Abstract
AIM
To investigate the impact of medication beliefs, illness perceptions and quality of life on medication adherence in people with decompensated cirrhosis.
METHODS
One hundred adults with decompensated cirrhosis completed a structured questionnaire when they attended for routine outpatient hepatology review. Measures of self-reported medication adherence (Morisky Medication Adherence Scale), beliefs surrounding medications (Beliefs about Medicines Questionnaire), perceptions of illness and medicines (Brief Illness Perception Questionnaire), and quality of life (Chronic Liver Disease Questionnaire) were examined. Clinical data were obtained via patient history and review of medical records. Least absolute shrinkage and selection operator and stepwise backwards regression techniques were used to construct the multivariable logistic regression model. Statistical significance was set at alpha = 0.05.
RESULTS
Medication adherence was “High” in 42% of participants, “Medium” in 37%, and “Low” in 21%. Compared to patients with “High” adherence, those with “Medium” or “Low” adherence were more likely to report difficulty affording their medications (P < 0.001), lower perception of treatment helpfulness (P = 0.003) and stronger medication concerns relative to medication necessity beliefs (P = 0.003). People with “Low” adherence also experienced greater symptom burden and poorer quality of life, including more frequent abdominal pain (P = 0.023), shortness of breath (P = 0.030), and emotional disturbances (P = 0.050). Multivariable analysis identified having stronger medication concerns relative to necessity beliefs (Necessity-Concerns Differential ≤ 5, OR = 3.66, 95%CI: 1.18-11.40) and more frequent shortness of breath (shortness of breath score ≤ 3, OR = 3.87, 95%CI: 1.22-12.25) as independent predictors of “Low”adherence.
CONCLUSION
The association between “Low” adherence and patients having strong concerns or doubting the necessity or helpfulness of their medications should be explored further given the clinical relevance.
Keywords: Medication adherence, Medication beliefs, Illness perceptions, Quality of life, Liver cirrhosis
Core tip: Medication non-adherence is common in people with decompensated cirrhosis but the impact that patients’ medication beliefs and illness perceptions have on adherence is under-recognised. Clinician engagment with non-adherent patients should include open discussion of medications and liver disease. Acknowledgement of patient concerns surrounding their medicines, with positive reinforcement of medication necessity in terms of disease management may improve adherence behaviour and patients’ quality of life.
INTRODUCTION
People with decompensated cirrhosis require intensive inpatient and outpatient management, experience poor quality of life (QoL), and have a median survival of approximately two years. While liver transplantation is a viable treatment for end-stage liver disease, this is not an option for many patients. A complex regimen of medications is usually prescribed to manage complications of portal hypertension and liver insufficiency, however medication mismanagement and non-adherence is relatively common among patients with decompensated cirrhosis.
Medication adherence is defined by the World Health Organisation (WHO) as “the extent to which a person’s behaviour - taking medication, following a diet, and/or executing lifestyle changes - corresponds with agreed recommendations from a health care provider”[1]. Similar to other chronic diseases where approximately 50% of patients are thought to be non-adherent, up to 70% of patients with cirrhosis identify as having “Low” or “Medium” levels of medication adherence[2].
Non-adherence with medications has been associated with increased mortality in diabetes, coronary heart disease and heart failure[3,4]. It has been estimated that between 22% and 37% of 30-d readmissions among patients with decompensated cirrhosis may be potentially preventable with improved management of pharmacotherapy[5,6]. For example, non-adherence with lactulose, a non-absorbable disaccharide syrup used in the treatment of hepatic encephalopathy (HE), is reported to be as high as 69%[7], and has been associated with approximately 36% of potentially preventable 30-d readmissions[6]. Intentional non-adherence due to adverse effects (diarrhoea, flatulence and abdominal pain)[7] or misunderstanding of the indication[6] carries a 3-fold risk of HE recurrence[8]; a substantial, potentially preventable burden on patients, carers and the healthcare system[9]. The one-year survival probability following an episode of overt HE is 42%[10], and persisting cognitive impairment or covert HE[11] may in turn lead to unintentional non-adherence with other medications.
