Abstract
Acute coronary syndromes (ACS) remain life-threatening disorders that are associated with high morbidity and mortality. Dual-antiplatelet therapy with aspirin and clopidogrel has shown to reduce cardiovascular events in patients with ACS. However, there is substantial inter-individual variability in response to clopidogrel treatment in addition to prolonged recovery of platelet reactivity as a result of irreversible binding to P2Y12 receptors. This high inter-individual variability in treatment response has primarily been associated with genetic polymorphisms in the genes encoding for cytochrome (CYP) 2C19 that affect clopidogrel’s pharmacokinetics. While FDA has issued a boxed warning for CYP2C19 poor metabolizers due to a potentially reduced efficacy in these patients, results from multivariate analyses suggest that additional factors, including age, sex, obesity, concurrent diseases and drug-drug interactions, may all contribute to the overall between-subject variability in treatment response. However, the extent to which each of these factors contributes to the overall variability and how they are interrelated is currently unclear. The objective of this review article is to provide a comprehensive update on the different factors that influence clopidogrel’s pharmacokinetics and pharmacodynamics and how they mechanistically contribute to inter-individual differences in response to clopidogrel treatment.
1. Introduction
Cardiovascular disease (CVD) is currently the leading cause of death worldwide [1]. Many CVD patients develop acute coronary syndrome (ACS), a life threatening condition encompassing myocardial infarction (MI) with or without ST-segment elevation (STEMI/NSTEMI), or unstable angina [1]. Approximately 1.2 million ACS patients are being hospitalized in the United States every year for cardiovascular events [2]. Elevated platelet aggregation and subsequent thrombus formation play a critical role in the pathophysiology of these patients. As a consequence, safe and effective antiplatelet therapy is essential for reducing the high morbidity and mortality of this disease [3]. Clopidogrel (Plavix®), which was the second largest selling branded drug in the US in 2010 with $8.8 billion in sales, is an irreversible P2Y12 receptor antagonist indicated for reduction of arteriosclerotic events in patients with recent stroke or MI, and established peripheral arterial disease [4, 5]. Clopidogrel is a second generation thienopyridine that has largely replaced ticlopidine, a first generation thienopyridine with similar efficacy, due to improved tolerability, reduced incidence of haematological side effects, more rapid onset of action and a convenient (once-daily) dosing regimen [6]. In recent years, dual antiplatelet therapy with aspirin and P2Y12 receptor antagonists clopidogrel, prasugrel or ticagrelor has become the clinical gold standard for patients with ACS and/or undergoing percutaneous coronary interventions (PCI) due to the significant improvement of long-term clinical outcome [1, 3, 7–9]. Although clopidogrel is safe and effective in many patients, there is substantial variability in treatment response between individuals [10]. Some of these patients continue to have cardiovascular events despite clopidogrel treatment [11]. This lack of efficacy has, in part, been attributed to the reduced response to clopidogrel in patients, resulting in high on-treatment platelet reactivity (HPR) and the development of atherothrombotic complications [3]. This relative non-responsiveness to clopidogrel therapy has been coined “clopidogrel resistance” and is thought to affect 5–44% of patients receiving standard-dose clopidogrel treatment [11]. On the other hand, some patients also experience drug-induced bleeding due to excessive platelet inhibition [7].
Clopidogrel is an inactive pro-drug that requires enzymatic conversion into its active metabolite by a series of cytochrome P450 (CYP) enzymes [12]. Clinical evidence suggests that patients with deficient CYP2C19 activity (e.g. poor metabolizers or as a result of drug-drug interactions) have remarkably higher on-treatment platelet reactivity, which puts them at an increased risk of ischemic events following the standard dosing regimen, prompting the U.S. FDA to issue a boxed warning [13–16]. However, the results from a multivariate analysis of the Pharmacogenomics of Antiplatelet Intervention (PAPI) study revealed that CYP2C19 polymorphisms are responsible for about 12% of the between-subject variability in response to clopidogrel treatment, whereas age or body mass index (BMI) accounted for 3.8% and 2.3% of the variability, respectively [14]. Similar findings have been reported from other studies, which all indicate that, in addition to CYP2C19 polymorphism, multiple demographic and disease risk factors contribute to the interindividual variability in response to clopidogrel treatment [15–19]. However, the underlying mechanisms related to each of these intrinsic and extrinsic factors are not yet fully understood. It should be noted at this point though that the also the assays that have been used to determine the response to clopidogrel treatment are subject to substantial between-assay variability.
The objective of this review is to comprehensively evaluate the different sources of variability in PK and PD and how they mechanistically relate to interindividual differences in response to clopidogrel treatment. We attempt to do so in a systematic fashion by providing an overview of the known genetic and non-genetic factors that contribute to interindividual differences in clopidogrel’s pharmacokinetics (PK) and pharmacodynamics (PD) and to how they relate to clinical outcome.
2. Clopidogrel pharmacokinetics and pharmacodynamics
Following oral administration, about 50% of the dose is absorbed from the intestine based on urinary metabolites data [20]. Results from in vitro studies show that the uptake of clopidogrel into Caco-2 cells is limited by P-glycoprotein (P-gp, ABCB1), which suggests that P-gp may affect the intestinal absorption and oral bioavailability of clopidogrel [21]. Once delivered to the liver, a number of cytochrome P450 (CYP) enzymes, including CYP2C19, CYP1A2, CYP2B6, CYP2C9 and CYP3A4, mediate the bioactivation of clopidogrel via a two-step process. First, 2-oxo-clopidogrel, an intermediate and pharmacologically inactive metabolite, is formed, which is then further converted into the pharmacologically active metabolite R-130964 (clop-AM) [12]. At the same time, a large portion of the absorbed clopidogrel (at least 85 – 90%) undergoes first-pass metabolism in the liver, where it is hydrolyzed by carboxylesterase 1 (CES1) to the inactive carboxylic acid metabolite SR26334 [22, 23]. As a consequence, only about 2% of the administered clopidogrel dose is converted to clop-AM and reaches the systemic circulation [20]. It should be noted at this point that CES1 also hydrolyzes 2-oxo-clopidogrel and clop-AM [23, 24].
In vitro enzyme kinetics studies revealed that CYP1A2 (35.8%), CYP2B6 (19.4%) and CYP2C19 (44.9%) contribute to the formation of 2-oxo-clopidogrel, whereas CYP2B6 (32.9%), CYP2C9 (6.79%), CYP2C19 (20.6%) and CYP3A4 (39.8%) contribute to the formation of active metabolite clop-AM, respectively [12]. It is estimated that CYP2C19 contributes to about 50% to the overall formation of clop-AM from clopidogrel and thus plays a substantial role in clopidogrel’s bioactivation, whereas the other isozymes contribute to a lesser extent. There is conflicting data available in the literature on whether or not these biotransformation pathways can be saturated, i.e. whether or not clopidogrel and its active metabolite exhibit linear pharmacokinetics. While data from a variety of studies suggests that clopidogrel and its major inactive metabolite SR26334 exhibited linear PK across a wide range of doses (50 – 900 mg) [25–27], data by Wallentin et al. [28] and Collet et al. [29] reported a ~4-fold and ~2-fold increase in clop-AM AUC when increasing the clopidogrel dose from 75 mg to 600 mg and from 300 mg to 900 mg, respectively, Horenstein et al. reported that an increase in the clopidogrel dose from 75 to 150 or 300 mg led to ~1.5- and ~2.2-fold increase of clop-AM AUC in CYP2C19 extensive, intermediate and poor metabolizers, respectively [30]. These findings support the presence of nonlinearity in clopidogrel’s bioactivation processes.
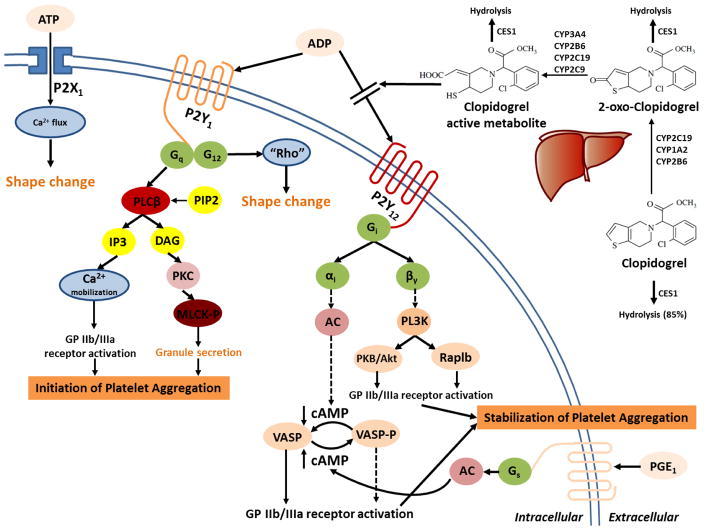
Upon activation, clopidogrel exhibits its pharmacodynamic effect by specifically and irreversibly binding to P2Y12, a subtype of the adenosine diphosphate (ADP) receptor, on the surface of platelets [3, 31]. P2Y12 is a Gi-protein coupled receptor. Activation of the P2Y12 receptor triggers a complex cascade of intracellular events, resulting in reduced protein kinase (PKA) phosphorylation of vasodilator-stimulated phosphoprotein (VASP) and subsequent activation of the glycoprotein (GP) IIb/IIIa receptor, granule release, amplification of platelet aggregation, and stabilization of the platelet aggregate (Fig. 1). Irreversible binding of clop-AM to the P2Y12 receptor consequently results in an inactivation of the GP IIb/IIIa receptor and destabilization of the thrombus for the lifespan of the platelets [3, 10, 31]. It should be noted though that other physiological agonists, such as thromboxane A2 (cf. aspirin), thrombin, collagen, serotonin (5-HT), also contribute to platelet activation. Therefore, any factors influencing the P2Y12-dependent and/or P2Y12-independent signal transduction pathways that impact the platelet activation should be considered when evaluating the responsiveness of patients to clopidogrel treatment and clopidogrel resistance.
Figure 1.
Clopidogrel is an orally administered pro-drug. In the liver, approximately 15% of absorbed clopidogrel is metabolized by the cytochrome P450 (CYP) system to generate its active metabolite via a 2-step bioactivation process, whereas the rest 85% is hydrolyzed by carboxylesterase 1 (CES1) to an inactive carboxylic acid derivative. CES1 also catalyzes the hydrolysis of the intermediate metabolite 2-oxo-clopidogrel and the active metabolite. The active metabolite binds to the adenosine diphosphate (ADP) P2Y12 receptor on the surface of platelet and leads to an irreversible inhibition of platelet aggregation. ADP binds to the Gq-coupled P2Y1 receptor, which activates phospholipase C (PLC) that forms inositol triphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol bisphosphate (PIP2). IP3 causes mobilization of intracellular calcium, whereas DAG activates protein kinase C (PKC) and leads to phosphorylation of myosin light chain kinase (MLCK-P). These two processes both lead to initiation of platelet aggregation. On the other hand, activation of P2Y1 receptor coupled G12, another G-protein, which activates the “Rho” protein, as well as activation of P2X1 receptor by adenosine triphosphate (ATP), which causes extracellular calcium influx, both lead to change of platelet shape. Activation of the Gi-coupled P2Y12 receptor by ADP leads to release of the αi and βγ subunits, which ultimately lead to stabilization of platelet aggregation. The αi subunit inhibits adenylyl cyclase (AC), which decreases intracellular levels of cyclic adenosine monophosphate (cAMP), reduces cAMP-mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP) (VASP-P), and modulates activation of glycoprotein (GP) IIb/IIIa receptor. The βγ subunit activates the phosphatidylinositol 3-kinase (PI3K), which in turn, cause activation of a serine-threonine protein kinase B (PKB/Akt) and of Rap1b GTP binding proteins and causes activation of GP IIb/IIIa receptor. In addition, Prostaglandin E1 (PGE1) elevates cAMP and VASP-P levels via activation of AC. Solid arrows represent activation, dashed arrows represent inhibition (This figure is modified from Angiolillo, DJ et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. Am Coll Cardiol. 2007 Apr 10;49(14):1505–16 [3]).
3. Assays used to determine clopidogrel resistance and HPR
Several ex vivo platelet function assays have been developed to assess patients’ responsiveness to clopidogrel treatment and, ultimately, to determine which patient is at an increased ischemic risk [22]. Light transmittance aggregometry (LTA) and a variety of other methods measure overall platelet function [3, 22], whereas the vasodilator stimulated phosphoprotein—platelet reactivity index (VASP-PRI) assay specifically determines clopidogrel induced P2Y12 inhibition [32–34]. Each of these assays has its advantages and limitations and none of them has been fully standardized or readily accepted for determining clopidogrel non-responsiveness [3, 11]. This is due to the fact that different agonists at in part different doses were used, lack of reproducibility and comparability between assays, and that different cut-off values for defining HPR were applied making a direct comparison of the different tests with respect to the determined impact on platelet reactivity and corresponding efficacy and safety outcomes difficult [22].
