have resulted in the current low smoking prevalence of 12.2% (21), and consequently the overall decline in lung cancer incidence for men over time that was observed in this and previous studies (1). However, the results of this study suggest that there may be population subgroups who have been more resistant to tobacco control efforts. Reducing inequalities in tobacco smoking among these population subgroups requires targeted interventions. Population wide strategies such as tax increases, mass media and smoke-free legislation have been found to be effective in reducing smoking across population groups, but particularly for lower socioeconomic groups (18). These policies have also been crucial in the Australian context, however our results suggest additional interventions are required. Improving access to tools that are known to help people quit, such as counselling, medications and behavioural interventions, could be key to reducing smoking rates in low socioeconomic areas in Australia (22).
Approximately 16.5% of lung cancers in Australian men are not attributable to tobacco use (4), and several environmental and occupational factors may be associated with an increased risk of lung cancer among lifelong never smokers. These include passive exposure to tobacco smoke, exposure to asbestos, radon and other ionizing radiation, and indoor air pollution (23). Previous Australian research has found that those from lower socioeconomic groups are at higher risk of exposure to environmental tobacco smoke (2), and higher proportions of men in low socioeconomic groups work in areas high in occupational risk factors for lung cancer, such as radioactive ores (e.g. uranium), chromium compounds, nickel, arsenic, soot, tar, asbestos, or diesel fumes. Furthermore, a meta-analysis revealed that the association of lower level of education with an increased risk of lung cancer persisted after adjustment for smoking (12).
There is a potential limitation to this study, as aggregated area-level data were used to assign individual cases to SES quintiles. While the area-level measure has been reviewed extensively and validated as a measure of SES (11), area-level measures do not necessarily reflect the SES of each individual living within those areas. However, previous studies have demonstrated the value of area-based socioeconomic measures in evaluating health inequalities (24). Furthermore, area-based measures may be particularly useful in studies of smoking-related health inequality, because the interventions and policies influencing neighbourhood characteristics are closely related to the prevalence of smoking in that area (25). Therefore, despite these concerns about our classification of SES, and even allowing for migration across areas over the lifetime (given the 20–30 year lag between smoking and lung cancer incidence), we are confident that our results do show that area-level SES at the time of diagnosis is significantly associated with lung cancer incidence.
Conclusions
This study highlights an increasing disparity in lung cancer incidence across socioeconomic groups, in both relative and absolute terms, among Australian men. To reduce the growing number of “potentially preventable” lung cancers amongst Australian men in the future, potential inequities in exposure to smoking and other lung cancer risk factors should be addressed.
Acknowledgements
We thank the NSW Cancer Registry for providing the data for this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Australian Institute of Health and Welfare. Cancer in Australia 2017. Canberra: AIHW, 2017.
- 2.Scollo MM, Winstanley MH. Tobacco in Australia: Facts and Issues. Melbourne: Cancer Council Victoria, 2012. Available online: http://www.tobaccoinaustralia.org.au/
- 3.Australian Institute of Health and Welfare. 2010 National drug strategy household survey report. Canberra: AIHW, 2011.
- 4.Pandeya N, Wilson LF, Bain CJ, et al. Cancers in Australia in 2010 attributable to tobacco smoke. Aust N Z J Public Health. 2015;39:464–70. doi: 10.1111/1753-6405.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith D, Taylor R, Coates M. Socioeconomic differentials in cancer incidence and mortality in urban New South Wales, 1987-1991. Aust N Z J Public Health. 1996;20:129–37. doi: 10.1111/j.1753-6405.1996.tb01806.x. [DOI] [PubMed] [Google Scholar]
- 6.Harper S, Lynch J, Meersman SC, et al. An overview of methods for monitoring social disparities in cancer with an example using trends in lung cancer incidence by area-socioeconomic position and race-ethnicity, 1992-2004. Am J Epidemiol. 2008;167:889–99. doi: 10.1093/aje/kwn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messer LC. Invited commentary: measuring social disparities in health -- what was the question again? Am J Epidemiol. 2008;167:900–4. doi: 10.1093/aje/kwn019. [DOI] [PubMed] [Google Scholar]
- 8.Yu XQ, O’Connell DL, Gibberd RW, et al. Trends in survival and excess risk of death after diagnosis of cancer in 1980-1996 in New South Wales, Australia. Int J Cancer. 2006;119:894–900. doi: 10.1002/ijc.21909. [DOI] [PubMed] [Google Scholar]
- 9.Tracey E, Baker D, Chen W, et al. Cancer in New South Wales: Incidence, Mortality and Prevalence, 2005. Sydney: Cancer Institute NSW, 2007.