Non-adherence and mismanagement of diuretic therapy, which is prescribed in the management of abdominal ascites, peripheral oedema or pleural complications, contributes to 55% of potentially preventable 30-d readmissions in people with decompensated cirrhosis[6]. While prevalence has not been reported in cirrhosis, 30%-66% of patients prescribed loop diuretics in the management of heart failure are non-adherent with therapy[12,13]. Incorrect use of diuretics in cirrhosis can lead to severe electrolyte disturbances and renal impairment, and may contribute to a requirement for recurrent large volume paracentesis. Bacterial infections in people with decompensated cirrhosis are common and often precipitate further deterioration. Spontaneous bacterial peritonitis (SBP) carries a mortality rate of 31.5% at one month and 66.2% at one year[14], with a one-year recurrence rate of 61% without prophylaxis[15]. Prophylaxis with antibiotic therapy considerably reduces SBP recurrence, development of hepatorenal syndrome, and improves probability of one year survival[15], however adherence with long-term antibiotics is known to be low in other patient groups[16,17].
The WHO has identified non-adherence as an international priority in the prevention of patient harm and optimisation of limited health resources[1]. However, medication adherence is a complex health behaviour that is the result of numerous interacting dynamic variables including health literacy, self-efficacy, psychological perceptions of medicines and disease, quality of life, and other internal and external barriers[1]. Previous studies in liver disease patients have identified that side effects and changes to routine are the most commonly cited reasons for non-adherence[7,18]. Illness perceptions have been shown to influence patients’ confidence to self-manage alcoholic liver disease and limit self-efficacy[19], while self-perceived disease stigma can affect medical-care seeking behaviours and impact on QoL[20]. However there is limited information available about how perceptions and beliefs surrounding medications and liver disease affect adherence behaviours in people with decompensated cirrhosis. Identifying potentially-modifiable factors that influence adherence behaviour is important, as strategies to support adherence can be tailored to individual patients’ needs.
The aim of this study was to: (1) investigate medication non-adherence in a cohort of ambulatory patients with decompensated cirrhosis; and (2) to identify the effect of patients’ medication beliefs, illness perceptions, quality of life and clinical and demographic factors on medication non-adherence.
MATERIALS AND METHODS
A convenience sample of adult patients with cirrhosis who had experienced a decompensating event (abdominal ascites, spontaneous bacterial peritonitis, hepatic hydrothorax, encephalopathy or variceal bleeding) within the preceding two years were invited to participate when they attended routine outpatient follow-up at the Princess Alexandra Hospital in Brisbane, Australia, between February and October 2016. Patients were excluded if they were less than 18 years of age, unable to provide informed consent, undergoing transplant workup, or receiving intensive management by the palliative care team.
A structured questionnaire was used to obtain measures of medication adherence, quality of life, medication beliefs and illness perceptions. Questionnaires were completed independently by the patient or with the assistance of a carer, family member, or study coordinator, according to patient preference. Clinical data was collected from patients and/or their medical records, including standard biochemical and serological assays and liver imaging to confirm the diagnosis of cirrhosis and decompensation history. Socio-demographic items included patient-reported individual-based measures of education level and employment status, and residential area-based measures (Index for Relative Socioeconomic Disadvantage[21] and the Accessibility/Remoteness Index of Australia[22] for classification of remoteness of residence).
Medication adherence
Self-reported medication adherence was examined using the 8-Question Morisky Medication Adherence Scale (MMAS-8) which contains seven questions with yes/no alternatives, and one question which features a 5-point Likert scale. The scores from completed questionnaires are categorised into “High” (score = 8), “Medium” (score 6 to < 8) and “Low” (score < 6) adherence groups[23-25].