Measurement of ADP-induced platelet aggregation in platelet-rich plasma by LTA assay has long been the gold-standard for assessing platelet function in relation to clinical outcome. In most studies, 5, 10 or 20 μmol/L of ADP was employed and respective cut-off values have been proposed to define HPR [22]. The specificity of LTA assay is confounded by the fact that other ADP receptor subtypes (e.g. P2Y1) can also contribute to platelet aggregation [3]. The utility of LTA assay is also limited by its labor intensive setup, operator dependent results and inconsistency between laboratories [22, 35]. The VerifyNow P2Y12 assay, on the other hand, is a fast and standardized point-of-care method that determines platelet-induced aggregation in the whole blood by ADP and prostaglandin E1 (PGE1) in order to increase the specificity to P2Y12 pathway. However, the experimental results of VerifyNow P2Y12 assay may be influenced by non-platelet blood components (e.g. hemoglobin) [3, 32]. The same holds true for other point-of-care whole blood platelet tests, such as the Impact-R, PFA-100, the Plateletworks tests and Multiplate analyzer [3, 22, 36]. All of these point-of-care assays are relatively new and in need of more extensive qualification. Alternatively, the VASP-PRI assay measures the phosphorylation state of VASP, a specific intracellular marker of the residual P2Y12 receptor reactivity, using flow cytometry [32–34]. However, this assay is time consuming and requires experienced staff [22, 36]. It has also been reported that the sensitivity and specificity of VASP-PRI assay in prediction of cardiovascular adverse events was lower than the ADP-stimulated platelet function assays, suggesting that specific determination of VASP pathway may overlook the contribution of alternative mechanism to platelet activation [15, 37].
In addition, a given assay may yield different results following multiple measurements in the same subject, which further complicates the establishment of a robust link between ex vivo assay readouts and HPR. For example, results from the recent ELEVATE-TIMI 56 study indicate that the response of 16 to 20% of the patients receiving a 75 mg clopidogrel maintenance dose differed when measured at different times. In fact, 33 to 50% of the patients who were originally classified as non-responder at first before they had to be re-classified as responder following a second measurement using the same assay, vice versa; and about 40% of the patients showed a larger than 40 scores change in PRU following serial measurement using the VerifyNow P2Y12 assay [38].
4. Covariates that affect clopidogrel dose/PK/PD
4.1 Demographic factors
4.1.1 Age
Several studies reported a significant association between older age and higher prevalence of high on-treatment platelet reactivity following clopidogrel treatment [14, 19, 39–41]. On the other hand, clinical outcome studies revealed that old age is associated with a substantial increase in both cardiovascular events [5, 16, 42–44] and bleeding [45, 46] following clopidogrel treatment. These findings suggest that dose adjustment may become more necessary in the elderly than in younger patients to optimize platelet inhibition while avoiding bleeding events.
4.1.2 Body weight
Obesity has been shown to significantly affect clopidogrel response. Several studies report that BMI or body weight is associated with HPR in both patients and healthy subjects [14, 41, 47]. A recent clinical PK study reported that, compared to patients with lower body weight (56.4 ± 3.7 kg), patients with higher body weight (84.7 ± 14.9 kg) had about 30% lower clop-AM plasma AUC, which ultimately led to a higher on-treatment platelet reactivity in these obese patients (VerifyNow P2Y12 reaction reading in obese patients was 207 PRU, whereas that in patients with normal weight was 152 PRU) [48]. This variability can at least partially be attributed to the lower body weight normalized dose in higher body weight patients compared to lower body weight ones (1.33 vs. 0.89 mg/kg). In addition, down regulation of CYP enzymes in obese subjects (e.g. CYP2C9, CYP2C19 and CYP3A4) may also play role as it leads to reduced bioactivation [49]. Some recent studies also showed that expression level of CES1, which governs clopidogrel elimination from the body, was significantly elevated in obese subjects and could be reversed by diet-induced weight loss [50, 51], indicating that the impact of obesity in clopidogrel response may be associated with multiple mechanisms. However, the link between obesity and treatment outcome seems inconclusive and an “obesity paradox” has been reported from several clinical investigations, where patients with lower BMI (normal and underweight) had an increased risk of bleeding as well as elevated clinical outcome including death and MI than the obese ones, due to the fact that, in these studies, higher BMI was associated with a trend towards male patients with younger ages, who usually show the tendency of seeking for medical care earlier and more aggressive initial management compared to older subjects [16, 42, 45, 52, 53]. On the other hand, a LEADERS trial that was conducted in clopidogrel discharged patients reported that, the obese individuals (BMI > 30 kg/m2) had significantly more major adverse cardiac events than those non-obese ones[54]. It was noteworthy that the obese patients involved in this study had significantly higher rate of diabetes mellitus, which itself also has been shown to significantly impact clopidogrel resistance [55, 56]. Thus, a more thorough investigation may be necessary for distinguishing the true contribution of obesity in clopidogrel resistance.
4.1.3 Sex
The impact of sex on clopidogrel’s efficacy and safety has been investigated in different clinical settings. It has been reported that the systemic clop-AM exposure was similar in men and women [19]. Many clinical studies revealed that clopidogrel PD was not different between males and females [14, 39, 57], whereas some others reported a significant decreased risk of HPR in males compared to females [40, 58]. Results from the FAST-MI clinical trial and another clinical study conducted in patients undergoing PCI both reported that females had lower risk of cardiovascular events than males (including death, MI or stroke) [16, 42]. Female sex was associated with an increase in bleeding from REPLACE-2 and ISAR-REACT 3 clinical trials [45, 59], indicating that sex might play a role in clopidogrel clinical outcome, which may be associated with the “one size fits all” dosing of clopidogrel in all patients and the relatively lower body weight of female patients compared to male patients. On the other hand, results from several other clinical studies suggest that, compared to other factors, the impact of sex on clopidogrel clinical outcome is minimal [5, 43, 52, 60, 61].
4.2 Genetic polymorphisms
4.2.1 Genetic polymorphisms that affect clopidogrel PK
4.2.1.1 ABCB1
The ABCB1 C3435T mutation has been associated with changes in the intestinal efflux of drugs and thus their oral bioavailability [62]. However, its impact on clopidogrel’s PK/PD and clinical outcome remains controversial. A clinical PK study conducted in patients undergoing PCI reported that following administration of a single clopidogrel loading dose (300 mg or 600 mg), peak plasma concentrations (Cmax) and area under the curve (AUC) of clopidogrel and clop-AM were significantly lower in 3435T/T homozygotes than that in 3435C/T heterozygotes and C/C (wild type) homozygotes, suggesting a change in oral bioavailability due to enhanced clopidogrel efflux for the C3435T mutation [21]. However, these results could not be reproduced by subsequent studies following clopidogrel 75 or 150 mg maintenance doses [16, 19]. Similarly, several studies in both healthy adults and patients undergoing PCI failed to show a clear correlation between C3435T polymorphism and HPR following either loading or maintenance doses of clopidogrel [19, 39, 60, 63]. The association between C3435T mutation and cardiovascular risk is also inconsistent [16, 60, 64, 65]. These conflicting findings on the impact of ABCB1 C3435T mutation for cardiovascular outcome was evaluated in two meta-analyses showing that this mutation is unlikely to play a major in between subject variability in response to clopidogrel treatment [66, 67].
4.2.1.2 CES1
Hepatic CES1 is a serine hydrolase with broad substrate spectrum, which is involved in biotransformation of both endobiotic and xenobiotic substrates. In addition to its role in cholesterol metabolism and trafficking, CES1 also processes metabolism and bioactivation of numerous drugs, such as clopidogrel, methylphenidate, and oseltamivir [23]. A recent in vitro study reported that hydrolysis of clopidogrel and 2-oxo-clopidogrel was completely impaired in CES1 G143E allelic isozyme; suppression of CES1 activity greatly enhanced generation of 2-oxo-clopidogrel and clop-AM from clopidogrel in human liver S9 fraction [23]. Consistently, further analysis of PAPI study data revealed that, following clopidogrel treatment, CES1 143E-allele carriers have significantly higher clop-AM levels resulting in a more pronounced PD response than patients that are homozygous for CES1 143G (wild-type) [68]. In patients with acute coronary disease under clopidogrel treatment, the on-treatment platelet reactivity in the individuals carrying the CES1 143E-allele was also significantly lower than that in 143G-homozygotes [68]. On the other hand, the CES1A -816A/C allele, which has been reported to cause significantly enhanced transcriptional activity of CES gene [69], has been found to be associated with either significantly increased or reduced on treatment platelet reactivity in patients with coronary heart disease [70, 71]. These findings suggests that more research needs to be done to conclusively characterize the impact of genetic polymorphism in CES1 on clopidogrel response.
4.2.1.3 CYP enzymes
CYP2C19 is one of the most important polymorphic CYP enzymes across different populations. To date, over 20 genetic variants have been identified for the CYP2C19 gene. CYP2C19*2 (G681A) and CYP2C19*3 (G636A) mutations are the two most functionally important variants, which in combination account for more than 90% of CYP2C19 loss-of-function (LOF) alleles, whereas other CYP2C19 LOF alleles occur far less frequently [72, 73]. On the other hand, the gain-of-function mutation CYP2C19*17 (C806T) has been associated with elevated enzyme expression and thus increased catalytic capacity [74]. The PAPI study and several other clinical PK/PD investigations conducted in healthy volunteers all revealed that the subjects carrying CYP2C19 reduced-function alleles (e.g. CYP2C19*2 or CYP2C19*3) had significantly lower systemic exposure to clop-AM and anti-platelet aggregation effect compared to wild-type individuals [4, 30, 75–77]. Similarly, studies conducted in patients with cardiovascular diseases treated with clopidogrel all reported that CYP2C19 reduced-function alleles were associated with significantly higher on-treatment platelet reactivity [17, 18, 39, 58, 78–82] and worse clinical outcomes including cardiovascular death, MI, stroke and ST [14, 16, 18, 65, 78, 81, 82], as confirmed by several meta-analyses [83–85]. In comparison, the impact of CYP2C19*17 mutation on clopidogrel PK may be minimal, as shown in PAPI study, which reported that clop-AM levels were similar in subjects carrying CYP2C19*17 alleles and the corresponding peers carrying CYP2C19*1 allele [77]. Conflicting results have been also reported in regard to the association between CYP2C19*17 allele and enhancement of platelet inhibition, reduction of major cardiovascular risk or increase in bleeding events from different studies [15, 65, 74, 77, 83, 86, 87]. A recent pharmacogenetic study identified a linkage between CYP2C19*17 allele and CYP2C19*4, a loss-of-function mutation, which suggest that the high metabolic capacity of CYP2C19*17 carriers is altered if these subjects show also the CYP2C19*4B haplotype [88]. As a result, further studies are needed to fully delineate the overall impact of new CYP2C19 genotype/haplotype on clopidogrel’s treatment response.
No clear association was found between genetic polymorphism of CYP2B6, CYP2C9, CYP1A2 and CYP3A4, which all contribute to clopidogrel bioactivation to some extent in vitro [12], and clopidogrel PK, PD as well as clinical outcome. Results from in vitro studies indicate that both clopidogrel and 2-oxo-clopidogrel irreversibly inhibit CYP2B6 [89, 90]. An in vivo study also revealed that repeated dose (4 days) of clopidogrel significantly suppressed the CYP2B6-catalyzed bupropion hydrolyzation in healthy adults [91], suggesting that long term exposure to clopidogrel may suppress the function of CYP2B6, which consequentially attenuates the impact of CYP2B6 polymorphism. Consistently, reports from two clinical studies showed that the CYP2B6 reduced-function alleles (*1B, *1C, *5, *6, *9 or *13) had a significant impact on clopidogrel bioactivation and PD following short-term clopidogrel treatments, but didn’t impact the long-term clopidogrel PD or clinical outcome [18, 39]. Conflicting findings have been reported when assessing the impact of CYP2C9 polymorphism on clopidogrel PK, PD and clinical outcome [18, 61, 75, 80]. No significant association with CYP1A2 polymorphism has been reported [17, 18, 75, 80]. Inconsistent results have also been reported for the impact of CYP3A4/5 polymorphism on clopidogrel PD or clinical outcome [18, 61, 75, 80, 81, 92, 93]. Nevertheless, a clinical PK study conducted in healthy volunteers revealed that CYP3A4 inhibitor itraconazole showed stronger inhibitory effect on clopidogrel PD in healthy volunteers carrying CYP3A5 non-expressor genotype than those carrying CYP3A5 expressor genotype [93], another CROSS-VERIFY clinical study also reported that calcium channel blocker amlodipine, which is a CYP3A4 inhibitor, only exhibited adverse effect on clopidogrel response and clinical outcome in CYP3A5 non-expressors as CYP3A5 may act as a “backup system” once CYP3A4 is inhibited [94], suggesting a potential interplay between CYP3A4 and CYP3A5 functional variations.