- 10.Currow D, Thomson W. Cancer in NSW: Incidence report 2009. Sydney: Cancer Institute NSW, 2014.
- 11.Australian Bureau of Statistics. Socio-Economic Indexes for Areas (SEIFA) 2001 - Technical Paper. Canberra: ABS, 2004 (ABS catelogue no. 2039.0.55.001).
- 12.Sidorchuk A, Agardh EE, Aremu O, et al. Socioeconomic differences in lung cancer incidence: a systematic review and meta-analysis. Cancer Causes Control. 2009;20:459–71. doi: 10.1007/s10552-009-9300-8. [DOI] [PubMed] [Google Scholar]
- 13.Yu XQ, Luo Q, Kahn C, et al. Contrasting temporal trends in lung cancer incidence by socioeconomic status among women in New South Wales, Australia, 1985–2009. Lung Cancer. 2017;108:55–61. doi: 10.1016/j.lungcan.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Silcocks P. Estimating confidence limits on a standardised mortality ratio when the expected number is not error free. J Epidemiol Community Health. 1994;48:313–7. doi: 10.1136/jech.48.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3:242–7. doi: 10.1136/tc.3.3.242>. [DOI] [Google Scholar]
- 16.Schaap MM, Kunst AE. Monitoring of socio-economic inequalities in smoking: learning from the experiences of recent scientific studies. Public Health. 2009;123:103–9. doi: 10.1016/j.puhe.2008.10.015S0033-3506(08)00307-7. [DOI] [PubMed] [Google Scholar]
- 17.White V, Hill D, Siahpush M, et al. How has the prevalence of cigarette smoking changed among Australian adults? Trends in smoking prevalence between 1980 and 2001. Tob Control. 2003;12(Suppl 2):ii67–74. doi: 10.1136/tc.12.suppl_2.ii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenhalgh E, Bayly M, Winstanley M. Trends in the prevalence of smoking by socio-economic status. In: Scollo M, Winstanley M, eds. Tobacco in Australia: Facts and issues. Melbourne: Cancer Council Victoria, 2015.
- 19.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–70. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irvin Vidrine J, Reitzel LR, Wetter DW. The role of tobacco in cancer health disparities. Curr Oncol Rep. 2009;11:475–81. doi: 10.1007/s11912-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Australian Institute of Health and Welfare. National Drug Strategy Household Survey (NDSHS) 2016 key findings. Canberra: AIHW, 2017 [13/06/2017]. Available online: http://www.aihw.gov.au/alcohol-and-other-drugs/data-sources/ndshs-2016/key-findings/
- 22.Carlsten C, Halperin A, Crouch J, et al. Personalized medicine and tobacco-related health disparities: is there a role for genetics? Ann Fam Med. 2011;9:366–71. doi: 10.1370/afm.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–45. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu XQ, O’Connell DL, Gibberd RW, et al. Assessing the impact of socio-economic status on cancer survival in New South Wales, Australia 1996-2001. Cancer Causes Control. 2008;19:1383–90. doi: 10.1007/s10552-008-9210-1. [DOI] [PubMed] [Google Scholar]
- 25.Siahpush M, Borland R. Socio-demographic variations in smoking status among Australians aged ≥18: multivariate results from the 1995 National Health Survey. Aust N Z J Public Health. 2001;25:438–42. [PubMed] [Google Scholar]