Medication beliefs
The Beliefs about Medications Questionnaires (BMQ-General and BMQ-Specific) were used to elicit patients’ beliefs about medications. Participants responded to eighteen statements across four domains using a 5-point Likert scale, from “strongly disagree” (score = 1) to “strongly agree” (score = 5). The BMQ-General contains 4 items that examine beliefs about Overuse of medicines by doctors, and 4 items about medication Harms. The BMQ-Specific contains 5 items in the Necessity domain and 5 items in the Concerns domain, which elicit patients’ respective necessity and concern beliefs about the medicines prescribed for their liver disease. Scores derived from the Concerns domain can be subtracted from the Necessity domain to give a Necessity-Concerns Differential (N-C differential)[26].
Illness perceptions
The Brief Illness Perceptions Questionnaire (Brief-IPQ) was used to examine the strength of patients’ perceptions about liver cirrhosis. The Brief-IPQ contains eight items, including identity (severity of symptoms) and consequences of disease on daily life, personal control and treatment control over disease, timeline for disease duration, self-perceived coherence or understanding of the disease, in addition to concerns and emotional representation that are caused by the disease. Patients self-measure each item on a scale from 0 to 10, where one end of the scale represents a benign perception and the other represents a threatening perception of illness[27].
Quality of life
The Chronic Liver Disease Questionnaire (CLDQ) was chosen to measure health related quality of life as it comprises specific domains relevant to the study population[28]. The full CLDQ contains 29 items across six domains; however responses to the twenty-ninth item have been excluded from analysis due to inapplicability to the majority of study participants. The twenty-ninth item pertains to worry about the availability of a liver if the patient requires a liver transplant; however patients being actively assessed for liver transplant were excluded from the study.
The shortened CLDQ used for the present study therefore contained eight questions related to Emotional Function, five questions within the domains of Systemic Symptoms and Fatigue, three questions within the domains of Abdominal Symptoms and daily Activity, and four items related to Worry. Individual item scores range from 1 to 7 and domain scores are calculated by averaging item scores within each domain. Higher scores represent better perceived health-related quality of life.
Data analysis:Statistical review of the study was performed by a biomedical statistician. Data was analysed using IBM® SPSS© Version 20.0. Where a question was missed within a validated tool, but at least 85% of the tool had otherwise been correctly completed, the missing value was imputed based on the mean or median response to that question, according to data skew. Imputation was required for a single value for two participants: one Brief IPQ-timeline (imputed median score 10) and one CLDQ-anxiety (imputed mean value 4).
Continuous and normally-distributed variables are presented as mean ± SD. Differences between groups were analysed by one-way ANOVA. Non-normally distributed data are presented as median (range) and have been analysed using the Kruskal-Wallis H test. Categorical data are presented as proportional percent and analysed using Pearson’s chi-squared (χ2), Fisher’s Exact test or Linear-by-Linear Associated test for trend as denoted. Statistical significance was set at alpha = 0.05.
The relationship between “Low” medication adherence and patients’ clinical, demographic and self-reported medication beliefs, illness perceptions and QoL were determined by calculating the odds ratio (OR) and 95%CI. Continuous variables were systematically assessed to identify optimal cut-points. Least absolute shrinkage and selection operator and stepwise backwards regression techniques were used to construct the final multivariable logistic regression model. Both methodologies identified the same significant factors for inclusion in the final model. The Hosmer-Lemeshow test was used to assess goodness-of-fit. Interactions between individual variables were not found to be statistically significant.
RESULTS
Demographic and clinical
One-hundred and thirty-four eligible patients were invited to participate in the study (Figure 1). Fourteen patients declined. Five patients indicated that they did not take medicines for their liver disease and thus did not complete essential components of the questionnaires. Of these five patients, three were unaware that their current pharmacotherapy included treatment for hepatic decompensation events, and two had ceased their therapy without medical advice. An additional fifteen patients incompletely filled out the questionnaires for other reasons.
Figure 1.