4.2.1.4 Paraoxonase-1 (PON1)
Paraoxonase-1 (PON1) is an aromatic esterase that is thought to have antioxidant and cardioprotective properties [95]. It has been reported that its gain-of-function mutation Q192R and elevated PON1 activity were both associated with a significantly lower incidence of major adverse cardiovascular events [95]. Bouman et al. first reported that PON1 Q192R mutation was identified as a new determinant in converting clopidogrel to clop-AM and risk of ST in patients undergoing PCI [24]. However, inconsistent results were observed in several subsequent studies when assessing the association between PON1 Q192R mutation and clop-AM formation, antiplatelet activity or clinical outcome [65, 76, 79, 82, 96–98], suggesting that the role of PON1 on clopidogrel resistance may need further investigation.
4.2.2 Genetic Polymorphisms that affect clopidogrel PD
The interplay between ADP and P2Y12 receptor that is located on the surface of platelet plays an essential role in platelet activation [3, 22]. To date, several P2Y12 gene mutations have been identified and investigated with respect to their impact on clopidogrel resistance. However, their association with clopidogrel resistance is inconclusive. It has been reported that the frequency of the P2Y12 H2 haplotype (consisting of intronic [i]-C139T, [i]-T744C, [i]-ins801A and G52T SNPs) was significantly higher in coronary artery disease (CAD) patients [99]. Although some clinical PK/PD studies revealed that the H2 haplotype was associated with HPR in both healthy subjects and patients undergoing PCI [100, 101], such relationship couldn’t be demonstrated in several other studies [81, 102, 103]. H2 haplotype also failed to show impact on worse clinical outcome in clopidogrel treated patients undergoing PCI [104, 105]. Similarly, inconsistent results have also been reported when assessing the association between the P2Y12 C34T mutation and clopidogrel PD or clinical outcome [16, 39, 106, 107]. A study conducted in Chinese ACS-PCI patients receiving clopidogrel treatment reported that the impact of P2Y12 C34T mutation on clinical outcomes became significant only in patients also carrying CYP2C19*2 (G681A) allele [107]. Rudez et al. reported that the s6787801 mutation (c.−217+2739T>C) of P2Y12 receptor was associated with significantly lower on-treatment platelet reactivity in 1031 clopidogrel treated CAD patients under PCI [108]. However, their one-year clinical follow-up study failed to show the impact of such mutation in cardiovascular events [109], whereas two other studies suggested that this mutation might be associated with a significantly increased HPR or rate of target vessel revascularization in patients undergoing PCI [57, 105]. Therefore, further studies are necessary for the establishment of the relation of P2Y12 receptor genetic polymorphisms and clopidogrel non-responsiveness.
The ITGB3 gene encodes for the integrin β3 of the GP IIb/IIIa platelet receptor, which is the major membrane receptor for platelet aggregation. Inconsistent results have been reported when evaluating the association between ITGB3 and clopidogrel resistance. One study reported that ITGB3 PLA2 mutation carriers showed higher on-treatment platelet reactivity following clopidogrel loading dose (300 mg) [110]. Another study revealed that ITGB3 mutation was associated with decreased risk of early ST [104]. However, results from other studies suggested that there was no association between ITGB3 PLA2 mutation and clopidogrel response [16, 111]. In addition to P2Y12 receptors, ADP also stimulates platelet P2Y1 receptors, which causes platelet conformational change and initiate weak and transient platelet aggregation [3]. Yet, no association was observed between P2Y1 A1622G genotype and altered clopidogrel response in patients [61, 111, 112].
4.2.3 Race
Race is probably the most important demographic covariate that explains differences in response to clopidogrel treatment as it not only accounts for genetic differences between subjects but also for other associated factors, such as diet, life style, comorbidity and medical practice [113]. It is well-known that allele frequencies of CYP2C19 variants are subject to significant interracial differences. CYP2C19*2, the most frequent LOF allele, for example, is present in 13% of Caucasians, 20% of African-Americans and 28% of Asians. Other LOF mutations, such as CYP2C19*3, are also more prevalent in Asians compared to other racial groups (~5% vs. <1%) [72, 73]. CYP2C19*17, a gain-of-function mutation, on the other hand, is expressed to a lesser extent in Asians (~6%) compared to African-Americans (~18%) or Caucasians (~16%) [72]. In addition to CYP2C19, LOF mutations in other CYP enzymes involved in clopidogrel’s biotransformation, such as CYP2C9*2 and *3, have also been reported to vary by race [114]. Substantial differences have also been reported in the allelic frequency of ABCB1 C3435T mutations in European Americans (62%) and in African Americans (13%) [115]. As a result, the prevalence of clopidogrel resistance is expected to be higher in Asians compared to Caucasians. In fact, several clinical studies conducted in Chinese, Japanese and Korean patients revealed that the frequency of clopidogrel resistance in Asian population ranged from 20 to 65%, which was remarkably higher than the results obtained from clinical trials that majorly included Caucasian patients [116]. However, direct comparison of these study result may not be feasible, since these studies have relatively small sample sizes, applied different HPR cut-off values, or utilized different clinical settings. In addition, difference in body weight, diet, life style and comorbidities in different racial groups should be also taken into consideration when investigating the impact of race factor in clopidogrel resistance.
4.3 Drug-Drug Interactions
Patients undergoing clopidogrel treatment are often required to take concomitant medication. Therefore, the PK and PD level drug-drug interactions (DDI) that affect plasma levels of clop-AM and platelet activation & aggregation may consequently all contribute to differences in clopidogrel response and clinical outcome.
4.3.1 Proton pump inhibitors
Since gastrointestinal (GI) bleeding is a common side effect of clopidogrel, in particular when combined with aspirin [117], proton pump inhibitors (PPIs) are often co-prescribed with clopidogrel and aspirin, which significantly decreased the drug-induced gastrointestinal bleeding [117, 118]. In vitro studies revealed that some of the PPIs, such as omeprazole, esomeprazole and lansoprazole, but not pantoprazole are mechanism-dependent inhibitors of CYP2C19, which suppress clopidogrel’s bioactivation [12, 119]. Clinical PK/PD studies conducted in healthy subjects and patients all confirmed that concurrent omeprazole led to significant decrease in systemic exposure to clop-AM as well as in suppression of antiplatelet activity [120–123]. On the other hand, esomeprazole, but not other PPIs including dexlansoprazole and pantoprazole, significantly interfered with clopidogrel PK and PD [120–122]. Conflicting results have been reported when assessing impact of lansoprazole on clopidogrel PD [121, 123–125]. Interestingly, the inhibitory effect of omeprazole and lansoprazole on clopidogrel PD was diminished in CYP2C19*2 carriers, suggesting the impact of PPIs on clopidogrel response may be dependent on CYP2C19 genotype [123, 125]. In 2009 and 2011, the FDA issued warnings to avoid concomitant use of omeprazole or esomeprazole with clopidogrel “because of the effect on clopidogrel’s active metabolite levels and anti-clotting activity” [126, 127]. However, the impact of concomitant PPI on clopidogrel clinical outcome still remains controversial and the degree of interaction between PPIs and clopidogrel seems to depend on the PPI [118, 128, 129]. Although several recent meta-analyses showed that there is no clinically significant interaction between clopidogrel and PPI, which suggests that this combination is a safe treatment choice for patients at high risk of GI bleeding, these analyses face the following limitations: inclusion of lower risk population (e.g. only 42% were taking clopidogrel for ACS [128]), usage of fixed dose formulations or early termination of the study. [130–132]. As a result, the findings of these meta-analyses should be interpreted with caution and preference may be given to PPIs that minimally inhibit CYP2C19 for patients taking clopidogrel who are considered to be at increased risk of upper gastrointestinal bleeding.
4.3.2 Statins
Clopidogrel is often co-prescribed with 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors or statins. Concerns that these statins could potentially affect clopidogrel bioactivation and response have been voiced in recent years due to the fact that some lipophilic statins (e.g. atorvastatin, lovastatin and simvastatin) are majorly metabolized by CYP3A4. Other statin drugs including atorvastatin, simvastatin, rosuvastatin, lovastatin and pravastatin can induce CYP2B6 and CYP2C9 in addition to CYP3A4 via activation of the pregnane X receptor (PXR) [133, 134]. Two clinical studies reported that continuous treatment of 80 mg atorvastatin significantly enhanced clopidogrel bioactivation and efficacy in both healthy volunteers and PCI patients with or without diabetes mellitus [135, 136]. Several other clinical studies also suggested that atorvastatin didn’t show negative effect or even showed positive effect on the antiplatelet activity of clopidogrel in patients [137–145]. Similarly, no other statins including lovastatin and simvastatin, fluvastatin, rosuvastatin or pravastatin have shown a significant effect on clopidogrel’s antiplatelet activity [137–144]. These findings are in agreement with most clinical reports showing that concomitant atorvastatin and other statin drugs have no negative impact on clinical outcome in patients taking clopidogrel [141, 142, 145–148].
4.3.3 Calcium channel blockers
Some of calcium channel blockers (CCBs) including amlodipine, nicardipine and verapamil are CYP3A4 substrates and inhibitors [149]. It has been reported that concurrent use of CCBs was associated with significant decrease of the antiplatelet potency of clopidogrel and increase of cardiovascular risk in CAD patients [63, 150]. However, several subsequent studies showed that CCBs didn’t affect clopidogrel’s antiplatelet effect or clinical outcome in ACS patients [151–153]. Interestingly, the prospective POPular study reported that, in CAD patients undergoing PCI, concurrent CCBs were only significantly associated with both HPR and increased cardiovascular events (death, non-fatal MI, ST and ischemic stroke) in patients, who were CYP2C19*2-carriers but not in those, who were CYP2C19*2-noncarriers [154]. Similarly, a CROSS-VERIFY clinical study also revealed that amlodipine only had a significant impact on clopidogrel response and clinical outcome in CYP3A5 non-expressors [94], both indicating that further prospective research is still needed to conclusively determine the clinical significance of clopidogrel-CCBs interactions.
4.3.4 CYP inhibitor and inducers that may interact with clopidogrel
Since the pharmacological effect of clopidogrel is closely linked to its bioactivation via CYP enzymes, other concomitant medications that suppress the activity of relevant CYP enzymes (e.g. CYP2C19, CYP3A4, CYP2C9, CYP2B6 and CYP1A2) may interrupt the antiplatelet activity of clopidogrel and, thus, negatively impact clinical outcome. For example, platelet inhibition was significantly reduced when clopidogrel was co-administered with sulfonylureas (CYP2C9 substrates) [155], phenprocoumon (CYP3A4 and CYP2C9 substrate) [156] or other CYP3A4 inhibitors, such as ketoconazole, erythromycin and troleandomycin [157, 158]. It has been shown that the concurrent intake of grapefruit juice causes a more than 80% decrease in clopidogrel bioactivation due to a suppression of CYP2C19 in addition to its well-established effect on CYP3A4 [159]. Interestingly enough, the CYP3A4 inhibitor itraconazole showed a stronger inhibitory effect on clopidogrel PD in healthy volunteers carrying the CYP3A5 non-expressor genotype than in those carrying the CYP3A5 expressor genotype [93].
Since most enzymes (e.g. CYP3A4, CYP2C19, CYP2B6 and CYP1A2) involved in clopidogrel bioactivation are regulated by xenobiotic receptors including aryl hydrocarbon receptor (AhR), PXR and constitutive androstane receptor (CAR) [160], any xenobiotic that can activate these xenobiotic receptors has the potential to enhance the antiplatelet effect of clopidogrel via upregulation of enzymatic activity. At the same time, the risk of experiencing bleeding events can also expected to be higher for these drug-drug interactions. For example, rifampicin, a potent PXR and CAR ligand, has been shown to significantly promote the antiplatelet activity of clopidogrel [158]. Concordantly, St John’s wort, a PXR ligand, remarkably induced CYP3A4 activity and magnified the antiplatelet activity of clopidogrel in both healthy volunteers and post-coronary stent patients [161]. Smoking is known to cause significant induction of CYP1A2 activity via AhR pathway [160]. Several studies showed that smokers exhibited enhanced platelet inhibition [144, 162, 163]. However, the association between smoking and clopidogrel clinical outcome is inconclusive, as summarized by a recent review paper [163].
4.3.5 Anticoagulants
Blood clots are the result of elevated platelet aggregation and activation of coagulation system [164]. Blockage of both systems by anticoagulants (e.g. warfarin) and antiplatelet agents (e.g. aspirin and clopidogrel) as, for example during triple antithrombotic therapy (anticoagulant drugs + clopidogrel + aspirin) recommended for atrial fibrillation patients presenting with ACS and/or PCI [165], causes an increase in antithrombotic efficacy in addition to an increased bleeding risk. This expectation is confirmed by the results from two meta-analyses that showed that this drug combination resulted in a more efficacious protection from major cardiovascular risk but also remarkably elevated bleeding events compared to antiplatelet or anticoagulant treatment alone [166, 167]. The results from WOEST clinical trial also reported that, compared with the triple antithrombotic therapy, dual antithrombotic therapy (clopidogrel + anticoagulant) significantly decreases the risk of bleeding complications while leaving the rate of thrombotic events unchanged [168]. These findings indicate that aspirin may have to be excluded from combination therapy in order to achieve the desired benefit/risk profile.
4.3.6 Selective serotonin reuptake inhibitors
It has been reported that about 20% of CVD patients suffer from depression, which is frequently treated with selective serotonin reuptake inhibitors (SSRIs, e.g. fluoxetine, citalopram and sertraline) [169]. SSRIs inhibit the serotonin transporter in the central nervous system and thereby suppress the uptake of synaptic serotonin into the presynaptic neuron. In the blood, SSRIs block the entry of serotonin into platelets, which leads to a depletion of intra-platelet serotonin stores, thereby reducing the efficiency of ADP-induced platelet aggregation [170]. Results from the SADHART trial ACS in patients with depression showed that in addition to antiplatelet regimens including aspirin and clopidogrel, concomitant sertraline was associated with a further reduction in platelet/endothelial activation, suggesting that SSRIs might bring additional advantage in CAD patients with comorbid depression [171]. On the other hand, a recent DDI study conducted with healthy adults revealed that concurrent fluoxetine significantly reduced clop-AM plasma levels and clopidogrel response [172] due to the inhibition of CYP2C9, CYP2C19 and CYP3A4 [12, 173]. Inconsistent results were reported from several clinical outcome studies. The ENRICHD clinical trial and two following clinical outcome studies reported that concurrent SSRIs was associated with a significant reduced risk of death or recurrent MI as well as an increased risk in bleeding in clopidogrel treated patients [174–176]. However, conflicting results were reported from several other studies [169, 177, 178], suggesting the necessity of further evaluation of the potential impact of SSRIs on clopidogrel antiplatelet treatment.
4.4 Comorbidities
4.4.1 Diabetes mellitus
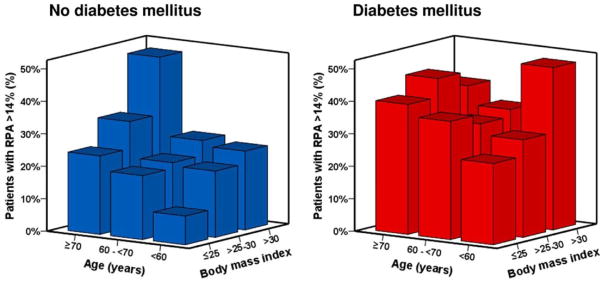
A significant portion of ACS patients (~30%) also suffer from diabetes mellitus (DM), which puts them at an increased atherothrombotic risk and higher mortality rates compared to their non-diabetic peers [44, 55, 56, 82, 179]. The EXCELSIOR study showed that DM was most relevant independent indicator of HPR next to the patient’s CYP2C19*2 carrier status and that individuals with DM had a significantly higher prevalence of HPR than non-diabetic subjects at all BMI and age groups (e.g. age ≥ 70 & BMI ≤ 25 or age ≤ 60 & BMI ≥ 30) (Figure 2) [41]. Although the exact mechanism is still unclear, several factors, such as endothelial dysfunction, increased coagulation, impaired fibrinolysis and platelet hyper-reactivity, contribute to prothrombotic conditions in DM patients and are summarized elsewhere [180]. A study conducted by Angiolillo et al. reported that a mutation (rs956115) of insulin receptor substrate-1 was associated with significantly higher prevalence of HPR and major adverse cardiac events in patients with type 2 DM and stable CAD following treatment of clopidogrel and aspirin [181]. On the other hand, Erlinge et al. reported that the poor clopidogrel response in patients with DM was attributed to the lower systemic exposure to clop-AM rather than changes in platelet response [182]. These findings were confirmed in a PK study conducted in healthy subjects and type 2 diabetes patients with CYP2C19 substrate R483, which showed that DM may cause a significant suppression of CYP2C19 catalytic capacity [183] and potentially increase clopidogrel resistance in DM patients. Consistently, a recent study conducted in ACS patients also reported that CYP2C19 loss-of-function mutation significantly impacted clopidogrel clinical outcome in non-DM individuals compared to DM patients [184].
Figure 2.
Prevalence of patients with high on-treatment platelet reactivity (HPR) according to age, body mass index and diabetes mellitus status. HPR was defined as residual platelet aggregation (RPA) > 14%, which was assessed by light transmittance aggregometry (LTA) assay with ADP 5 μmol/L following a 600 mg of clopidogrel loading dose in 760 patients undergoing elective coronary stent implantation [41].
4.4.2 Chronic kidney disease
Renal insufficiency (creatinine clearance < 70 mL/min) has been reported for 35 to 40% of ACS patients [185]. The impact of chronic kidney disease (CKD) on clopidogrel response has been studied by multiple investigators but the results remain inconclusive. This is partly due to the fact that different assays used provide different results. For example, CKD was determined to have a significant impact on HPR when using the VerifyNow P2Y12 assay, whereas no difference between patients with or without CKD was found when using the LTA assay or the VASP-PRI assay [186–191]. The differences in test results were attributed to varying hemoglobin levels in CKD patients, which may cause interference in this assay when whole blood is used [186–188]. On the other hand, CKD was found to be associated with an increased risk in clinical outcome (e.g. death, adverse cardiovascular events and ST) and bleeding clopidogrel treated patients [43, 44, 59, 192–195]. A recent meta-analysis also reported that the benefits of antiplatelet therapy in CKD patients are uncertain and potentially outweighed by bleeding hazards [196], suggesting that caution should be needed when CKD patients seek for antiplatelet therapy.
4.5 Patient compliance
Timely initiation of therapy and rigorous adherence to the prescribed treatment are imperative for successful management of ACS. Failure to comply with these requirements, e.g. in terms of delayed onset of therapy, failure in timely refill of prescriptions or premature discontinuation of clopidogrel or dual antiplatelet therapy, were identified as “hidden factors” that contribute to clopidogrel resistance, an elevated risk of adverse cardiac events and even death [5, 44, 197]. Ho et al. reported that delay in filling clopidogrel prescriptions resulted in significantly increased death/MI rates in patients following stent implantation [198]. Interventions, such as follow up by phone, checking refill histories or monitoring clinical registries, have resulted in significantly improved compliance to clopidogrel therapy [199, 200].
5. Summary and future perspectives
In recent years, variability in clopidogrel response has become an increasingly important clinical issue with potentially severe consequences [3]. Therefore, it becomes imperative to understand the key factors that contribute to the high between-subject variability in response to clopidogrel treatment, particularly to clopidogrel resistance. In this paper we systematically reviewed known pharmacokinetic, pharmacodynamic as well as genetic and non-genetic factors that contribute to interindividual differences in response to clopidogrel therapy and evaluated how they relate to clinical outcome (cf. Table 1 & 2).
Table 1.
Summary of factors that may affect clopidogrel pharmacokinetics, pharmacodynamics and clinical outcome.
| Potential Factors | Influence on Clopidogrel PK/PD | Influence on Clinical Outcome | |
|---|---|---|---|
| Demographics | |||
| Older Age | Higher on-treatment platelet reactivity [14, 19, 39–41]. | Increase in both cardiovascular events [5, 16, 42–44] and bleeding [45, 46]. | |
| Obesity | Lower systemic exposure to clop-AM [136] Higher on-treatment platelet reactivity [14, 41, 47]. |
Inconclusive (obesity paradox) [16, 42, 45, 52, 53]. | |
| Sex | Minimal/inconclusive [14, 19, 39, 57, 87] [40, 58]. | Minimal/inconclusive [16, 42] [5, 43, 45, 52, 59–61]. | |
| Pharmacogenetics | |||
| ABCB1 | C3435T | Minimal/inconclusive [16, 19, 21, 39, 60] | Minimal/inconclusive [16, 60, 64–67] |
| CES1 | G143E A-618C |
Lower on-treatment platelet reactivity [23, 68] Inconclusive [70, 71] |
N/A |
| CYP2C19 | G681A (*2) G636A (*3) |
Lower systemic exposure to clop-AM [4, 75–77] Higher on-treatment platelet reactivity [17, 18, 39, 58, 78–82] |
Increase of cardiovascular risk [14, 16, 18, 65, 78, 81–85] |
| C806T (*17) | Minimal/inconclusive [15, 77, 86, 87] | Minimal/inconclusive [15, 65, 74, 83, 87] | |
| CYP1A2 | *1C-1F, *7, *11, *16 and others | Minimal/inconclusive [17, 18, 75, 80] | Minimal/inconclusive [18] |
| CYP2B6 | *1B, *1C, *5, *6, *9, *11 and others | Minimal/inconclusive [18, 39] | Minimal/inconclusive [18] |
| CYP2C9 | C430T (*2) A1075C (*3) and others |
Minimal/inconclusive [18, 75, 80] | Minimal/inconclusive [18, 61] |
| CYP3A4/5 | *2, *3, *17 and others (CYP3A4) *2, *3, *6 and others (CYP3A5) |
Minimal/inconclusive [18, 75, 80, 81, 92, 93]. | Minimal/inconclusive [18, 61, 93]. |
| PON1 | Q192R | Minimal/inconclusive [24, 76, 79, 82, 96, 97], | Minimal/inconclusive [65, 79, 82, 96–98], |
| P2Y12 | H2 haplotype and others | Minimal/inconclusive [57, 100, 101, 104, 105, 108] | Minimal/inconclusive [16, 39, 105–107, 109] |
| Drug-Drug Interactions | |||
| Proton pump inhibitors | Lower systemic exposure to clop-AM [120, 121, 124] Higher on-treatment platelet reactivity [120–127]. |
Minimal impact on cardiovascular risk [118, 130–132] | |
| Statins | No negative effect on systemic exposure to clop- AM [135]. No negative effect on on-treatment platelet reactivity [127, 135, 137–145]. |
No negative effect on clinical outcome [141, 146–148] | |
| Calcium channel blockers | Minimal/inconclusive [63, 94, 150, 151, 154] | Minimal/inconclusive [94, 150–154] | |
| Anticoagulants | N/A | Decrease of cardiovascular risk while increase of bleeding events [166, 167] | |
| Antidepressants | Conflicting/inconclusive [171, 172] | Conflicting/inconclusive [169, 174–178] | |
| Comorbidities | |||
| Diabetes | Lower systemic exposure to clop-AM [182] Higher on-treatment platelet reactivity [41, 55, 82, 179, 181]. |
Increase of cardiovascular risk [41, 44, 56, 82, 181] | |
| Chronic kidney disease | Higher on-treatment platelet reactivity (more significant when using VerifyNow™ P2Y12 assay) [91, 186–191] | Increase in both cardiovascular events and bleeding [43, 44, 59, 192–196] | |
Table 2.
Summary of demographic, genetic, drug- and disease-mediated factors influencing antiplatelet therapy with clopidogrel.
| Potential factors | Influence on clopidogrel HPR | Influence on cardiovascular risk | Influence on bleeding risk | |
|---|---|---|---|---|
| Demographics | ||||
| Older age | ↑ | ↑ | ↑ | |
| Obesity | ↑↑ | ↑↓ | - | |
| Sex | ↔ | ↔ | ↔ | |
| Pharmacogenetics | ||||
| ABCB1 | C3435T | ↔ | ↔ | ↔ |
| CES1 | G143E A-618C |
↓ ↔ |
N/A ↔ |
N/A N/A |
| CYP2C19 | G681A (*2) G636A (*3) |
↑↑ | ↑↑ | ↓ |
| C806T (*17) | ↔ | ↔ | ↔ | |
| CYP1A2 | *1C-1F, *7, *11, *16 and others | ↔ | ↔ | ↔ |
| CYP2B6 | *1B, *1C, *5, *6, *9, *11 and others | ↔ | ↔ | ↔ |
| CYP2C9 | C430T (*2) A1075C (*3) and others |
↔ | ↔ | ↔ |
| CYP3A4/5 | *2, *3, *17 and others (CYP3A4) *2, *3, *6 and others (CYP3A5) |
↔ | ↔ | ↔ |
| PON1 | Q192R | ↔ | ↔ | ↔ |
| P2Y12 | H2 haplotype and others | ↔ | ↔ | ↔ |
| Drug-Drug Interactions | ||||
| Proton pump inhibitors | ↑ | ↔ | ↓ | |
| Statins | ↔ | ↔ | ↔ | |
| Calcium channel blockers | ↔ | ↔ | ↔ | |
| Anticoagulants | N/A | ↓ | ↑ | |
| Antidepressants | ↔ | ↔ | ↔ | |
| Comorbidities | ||||
| Diabetes | ↑↑ | ↑↑ | ↓ | |
| Chronic kidney disease | ↑ | ↑ | ↑↑ | |
N/A not available, ↑ and ↓ indicate an increase and decrease, respectively, ↔ indicates no effect.
To date, numerous clinical studies have been conducted to investigate the potential cause of clopidogrel resistance. Most of these studies were either conducted to answer one specific question (e.g. age, DDI) or to investigate the impact of multiple impact factors to a qualitative manner, as manifested by statistical significance. Our review of the literature clearly indicates that despite a multiplicity of research efforts, no clear cut answer is available yet that allows to sufficiently answer all the open questions and, ultimately, to reliably identify optimal treatment/dosing regimens for individual patients prior to the start of therapy. This in part due to the fact that suboptimal response to clopidogrel treatment, both in terms of efficacy and safety, is a multifactorial problem that is difficult to address in one-off clinical trials that evaluate only one or only a few factors at a time. The harmonized use of quantitative analysis strategies, such as population and physiologically based modeling and simulation approaches in corporation with systems biology/pharmacology modeling, may provide a quantitative characterization for the multiple genetic, demographic and disease risk factors that affect clopidogrel response, and the interaction between them with a dynamic manner. These quantitative approaches in combination with clinical trials may help to overcome this limitation as they allow to interpret and to compare information from head-to-head clinical trials and to evaluate the impact of different genetic and non-genetic factors as well as their interplay on clinical outcome. Once identified and qualified, these models have the potential to serve as bedside-ready decision support tools for physicians and other health care professionals for optimizing patients’ clopidogrel dosing regimens based on the individuals’ genetics, demographics, medication and disease history. The use of quantitative approaches may further allow to performing cost-effectiveness analyses for single as well as combination antiplatelet therapy and, ultimately, guide clinical and health-policy decision-making.
Key messages.
Multiple genetic and non-genetic factors contribute to the high inter-individual variability in clopidogrel’s dose-concentration-response relationship following oral administration of the standard dosing regimen (300 mg loading dose, 75 mg maintenance dose)
In order to understand the relative contribution of each of these factors to the overall variability in treatment response, sufficient understanding of the underlying pharmacokinetics (PK) and pharmacodynamics (PD) is needed
An understanding of the variability in PK and PD requires a mechanistic-based, quantitative analysis approach that integrates available information on the clinically relevant factors that impact clopidogrel’s PK and PD
Once established and qualified, this qualitative and quantitative link can then be used to translate genetic and clinical information into actionable dosing recommendations and thus help to personalize clopidogrel therapy on a patient-by-patient basis.
Acknowledgments
This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064. X-LJ, SS, LJL and SS have no potential conflict of interest that might be relevant to the contents of this review.
References
- 1.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011 Dec;32(23):2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007 Apr 10;49(14):1505–16. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Kelly RP, Close SL, Farid NA, Winters KJ, Shen L, Natanegara F, et al. Pharmacokinetics and pharmacodynamics following maintenance doses of prasugrel and clopidogrel in Chinese carriers of CYP2C19 variants. Br J Clin Pharmacol. 2012 Jan;73(1):93–105. doi: 10.1111/j.1365-2125.2011.04049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boggon R, van Staa TP, Timmis A, Hemingway H, Ray KK, Begg A, et al. Clopidogrel discontinuation after acute coronary syndromes: frequency, predictors and associations with death and myocardial infarction--a hospital registry-primary care linked cohort (MINAP-GPRD) Eur Heart J. 2011 Oct;32(19):2376–86. doi: 10.1093/eurheartj/ehr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshaghian S, Kaul S, Amin S, Shah PK, Diamond GA. Role of clopidogrel in managing atherothrombotic cardiovascular disease. Annals of internal medicine. 2007 Mar 20;146(6):434–41. doi: 10.7326/0003-4819-146-6-200703200-00008. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001 Aug 16;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 8.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012 Aug 14;60(7):645–81. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 9.American College of Emergency P, Society for Cardiovascular A Interventions. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Jan 29;61(4):e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Perry CG, Shuldiner AR. Pharmacogenomics of anti-platelet therapy: how much evidence is enough for clinical implementation? Journal of human genetics. 2013 Jun;58(6):339–45. doi: 10.1038/jhg.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbel PA, Tantry US. Clopidogrel resistance? Thromb Res. 2007;120(3):311–21. doi: 10.1016/j.thromres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010 Jan;38(1):92–9. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 13.FDA. FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. 2010. [Google Scholar]
- 14.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009 Aug 26;302(8):849–57. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siller-Matula JM, Delle-Karth G, Lang IM, Neunteufl T, Kozinski M, Kubica J, et al. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost. 2012 Apr;10(4):529–42. doi: 10.1111/j.1538-7836.2012.04639.x. [DOI] [PubMed] [Google Scholar]
- 16.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009 Jan 22;360(4):363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 17.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006 Oct 1;108(7):2244–7. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 18.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009 Jan 22;360(4):354–62. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 19.Frelinger AL, 3rd, Bhatt DL, Lee RD, Mulford DJ, Wu J, Nudurupati S, et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol. 2013 Feb 26;61(8):872–9. doi: 10.1016/j.jacc.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Sanofi-Aventis . PRODUCT MONOGRAPH: Plavix Clopidogrel 75 and 300 mg Tablets, Manufacturer’s Standard. 2011. [Google Scholar]
- 21.Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006 Nov;80(5):486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010 Sep 14;56(12):919–33. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Zhu HJ, Wang X, Gawronski BE, Brinda BJ, Angiolillo DJ, Markowitz JS. Carboxylesterase 1 as a determinant of clopidogrel metabolism and activation. J Pharmacol Exp Ther. 2013 Mar;344(3):665–72. doi: 10.1124/jpet.112.201640. [DOI] [PubMed] [Google Scholar]
- 24.Bouman HJ, Schomig E, van Werkum JW, Velder J, Hackeng CM, Hirschhauser C, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011 Jan;17(1):110–6. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 25.Peer CJ, Spencer SD, VanDenBerg DA, Pacanowski MA, Horenstein RB, Figg WD. A sensitive and rapid ultra HPLC-MS/MS method for the simultaneous detection of clopidogrel and its derivatized active thiol metabolite in human plasma. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2012 Jan 1;880(1):132–9. doi: 10.1016/j.jchromb.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Beckerath N, Taubert D, Pogatsa-Murray G, Schomig E, Kastrati A, Schomig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation. 2005 Nov 8;112(19):2946–50. doi: 10.1161/CIRCULATIONAHA.105.559088. [DOI] [PubMed] [Google Scholar]
- 27.Caplain H, Donat F, Gaud C, Necciari J. Pharmacokinetics of clopidogrel. Semin Thromb Hemost. 1999;25(Suppl 2):25–8. [PubMed] [Google Scholar]
- 28.Wallentin L, Varenhorst C, James S, Erlinge D, Braun OO, Jakubowski JA, et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008 Jan;29(1):21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- 29.Collet JP, Hulot JS, Anzaha G, Pena A, Chastre T, Caron C, et al. High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2) JACC Cardiovasc Interv. 2011 Apr;4(4):392–402. doi: 10.1016/j.jcin.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Horenstein RB, Madabushi R, Zineh I, Yerges-Armstrong LM, Peer CJ, Schuck RN, et al. Effectiveness of clopidogrel dose escalation to normalize active metabolite exposure and antiplatelet effects in CYP2C19 poor metabolizers. J Clin Pharmacol. 2014 Apr 7; doi: 10.1002/jcph.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Small DS, Farid NA, Payne CD, Konkoy CS, Jakubowski JA, Winters KJ, et al. Effect of intrinsic and extrinsic factors on the clinical pharmacokinetics and pharmacodynamics of prasugrel. Clinical pharmacokinetics. 2010 Dec;49(12):777–98. doi: 10.2165/11537820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Linden MD, Tran H, Woods R, Tonkin A. High platelet reactivity and antiplatelet therapy resistance. Semin Thromb Hemost. 2012 Mar;38(2):200–12. doi: 10.1055/s-0032-1301417. [DOI] [PubMed] [Google Scholar]
- 33.Ait-Mokhtar O, Bonello L, Benamara S, Paganelli F. High on treatment platelet reactivity. Heart Lung Circ. 2012 Jan;21(1):12–21. doi: 10.1016/j.hlc.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 34.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007 Nov 6;50(19):1822–34. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: a report from the platelet physiology subcommittee of the SSC of the ISTH. J Thromb Haemost. 2009 Jun;7(6):1029. doi: 10.1111/j.1538-7836.2009.03458.x. [DOI] [PubMed] [Google Scholar]
- 36.Sibbing D, Byrne RA, Bernlochner I, Kastrati A. High platelet reactivity and clinical outcome - fact and fiction. Thromb Haemost. 2011 Aug;106(2):191–202. doi: 10.1160/TH11-01-0040. [DOI] [PubMed] [Google Scholar]
- 37.Cuisset T, Frere C, Quilici J, Gaborit B, Castelli C, Poyet R, et al. Predictive values of post-treatment adenosine diphosphate-induced aggregation and vasodilator-stimulated phosphoprotein index for stent thrombosis after acute coronary syndrome in clopidogrel-treated patients. Am J Cardiol. 2009 Oct 15;104(8):1078–82. doi: 10.1016/j.amjcard.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Hochholzer W, Ruff CT, Mesa RA, Mattimore JF, Cyr JF, Lei L, et al. Variability of Individual Platelet Reactivity Over Time in Patients Treated With Clopidogrel: Insights From the ELEVATE-TIMI 56 Trial. J Am Coll Cardiol. 2014 Jul 29;64(4):361–8. doi: 10.1016/j.jacc.2014.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, et al. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J Am Coll Cardiol. 2012 May 29;59(22):1928–37. doi: 10.1016/j.jacc.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 40.Park JJ, Park KW, Kang J, Jeon KH, Kang SH, Ahn HS, et al. Genetic determinants of clopidogrel responsiveness in Koreans treated with drug-eluting stents. International journal of cardiology. 2013 Feb 10;163(1):79–86. doi: 10.1016/j.ijcard.2012.09.075. [DOI] [PubMed] [Google Scholar]
- 41.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010 Jun 1;55(22):2427–34. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 42.Carlquist JF, Knight S, Horne BD, Huntinghouse JA, Rollo JS, Muhlestein JB, et al. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb Haemost. 2013 Apr 8;109(4):744–54. doi: 10.1160/TH12-05-0336. [DOI] [PubMed] [Google Scholar]
- 43.Roth GA, Morden NE, Zhou W, Malenka DJ, Skinner J. Clopidogrel use and early outcomes among older patients receiving a drug-eluting coronary artery stent. Circulation Cardiovascular quality and outcomes. 2012 Jan;5(1):103–12. doi: 10.1161/CIRCOUTCOMES.111.962704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005 May 4;293(17):2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 45.Iijima R, Ndrepepa G, Mehilli J, Byrne RA, Schulz S, Neumann FJ, et al. Profile of bleeding and ischaemic complications with bivalirudin and unfractionated heparin after percutaneous coronary intervention. Eur Heart J. 2009 Feb;30(3):290–6. doi: 10.1093/eurheartj/ehn586. [DOI] [PubMed] [Google Scholar]
- 46.Cay S, Cagirci G, Aydogdu S, Balbay Y, Sen N, Maden O, et al. Safety of clopidogrel in older patients: a nonrandomized, parallel-group, controlled, two-centre study. Drugs & aging. 2011 Feb 1;28(2):119–29. doi: 10.2165/11586380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Barrera Ramirez C, Sabate M, Fernandez C, et al. Platelet aggregation according to body mass index in patients undergoing coronary stenting: should clopidogrel loading-dose be weight adjusted? J Invasive Cardiol. 2004 Apr;16(4):169–74. [PubMed] [Google Scholar]
- 48.Wagner H, Angiolillo DJ, Ten Berg JM, Bergmeijer TO, Jakubowski JA, Small DS, et al. Higher body weight patients on clopidogrel maintenance therapy have lower active metabolite concentrations, lower levels of platelet inhibition, and higher rates of poor responders than low body weight patients. J Thromb Thrombolysis. 2013 Sep 17; doi: 10.1007/s11239-013-0987-8. [DOI] [PubMed] [Google Scholar]
- 49.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clinical pharmacokinetics. 2012 May 1;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 50.Jernas M, Olsson B, Arner P, Jacobson P, Sjostrom L, Walley A, et al. Regulation of carboxylesterase 1 (CES1) in human adipose tissue. Biochemical and biophysical research communications. 2009 May 22;383(1):63–7. doi: 10.1016/j.bbrc.2009.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagashima S, Yagyu H, Takahashi N, Kurashina T, Takahashi M, Tsuchita T, et al. Depot-specific expression of lipolytic genes in human adipose tissues--association among CES1 expression, triglyceride lipase activity and adiposity. Journal of atherosclerosis and thrombosis. 2011;18(3):190–9. doi: 10.5551/jat.6478. [DOI] [PubMed] [Google Scholar]
- 52.Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010 Jun;3(6):660–8. doi: 10.1016/j.jcin.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Mak KH, Bhatt DL, Shao M, Haffner SM, Hamm CW, Hankey GJ, et al. The influence of body mass index on mortality and bleeding among patients with or at high-risk of atherothrombotic disease. Eur Heart J. 2009 Apr;30(7):857–65. doi: 10.1093/eurheartj/ehp037. [DOI] [PubMed] [Google Scholar]
- 54.Sarno G, Garg S, Onuma Y, Buszman P, Linke A, Ischinger T, et al. The impact of body mass index on the one year outcomes of patients treated by percutaneous coronary intervention with Biolimus- and Sirolimus-eluting stents (from the LEADERS Trial) Am J Cardiol. 2010 Feb 15;105(4):475–9. doi: 10.1016/j.amjcard.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 55.Ang L, Palakodeti V, Khalid A, Tsimikas S, Idrees Z, Tran P, et al. Elevated plasma fibrinogen and diabetes mellitus are associated with lower inhibition of platelet reactivity with clopidogrel. J Am Coll Cardiol. 2008 Sep 23;52(13):1052–9. doi: 10.1016/j.jacc.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 56.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007 Aug 15;298(7):765–75. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 57.Kassimis G, Davlouros P, Xanthopoulou I, Stavrou EF, Athanassiadou A, Alexopoulos D. CYP2C19*2 and other genetic variants affecting platelet response to clopidogrel in patients undergoing percutaneous coronary intervention. Thromb Res. 2012 Apr;129(4):441–6. doi: 10.1016/j.thromres.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 58.Bouman HJ, Harmsze AM, van Werkum JW, Breet NJ, Bergmeijer TO, Ten Cate H, et al. Variability in on-treatment platelet reactivity explained by CYP2C19*2 genotype is modest in clopidogrel pretreated patients undergoing coronary stenting. Heart. 2011 Aug;97(15):1239–44. doi: 10.1136/hrt.2010.220509. [DOI] [PubMed] [Google Scholar]
- 59.Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007 Nov 1;100(9):1364–9. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 60.Jaitner J, Morath T, Byrne RA, Braun S, Gebhard D, Bernlochner I, et al. No association of ABCB1 C3435T genotype with clopidogrel response or risk of stent thrombosis in patients undergoing coronary stenting. Circ Cardiovasc Interv. 2012 Feb 1;5(1):82–8. S1–2. doi: 10.1161/CIRCINTERVENTIONS.111.965400. [DOI] [PubMed] [Google Scholar]
- 61.Harmsze AM, van Werkum JW, Ten Berg JM, Zwart B, Bouman HJ, Breet NJ, et al. CYP2C19*2 and CYP2C9*3 alleles are associated with stent thrombosis: a case-control study. Eur Heart J. 2010 Dec;31(24):3046–53. doi: 10.1093/eurheartj/ehq321. [DOI] [PubMed] [Google Scholar]
- 62.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007 Jan 26;315(5811):525–8. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 63.Harmsze AM, Robijns K, van Werkum JW, Breet NJ, Hackeng CM, Ten Berg JM, et al. The use of amlodipine, but not of P-glycoprotein inhibiting calcium channel blockers is associated with clopidogrel poor-response. Thromb Haemost. 2010 May;103(5):920–5. doi: 10.1160/TH09-08-0516. [DOI] [PubMed] [Google Scholar]
- 64.Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010 Oct 16;376(9749):1312–9. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delaney JT, Ramirez AH, Bowton E, Pulley JM, Basford MA, Schildcrout JS, et al. Predicting clopidogrel response using DNA samples linked to an electronic health record. Clin Pharmacol Ther. 2012 Feb;91(2):257–63. doi: 10.1038/clpt.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su J, Xu J, Li X, Zhang H, Hu J, Fang R, et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis. PLoS One. 2012;7(10):e46366. doi: 10.1371/journal.pone.0046366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roe MT, Goodman SG, Ohman EM, Stevens SR, Hochman JS, Gottlieb S, et al. Elderly Patients with Acute Coronary Syndromes Managed Without Revascularization Insights into the Safety of Long-Term Dual Antiplatelet Therapy with Reduced-Dose Prasugrel vs. Standard-Dose Clopidogrel. Circulation. 2013 Jul 12; doi: 10.1161/CIRCULATIONAHA.113.002303. [DOI] [PubMed] [Google Scholar]
- 68.Lewis JP, Horenstein RB, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics. 2013 Jan;23(1):1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geshi E, Kimura T, Yoshimura M, Suzuki H, Koba S, Sakai T, et al. A single nucleotide polymorphism in the carboxylesterase gene is associated with the responsiveness to imidapril medication and the promoter activity. Hypertension research: official journal of the Japanese Society of Hypertension. 2005 Sep;28(9):719–25. doi: 10.1291/hypres.28.719. [DOI] [PubMed] [Google Scholar]
- 70.Xie C, Ding X, Gao J, Wang H, Hang Y, Zhang H, et al. The effects of CES1A2 A(-816)C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenet Genomics. 2014 Apr;24(4):204–10. doi: 10.1097/FPC.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 71.Zou JJ, Chen SL, Fan HW, Tan J, He BS, Xie HG. CES1A -816C as a genetic marker to predict greater platelet clopidogrel response in patients with percutaneous coronary intervention. J Cardiovasc Pharmacol. 2014 Feb;63(2):178–83. doi: 10.1097/FJC.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 72.Martis S, Peter I, Hulot JS, Kornreich R, Desnick RJ, Scott SA. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. The pharmacogenomics journal. 2012 Apr 10; doi: 10.1038/tpj.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41(12):913–58. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 74.Pare G, Mehta SR, Yusuf S, Anand SS, Connolly SJ, Hirsh J, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010 Oct 28;363(18):1704–14. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 75.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007 Dec;5(12):2429–36. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 76.Gong IY, Crown N, Suen CM, Schwarz UI, Dresser GK, Knauer MJ, et al. Clarifying the importance of CYP2C19 and PON1 in the mechanism of clopidogrel bioactivation and in vivo antiplatelet response. Eur Heart J. 2012 Feb 27; doi: 10.1093/eurheartj/ehs042. [DOI] [PubMed] [Google Scholar]
- 77.Lewis J, Stephens S, Horenstein R, O’Connell J, Ryan K, Peer C, et al. The CYP2C19*17 Variant is not Independently Associated with Clopidogrel Response. J Thromb Haemost. 2013 Jun 29; doi: 10.1111/jth.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009 Jan 24;373(9660):309–17. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 79.Hulot JS, Collet JP, Cayla G, Silvain J, Allanic F, Bellemain-Appaix A, et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv. 2011 Oct 1;4(5):422–8. doi: 10.1161/CIRCINTERVENTIONS.111.963025. [DOI] [PubMed] [Google Scholar]
- 80.Simon T, Bhatt DL, Bergougnan L, Farenc C, Pearson K, Perrin L, et al. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin Pharmacol Ther. 2011 Aug;90(2):287–95. doi: 10.1038/clpt.2011.127. [DOI] [PubMed] [Google Scholar]
- 81.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007 Dec;17(12):1057–64. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- 82.Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, Schulz S, et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J. 2011 Jul;32(13):1605–13. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 83.Zabalza M, Subirana I, Sala J, Lluis-Ganella C, Lucas G, Tomas M, et al. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012 Jan;98(2):100–8. doi: 10.1136/hrt.2011.227652. [DOI] [PubMed] [Google Scholar]
- 84.Sorich MJ, Polasek TM, Wiese MD. Challenges and Limitations in the Interpretation of Systematic Reviews: Making Sense of Clopidogrel and CYP2C19 Pharmacogenetics. Clin Pharmacol Ther. 2013 May 13; doi: 10.1038/clpt.2013.100. [DOI] [PubMed] [Google Scholar]
- 85.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010 Oct 27;304(16):1821–30. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gurbel PA, Shuldiner AR, Bliden KP, Ryan K, Pakyz RE, Tantry US. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J. 2011 Mar;161(3):598–604. doi: 10.1016/j.ahj.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010 Feb 2;121(4):512–8. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 88.Scott SA, Martis S, Peter I, Kasai Y, Kornreich R, Desnick RJ. Identification of CYP2C19*4B: pharmacogenetic implications for drug metabolism including clopidogrel responsiveness. The pharmacogenomics journal. 2012 Aug;12(4):297–305. doi: 10.1038/tpj.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H, Amunugama H, Ney S, Cooper N, Hollenberg PF. Mechanism-based inactivation of human cytochrome P450 2B6 by clopidogrel: involvement of both covalent modification of cysteinyl residue 475 and loss of heme. Mol Pharmacol. 2011 Nov;80(5):839–47. doi: 10.1124/mol.111.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishiya Y, Hagihara K, Ito T, Tajima M, Miura S, Kurihara A, et al. Mechanism-based inhibition of human cytochrome P450 2B6 by ticlopidine, clopidogrel, and the thiolactone metabolite of prasugrel. Drug Metab Dispos. 2009 Mar;37(3):589–93. doi: 10.1124/dmd.108.022988. [DOI] [PubMed] [Google Scholar]
- 91.Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005 Jun;77(6):553–9. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 92.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Cavallari U, Trabetti E, et al. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1895–900. doi: 10.1161/01.ATV.0000223867.25324.1a. [DOI] [PubMed] [Google Scholar]
- 93.Suh JW, Koo BK, Zhang SY, Park KW, Cho JY, Jang IJ, et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ. 2006 Jun 6;174(12):1715–22. doi: 10.1503/cmaj.060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park KW, Kang J, Park JJ, Yang HM, Lee HY, Kang HJ, et al. Amlodipine, clopidogrel and CYP3A5 genetic variability: effects on platelet reactivity and clinical outcomes after percutaneous coronary intervention. Heart. 2012 Sep;98(18):1366–72. doi: 10.1136/heartjnl-2012-301892. [DOI] [PubMed] [Google Scholar]
- 95.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008 Mar 19;299(11):1265–76. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park KW, Park JJ, Kang J, Jeon KH, Kang SH, Han JK, et al. Paraoxonase 1 gene polymorphism does not affect clopidogrel response variability but is associated with clinical outcome after PCI. PLoS One. 2013;8(2):e52779. doi: 10.1371/journal.pone.0052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reny JL, Combescure C, Daali Y, Fontana P Group PONM-A. Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost. 2012 Jul;10(7):1242–51. doi: 10.1111/j.1538-7836.2012.04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang YH, Lao HY, Wu H, Lai WH, Li XX, Yu XY, et al. Association of PON1 genotype and haplotype with susceptibility to coronary artery disease and clinical outcomes in dual antiplatelet-treated Han Chinese patients. Eur J Clin Pharmacol. 2013 Apr 23; doi: 10.1007/s00228-013-1516-6. [DOI] [PubMed] [Google Scholar]
- 99.Cavallari U, Trabetti E, Malerba G, Biscuola M, Girelli D, Olivieri O, et al. Gene sequence variations of the platelet P2Y12 receptor are associated with coronary artery disease. BMC Med Genet. 2007;8:59. doi: 10.1186/1471-2350-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003 Aug 26;108(8):989–95. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 101.Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. International journal of cardiology. 2009 Apr 17;133(3):341–5. doi: 10.1016/j.ijcard.2007.12.118. [DOI] [PubMed] [Google Scholar]
- 102.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Cavallari U, Trabetti E, et al. Lack of association between the P2Y12 receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb Res. 2005;116(6):491–7. doi: 10.1016/j.thromres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Cuisset T, Frere C, Quilici J, Morange PE, Saut N, Lambert M, et al. Role of the T744C polymorphism of the P2Y12 gene on platelet response to a 600-mg loading dose of clopidogrel in 597 patients with non-ST-segment elevation acute coronary syndrome. Thromb Res. 2007;120(6):893–9. doi: 10.1016/j.thromres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 104.Cayla G, Hulot JS, O’Connor SA, Pathak A, Scott SA, Gruel Y, et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011 Oct 26;306(16):1765–74. doi: 10.1001/jama.2011.1529. [DOI] [PubMed] [Google Scholar]
- 105.Rudez G, Pons D, Leebeek F, Monraats P, Schrevel M, Zwinderman A, et al. Platelet receptor P2RY12 haplotypes predict restenosis after percutaneous coronary interventions. Human mutation. 2008 Mar;29(3):375–80. doi: 10.1002/humu.20641. [DOI] [PubMed] [Google Scholar]
- 106.Ziegler S, Schillinger M, Funk M, Felber K, Exner M, Mlekusch W, et al. Association of a functional polymorphism in the clopidogrel target receptor gene, P2Y12, and the risk for ischemic cerebrovascular events in patients with peripheral artery disease. Stroke; a journal of cerebral circulation. 2005 Jul;36(7):1394–9. doi: 10.1161/01.STR.0000169922.79281.a5. [DOI] [PubMed] [Google Scholar]
- 107.Tang XF, Zhang JH, Wang J, Han YL, Xu B, Qiao SB, et al. Effects of coexisting polymorphisms of CYP2C19 and P2Y12 on clopidogrel responsiveness and clinical outcome in patients with acute coronary syndromes undergoing stent-based coronary intervention. Chinese medical journal. 2013 Mar;126(6):1069–75. [PubMed] [Google Scholar]
- 108.Rudez G, Bouman HJ, van Werkum JW, Leebeek FW, Kruit A, Ruven HJ, et al. Common variation in the platelet receptor P2RY12 gene is associated with residual on-clopidogrel platelet reactivity in patients undergoing elective percutaneous coronary interventions. Circ Cardiovasc Genet. 2009 Oct;2(5):515–21. doi: 10.1161/CIRCGENETICS.109.861799. [DOI] [PubMed] [Google Scholar]
- 109.Bouman HJ, van Werkum JW, Rudez G, Hackeng CM, Leebeek FW, ten Cate H, et al. The relevance of P2Y(12)-receptor gene variation for the outcome of clopidogrel-treated patients undergoing elective coronary stent implantation: a clinical follow-up. Thromb Haemost. 2012 Jan;107(1):189–91. doi: 10.1160/TH11-05-0306. [DOI] [PubMed] [Google Scholar]
- 110.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Sabate M, Fernandez C, et al. PlA polymorphism and platelet reactivity following clopidogrel loading dose in patients undergoing coronary stent implantation. Blood Coagul Fibrinolysis. 2004 Jan;15(1):89–93. doi: 10.1097/00001721-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 111.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y (12), P2Y (1) and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119(3):355–60. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Sibbing D, von Beckerath O, Schomig A, Kastrati A, von Beckerath N. P2Y1 gene A1622G dimorphism is not associated with adenosine diphosphate-induced platelet activation and aggregation after administration of a single high dose of clopidogrel. J Thromb Haemost. 2006 Apr;4(4):912–4. doi: 10.1111/j.1538-7836.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- 113.Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008 Sep;84(3):417–23. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- 114.Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ. Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics. 2010 Jun;11(6):781–91. doi: 10.2217/pgs.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001 Aug;70(2):189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 116.Hasan MS, Basri HB, Hin LP, Stanslas J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: why the Asian population requires special attention. The International journal of neuroscience. 2013 Mar;123(3):143–54. doi: 10.3109/00207454.2012.744308. [DOI] [PubMed] [Google Scholar]
- 117.Harrison RW, Mahaffey KW. Clopidogrel and PPI interaction: clinically relevant or not? Curr Cardiol Rep. 2012 Feb;14(1):49–58. doi: 10.1007/s11886-011-0233-y. [DOI] [PubMed] [Google Scholar]
- 118.Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. The American journal of gastroenterology. 2010 Dec;105(12):2533–49. doi: 10.1038/ajg.2010.445. [DOI] [PubMed] [Google Scholar]
- 119.Ohbuchi M, Noguchi K, Kawamura A, Usui T. Different effects of proton pump inhibitors and famotidine on the clopidogrel metabolic activation by recombinant CYP2B6, CYP2C19 and CYP3A4. Xenobiotica. 2012 Jul;42(7):633–40. doi: 10.3109/00498254.2011.653655. [DOI] [PubMed] [Google Scholar]
- 120.Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther. 2011 Jan;89(1):65–74. doi: 10.1038/clpt.2010.219. [DOI] [PubMed] [Google Scholar]
- 121.Frelinger AL, 3rd, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012 Apr 3;59(14):1304–11. doi: 10.1016/j.jacc.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 122.Fontes-Carvalho R, Albuquerque A, Araujo C, Pimentel-Nunes P, Ribeiro VG. Omeprazole, but not pantoprazole, reduces the antiplatelet effect of clopidogrel: a randomized clinical crossover trial in patients after myocardial infarction evaluating the clopidogrel-PPIs drug interaction. Eur J Gastroenterol Hepatol. 2011 May;23(5):396–404. doi: 10.1097/MEG.0b013e3283460110. [DOI] [PubMed] [Google Scholar]
- 123.Furuta T, Iwaki T, Umemura K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol. 2010 Sep;70(3):383–92. doi: 10.1111/j.1365-2125.2010.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Small DS, Farid NA, Payne CD, Weerakkody GJ, Li YG, Brandt JT, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol. 2008 Apr;48(4):475–84. doi: 10.1177/0091270008315310. [DOI] [PubMed] [Google Scholar]
- 125.Hulot JS, Wuerzner G, Bachelot-Loza C, Azizi M, Blanchard A, Peyrard S, et al. Effect of an increased clopidogrel maintenance dose or lansoprazole co-administration on the antiplatelet response to clopidogrel in CYP2C19-genotyped healthy subjects. J Thromb Haemost. 2010 Mar;8(3):610–3. doi: 10.1111/j.1538-7836.2009.03729.x. [DOI] [PubMed] [Google Scholar]
- 126.FDA Food and Drug Administration. Information for Healthcare Professionals: Update to the labeling of Clopidogrel Bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC) 2009 [cited; Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm.
- 127.FDA. PLAVIX (clopidogrel bisulfate) tablets Labeling Revision. 2011 [cited 2013 08/04]; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020839s055lbl.pdf.
- 128.Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010 Nov 11;363(20):1909–17. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 129.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009 Mar 4;301(9):937–44. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 130.Focks JJ, Brouwer MA, van Oijen MG, Lanas A, Bhatt DL, Verheugt FW. Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome- a systematic review. Heart. 2013 Apr;99(8):520–7. doi: 10.1136/heartjnl-2012-302371. [DOI] [PubMed] [Google Scholar]
- 131.Siller-Matula JM, Jilma B, Schror K, Christ G, Huber K. Effect of proton pump inhibitors on clinical outcome in patients treated with clopidogrel: a systematic review and meta-analysis. J Thromb Haemost. 2010 Dec;8(12):2624–41. doi: 10.1111/j.1538-7836.2010.04049.x. [DOI] [PubMed] [Google Scholar]
- 132.Gerson LB, McMahon D, Olkin I, Stave C, Rockson SG. Lack of significant interactions between clopidogrel and proton pump inhibitor therapy: meta-analysis of existing literature. Digestive diseases and sciences. 2012 May;57(5):1304–13. doi: 10.1007/s10620-011-2007-1. [DOI] [PubMed] [Google Scholar]
- 133.Feidt DM, Klein K, Hofmann U, Riedmaier S, Knobeloch D, Thasler WE, et al. Profiling induction of cytochrome p450 enzyme activity by statins using a new liquid chromatography-tandem mass spectrometry cocktail assay in human hepatocytes. Drug Metab Dispos. 2010 Sep;38(9):1589–97. doi: 10.1124/dmd.110.033886. [DOI] [PubMed] [Google Scholar]
- 134.Howe K, Sanat F, Thumser AE, Coleman T, Plant N. The statin class of HMG-CoA reductase inhibitors demonstrate differential activation of the nuclear receptors PXR, CAR and FXR, as well as their downstream target genes. Xenobiotica. 2011 Jul;41(7):519–29. doi: 10.3109/00498254.2011.569773. [DOI] [PubMed] [Google Scholar]
- 135.Farid NA, Small DS, Payne CD, Jakubowski JA, Brandt JT, Li YG, et al. Effect of atorvastatin on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel in healthy subjects. Pharmacotherapy. 2008 Dec;28(12):1483–94. doi: 10.1592/phco.28.12.1483. [DOI] [PubMed] [Google Scholar]
- 136.Leoncini M, Toso A, Maioli M, Angiolillo DJ, Giusti B, Marcucci R, et al. Pharmacodynamic effects of adjunctive high dose atorvastatin on double dose clopidogrel in patients with high on-treatment platelet reactivity depending on diabetes mellitus status. J Thromb Thrombolysis. 2013 Jul 14; doi: 10.1007/s11239-013-0966-0. [DOI] [PubMed] [Google Scholar]
- 137.Muller I, Besta F, Schulz C, Li Z, Massberg S, Gawaz M. Effects of statins on platelet inhibition by a high loading dose of clopidogrel. Circulation. 2003 Nov 4;108(18):2195–7. doi: 10.1161/01.CIR.0000099507.32936.C0. [DOI] [PubMed] [Google Scholar]
- 138.Serebruany VL, Midei MG, Malinin AI, Oshrine BR, Lowry DR, Sane DC, et al. Absence of interaction between atorvastatin or other statins and clopidogrel: results from the interaction study. Arch Intern Med. 2004 Oct 11;164(18):2051–7. doi: 10.1001/archinte.164.18.2051. [DOI] [PubMed] [Google Scholar]
- 139.Smith SM, Judge HM, Peters G, Storey RF. Multiple antiplatelet effects of clopidogrel are not modulated by statin type in patients undergoing percutaneous coronary intervention. Platelets. 2004 Dec;15(8):465–74. doi: 10.1080/0953710412331272532. [DOI] [PubMed] [Google Scholar]
- 140.Trenk D, Hochholzer W, Frundi D, Stratz C, Valina CM, Bestehorn HP, et al. Impact of cytochrome P450 3A4-metabolized statins on the antiplatelet effect of a 600-mg loading dose clopidogrel and on clinical outcome in patients undergoing elective coronary stent placement. Thromb Haemost. 2008 Jan;99(1):174–81. doi: 10.1160/TH07-08-0503. [DOI] [PubMed] [Google Scholar]
- 141.Geisler T, Schaeffeler E, Dippon J, Winter S, Buse V, Bischofs C, et al. CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics. 2008 Sep;9(9):1251–9. doi: 10.2217/14622416.9.9.1251. [DOI] [PubMed] [Google Scholar]
- 142.Malmstrom RE, Ostergren J, Jorgensen L, Hjemdahl P. Influence of statin treatment on platelet inhibition by clopidogrel - a randomized comparison of rosuvastatin, atorvastatin and simvastatin co-treatment. J Intern Med. 2009 Nov;266(5):457–66. doi: 10.1111/j.1365-2796.2009.02119.x. [DOI] [PubMed] [Google Scholar]
- 143.Wenaweser P, Eshtehardi P, Abrecht L, Zwahlen M, Schmidlin K, Windecker S, et al. A randomised determination of the Effect of Fluvastatin and Atorvastatin on top of dual antiplatelet treatment on platelet aggregation after implantation of coronary drug-eluting stents. The EFA-Trial. Thromb Haemost. 2010 Sep;104(3):554–62. doi: 10.1160/TH09-11-0765. [DOI] [PubMed] [Google Scholar]
- 144.Motovska Z, Widimsky P, Petr R, Bilkova D, Marinov I, Simek S, et al. Factors influencing clopidogrel efficacy in patients with stable coronary artery disease undergoing elective percutaneous coronary intervention: statin’s advantage and the smoking “paradox”. J Cardiovasc Pharmacol. 2009 May;53(5):368–72. doi: 10.1097/FJC.0b013e31819d616b. [DOI] [PubMed] [Google Scholar]
- 145.Wenaweser P, Windecker S, Billinger M, Cook S, Togni M, Meier B, et al. Effect of atorvastatin and pravastatin on platelet inhibition by aspirin and clopidogrel treatment in patients with coronary stent thrombosis. Am J Cardiol. 2007 Feb 1;99(3):353–6. doi: 10.1016/j.amjcard.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 146.Wienbergen H, Gitt AK, Schiele R, Juenger C, Heer T, Meisenzahl C, et al. Comparison of clinical benefits of clopidogrel therapy in patients with acute coronary syndromes taking atorvastatin versus other statin therapies. Am J Cardiol. 2003 Aug 1;92(3):285–8. doi: 10.1016/s0002-9149(03)00626-x. [DOI] [PubMed] [Google Scholar]
- 147.Lotfi A, Schweiger MJ, Giugliano GR, Murphy SA, Cannon CP. High-dose atorvastatin does not negatively influence clinical outcomes among clopidogrel treated acute coronary syndrome patients--a Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) analysis. Am Heart J. 2008 May;155(5):954–8. doi: 10.1016/j.ahj.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 148.Patti G, Tomai F, Melfi R, Ricottini E, Macri M, Sedati P, et al. Strategies of clopidogrel load and atorvastatin reload to prevent ischemic cerebral events in patients undergoing protected carotid stenting. Results of the randomized ARMYDA-9 CAROTID (Clopidogrel and Atorvastatin Treatment During Carotid Artery Stenting) study. J Am Coll Cardiol. 2013 Apr 2;61(13):1379–87. doi: 10.1016/j.jacc.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 149.Katoh M, Nakajima M, Shimada N, Yamazaki H, Yokoi T. Inhibition of human cytochrome P450 enzymes by 1,4-dihydropyridine calcium antagonists: prediction of in vivo drug-drug interactions. Eur J Clin Pharmacol. 2000 Feb-Mar;55(11–12):843–52. doi: 10.1007/s002280050706. [DOI] [PubMed] [Google Scholar]
- 150.Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008 Nov 4;52(19):1557–63. doi: 10.1016/j.jacc.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 151.Sarafoff N, Neumann L, Morath T, Bernlochner I, Mehilli J, Schomig A, et al. Lack of impact of calcium-channel blockers on the pharmacodynamic effect and the clinical efficacy of clopidogrel after drug-eluting stenting. Am Heart J. 2011 Mar;161(3):605–10. doi: 10.1016/j.ahj.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt M, Johansen MB, Robertson DJ, Maeng M, Kaltoft A, Jensen LO, et al. Use of clopidogrel and calcium channel blockers and risk of major adverse cardiovascular events. Eur J Clin Invest. 2012 Mar;42(3):266–74. doi: 10.1111/j.1365-2362.2011.02579.x. [DOI] [PubMed] [Google Scholar]
- 153.Good CW, Steinhubl SR, Brennan DM, Lincoff AM, Topol EJ, Berger PB. Is there a clinically significant interaction between calcium channel antagonists and clopidogrel?: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Circ Cardiovasc Interv. 2012 Feb 1;5(1):77–81. doi: 10.1161/CIRCINTERVENTIONS.111.963405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harmsze AM, van Werkum JW, Souverein PC, Breet NJ, Bouman HJ, Hackeng CM, et al. Combined influence of proton-pump inhibitors, calcium-channel blockers and CYP2C19*2 on on-treatment platelet reactivity and on the occurrence of atherothrombotic events after percutaneous coronary intervention. J Thromb Haemost. 2011 Oct;9(10):1892–901. doi: 10.1111/j.1538-7836.2011.04483.x. [DOI] [PubMed] [Google Scholar]
- 155.Harmsze AM, Van Werkum JW, Moral F, Ten Berg JN, Hackeng CM, Klungel OH, et al. Sulfonylureas and on-clopidogrel platelet reactivity in type 2 diabetes mellitus patients. Platelets. 2011;22(2):98–102. doi: 10.3109/09537104.2010.530359. [DOI] [PubMed] [Google Scholar]
- 156.Sibbing D, von Beckerath N, Morath T, Stegherr J, Mehilli J, Sarafoff N, et al. Oral anticoagulation with coumarin derivatives and antiplatelet effects of clopidogrel. Eur Heart J. 2010 May;31(10):1205–11. doi: 10.1093/eurheartj/ehq023. [DOI] [PubMed] [Google Scholar]
- 157.Farid NA, Payne CD, Small DS, Winters KJ, Ernest CS, 2nd, Brandt JT, et al. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007 May;81(5):735–41. doi: 10.1038/sj.clpt.6100139. [DOI] [PubMed] [Google Scholar]
- 158.Lau WC, Waskell LA, Watkins PB, Neer CJ, Horowitz K, Hopp AS, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation. 2003 Jan 7;107(1):32–7. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 159.Holmberg MT, Tornio A, Neuvonen M, Neuvonen PJ, Backman JT, Niemi M. Grapefruit juice inhibits the metabolic activation of clopidogrel. Clin Pharmacol Ther. 2014 Mar;95(3):307–13. doi: 10.1038/clpt.2013.192. [DOI] [PubMed] [Google Scholar]
- 160.Jiang XL, Gonzalez FJ, Yu AM. Drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. Drug Metab Rev. 2011 Feb;43(1):27–40. doi: 10.3109/03602532.2010.512294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lau WC, Welch TD, Shields T, Rubenfire M, Tantry US, Gurbel PA. The effect of St John’s Wort on the pharmacodynamic response of clopidogrel in hyporesponsive volunteers and patients: increased platelet inhibition by enhancement of CYP3A4 metabolic activity. J Cardiovasc Pharmacol. 2011 Jan;57(1):86–93. doi: 10.1097/FJC.0b013e3181ffe8d0. [DOI] [PubMed] [Google Scholar]
- 162.Ueno M, Ferreiro JL, Desai B, Tomasello SD, Tello-Montoliu A, Capodanno D, et al. Cigarette smoking is associated with a dose-response effect in clopidogrel-treated patients with diabetes mellitus and coronary artery disease: results of a pharmacodynamic study. JACC Cardiovasc Interv. 2012 Mar;5(3):293–300. doi: 10.1016/j.jcin.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 163.Bliden KP, Baker BA, Nolin TD, Jeong YH, Bailey WL, Tantry US, et al. Thienopyridine efficacy and cigarette smoking status. Am Heart J. 2013 May;165(5):693–703. doi: 10.1016/j.ahj.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 164.James SH. Hematology pharmacology: anticoagulant, antiplatelet, and procoagulant agents in practice. AACN advanced critical care. 2009 Apr-Jun;20(2):177–92. doi: 10.1097/NCI.0b013e3181a0b631. [DOI] [PubMed] [Google Scholar]
- 165.Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen JK, Cuisset T, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary--a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2010 Jun;31(11):1311–8. doi: 10.1093/eurheartj/ehq117. [DOI] [PubMed] [Google Scholar]
- 166.Gao F, Zhou YJ, Wang ZJ, Yang SW, Nie B, Liu XL, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. International journal of cardiology. 2011 Apr 1;148(1):96–101. doi: 10.1016/j.ijcard.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 167.Oldgren J, Wallentin L, Alexander JH, James S, Jonelid B, Steg G, et al. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J. 2013 Mar 6; doi: 10.1093/eurheartj/eht049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013 Mar 30;381(9872):1107–15. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 169.Labos C, Dasgupta K, Nedjar H, Turecki G, Rahme E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011 Nov 8;183(16):1835–43. doi: 10.1503/cmaj.100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bismuth-Evenzal Y, Gonopolsky Y, Gurwitz D, Iancu I, Weizman A, Rehavi M. Decreased serotonin content and reduced agonist-induced aggregation in platelets of patients chronically medicated with SSRI drugs. Journal of affective disorders. 2012 Jan;136(1–2):99–103. doi: 10.1016/j.jad.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 171.Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, van Zyl LT, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003 Aug 26;108(8):939–44. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 172.Delavenne X, Magnin M, Basset T, Piot M, Mallouk N, Ressnikoff D, et al. Investigation of drug-drug interactions between clopidogrel and fluoxetine. Fundamental & clinical pharmacology. 2013 Feb 17; doi: 10.1111/fcp.12021. [DOI] [PubMed] [Google Scholar]
- 173.Spina E, Scordo MG, D’Arrigo C. Metabolic drug interactions with new psychotropic agents. Fundamental & clinical pharmacology. 2003 Oct;17(5):517–38. doi: 10.1046/j.1472-8206.2003.00193.x. [DOI] [PubMed] [Google Scholar]
- 174.Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Archives of general psychiatry. 2005 Jul;62(7):792–8. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 175.Mortensen JK, Larsson H, Johnsen SP, Andersen G. Post stroke use of selective serotonin reuptake inhibitors and clinical outcome among patients with ischemic stroke: a nationwide propensity score-matched follow-up study. Stroke; a journal of cerebral circulation. 2013 Feb;44(2):420–6. doi: 10.1161/STROKEAHA.112.674242. [DOI] [PubMed] [Google Scholar]
- 176.Ziegelstein RC, Meuchel J, Kim TJ, Latif M, Alvarez W, Dasgupta N, et al. Selective serotonin reuptake inhibitor use by patients with acute coronary syndromes. The American journal of medicine. 2007 Jun;120(6):525–30. doi: 10.1016/j.amjmed.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 177.Maschino F, Hurault-Delarue C, Chebbane L, Fabry V, Montastruc JL, Bagheri H, et al. Bleeding adverse drug reactions (ADRs) in patients exposed to antiplatelet plus serotonin reuptake inhibitor drugs: analysis of the French Spontaneous Reporting Database for a controversial ADR. Eur J Clin Pharmacol. 2012 Nov;68(11):1557–60. doi: 10.1007/s00228-012-1268-8. [DOI] [PubMed] [Google Scholar]
- 178.Kim DH, Daskalakis C, Whellan DJ, Whitman IR, Hohmann S, Medvedev S, et al. Safety of selective serotonin reuptake inhibitor in adults undergoing coronary artery bypass grafting. Am J Cardiol. 2009 May 15;103(10):1391–5. doi: 10.1016/j.amjcard.2009.01.348. [DOI] [PubMed] [Google Scholar]
- 179.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005 Aug;54(8):2430–5. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 180.Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011 Feb 22;123(7):798–813. doi: 10.1161/CIRCULATIONAHA.109.913376. [DOI] [PubMed] [Google Scholar]
- 181.Angiolillo DJ, Bernardo E, Zanoni M, Vivas D, Capranzano P, Malerba G, et al. Impact of insulin receptor substrate-1 genotypes on platelet reactivity and cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2011 Jun 28;58(1):30–9. doi: 10.1016/j.jacc.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 182.Erlinge D, Varenhorst C, Braun OO, James S, Winters KJ, Jakubowski JA, et al. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol. 2008 Dec 9;52(24):1968–77. doi: 10.1016/j.jacc.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 183.Bogman K, Silkey M, Chan SP, Tomlinson B, Weber C. Influence of CYP2C19 genotype on the pharmacokinetics of R483, a CYP2C19 substrate, in healthy subjects and type 2 diabetes patients. Eur J Clin Pharmacol. 2010 Oct;66(10):1005–15. doi: 10.1007/s00228-010-0840-3. [DOI] [PubMed] [Google Scholar]
- 184.Mizobe M, Hokimoto S, Akasaka T, Arima Y, Kaikita K, Morita K, et al. Impact of CYP2C19 polymorphism on clinical outcome following coronary stenting is more important in non-diabetic than diabetic patients. Thromb Res. 2014 Apr 26; doi: 10.1016/j.thromres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 185.Basra SS, Tsai P, Lakkis NM. Safety and efficacy of antiplatelet and antithrombotic therapy in acute coronary syndrome patients with chronic kidney disease. J Am Coll Cardiol. 2011 Nov 22;58(22):2263–9. doi: 10.1016/j.jacc.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 186.Leng WX, Ren JW, Cao J, Cong YL, Cui H, Hu GL, et al. Chronic kidney disease--is it a true risk factor of reduced clopidogrel efficacy in elderly patients with stable coronary artery disease? Thromb Res. 2013 Mar;131(3):218–24. doi: 10.1016/j.thromres.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 187.Voisin S, Bongard V, Tidjane MA, Lhermusier T, Carrie D, Sie P. Are P2Y12 reaction unit (PRU) and % inhibition index equivalent for the expression of P2Y12 inhibition by the VerifyNow assay? Role of haematocrit and haemoglobin levels. Thromb Haemost. 2011 Aug;106(2):227–9. doi: 10.1160/TH11-01-0046. [DOI] [PubMed] [Google Scholar]
- 188.Tello-Montoliu A, Ferreiro JL, Kodali MK, Ueno M, Tomasello SD, Rollini F, et al. Impact of renal function on clopidogrel-induced antiplatelet effects in coronary artery disease patients without diabetes mellitus. J Thromb Thrombolysis. 2012 Nov 11; doi: 10.1007/s11239-012-0828-1. [DOI] [PubMed] [Google Scholar]
- 189.Motovska Z, Odvodyova D, Fischerova M, Stepankova S, Maly M, Morawska P, et al. Renal function assessed using cystatin C and antiplatelet efficacy of clopidogrel assessed using the vasodilator-stimulated phosphoprotein index in patients having percutaneous coronary intervention. Am J Cardiol. 2012 Mar 1;109(5):620–3. doi: 10.1016/j.amjcard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 190.Cuisset T, Frere C, Moro PJ, Quilici J, Pons C, Gaborit B, et al. Lack of effect of chronic kidney disease on clopidogrel response with high loading and maintenance doses of clopidogrel after Acute Coronary Syndrome. Thromb Res. 2010 Nov;126(5):e400–2. doi: 10.1016/j.thromres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 191.Baber U, Bander J, Karajgikar R, Yadav K, Hadi A, Theodoropolous K, et al. Combined and independent impact of diabetes mellitus and chronic kidney disease on residual platelet reactivity. Thromb Haemost. 2013 May 16;110(1) doi: 10.1160/TH13-01-0004. [DOI] [PubMed] [Google Scholar]
- 192.Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, Mak KH, et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial) Am J Cardiol. 2009 May 15;103(10):1359–63. doi: 10.1016/j.amjcard.2009.01.342. [DOI] [PubMed] [Google Scholar]
- 193.Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, et al. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008 Apr;155(4):687–93. doi: 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 194.Keltai M, Tonelli M, Mann JF, Sitkei E, Lewis BS, Hawken S, et al. Renal function and outcomes in acute coronary syndrome: impact of clopidogrel. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2007 Apr;14(2):312–8. doi: 10.1097/01.hjr.0000220582.19516.a6. [DOI] [PubMed] [Google Scholar]
- 195.Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011 Jan 25;57(4):399–408. doi: 10.1016/j.jacc.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 196.Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Annals of internal medicine. 2012 Mar 20;156(6):445–59. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 197.Bauer T, Gitt AK, Junger C, Zahn R, Koeth O, Towae F, et al. Guideline-recommended secondary prevention drug therapy after acute myocardial infarction: predictors and outcomes of nonadherence. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010 Oct;17(5):576–81. doi: 10.1097/HJR.0b013e328338e5da. [DOI] [PubMed] [Google Scholar]
- 198.Ho PM, Tsai TT, Maddox TM, Powers JD, Carroll NM, Jackevicius C, et al. Delays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of care. Circulation Cardiovascular quality and outcomes. 2010 May;3(3):261–6. doi: 10.1161/CIRCOUTCOMES.109.902031. [DOI] [PubMed] [Google Scholar]
- 199.Krueger KP, Felkey BG, Berger BA. Improving adherence and persistence: a review and assessment of interventions and description of steps toward a national adherence initiative. Journal of the American Pharmacists Association: JAPhA. 2003 Nov-Dec;43(6):668–78. doi: 10.1331/154434503322642598. quiz 78–9. [DOI] [PubMed] [Google Scholar]
- 200.Rinfret S, Rodes-Cabau J, Bagur R, Dery JP, Dorais M, Larose E, et al. Telephone contact to improve adherence to dual antiplatelet therapy after drug-eluting stent implantation. Heart. 2013 Apr;99(8):562–9. doi: 10.1136/heartjnl-2012-303004. [DOI] [PubMed] [Google Scholar]