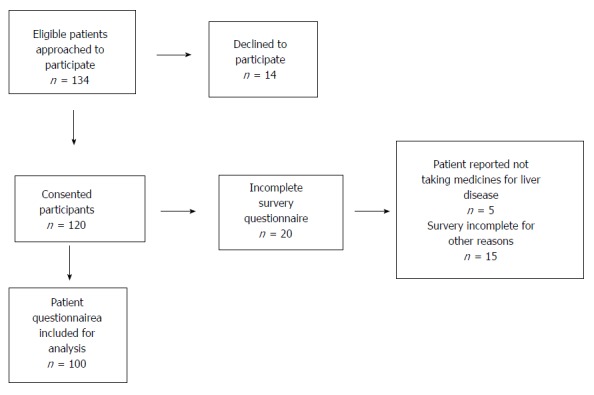
Participant recruitment flow diagram.
Of the 100 patients who completed the questionnaire tools, the majority (65.0%) were male and the mean age was 58.4 ± 10.2 years. Primary disease aetiology was alcoholic liver disease (ALD) in 49 patients, hepatitis C (HCV) in 35, non-alcoholic fatty liver disease in 9, primary sclerosing cholangitis in two, hepatitis B in one, autoimmune hepatitis in one, drug-induced liver injury in one, alpha-1-antitrypsin deficiency in one and unknown in one. Of the 51 patients who were not considered to have ALD, alcohol was a documented cofactor in 21 patients (41.2%).
Forty-two participants (42%) were categorised as having “High” medication adherence, 37% with “Medium” adherence, and 21% with “Low” adherence. Demographic and clinical characteristics of patients, and their association with medication adherence, are presented in Table 1. Male gender (P = 0.015) and inability to afford medications (P < 0.001) were associated with lower levels of medication adherence. Self-reported ability to afford medicines could not be predicted by employment status, relative sociodemographic disadvantage, or other sociodemographic factors (P > 0.05).
Table 1.
Demographic and clinical factors in patients with high, medium and low medication adherence n (%)
| Demographic and clinical variables | All patients |
Medication adherence ranking |
P value | |||
| High | Medium | Low | ||||
| (n = 42) | (n = 37) | (n = 21) | ||||
| Age | 58.4 ± 10.2 | 57.9 ± 10.9 | 58.2 ± 9.0 | 57.8 ± 10.8 | 0.787 | |
| Male gender | 65 (65.0) | 21 (50.0) | 30 (81.1) | 14 (66.7) | 0.015 | |
| Primary aetiology | ALD | 49 (48.0) | 19 (45.2) | 19 (51.4) | 11 (52.4) | 0.842 |
| HCV | 35 (33.0) | 14 (33.3) | 13 (35.1) | 8 (38.1) | 0.963 | |
| Other | 16 (16.0) | 9 (21.5) | 5 (13.5) | 2 (9.5) | 0.383 | |
| 1Child-Turcotte Pugh class | A | 24 (24.0) | 6 (14.3) | 12 (32.4) | 6 (28.6) | 0.684 |
| B | 59 (59.0) | 29 (69.0) | 21 (56.8) | 9 (42.8) | ||
| C | 17 (17.0) | 7 (16.7) | 4 (10.8) | 6 (28.6) | ||
| MELD score | 14.4 ± 5.2 | 14.6 ± 4.6 | 14.2 ± 5.1 | 14.2 ± 6.7 | 0.936 | |
| Ascites at review (incl. suppressed by medication) | 80 (80.0) | 37 (88.1) | 28 (75.7) | 15 (71.4) | 0.187 | |
| Encephalopathy at review (incl. suppressed by medication) | 36 (36.0) | 12 (28.6) | 13 (35.1) | 11 (52.4) | 0.184 | |
| Hepatocellular carcinoma | 8 (8.0) | 3 (7.1) | 3 (8.1) | 2 (9.5) | 1.00 | |
| Number of self-reported medicines | 7.1 ± 3.5 | 7.2 ± 3.7 | 7.1 ± 3.6 | 6.9 ± 3.1 | 0.923 | |
| Number of comorbidities | 5.5 ± 2.8 | 5.4 ± 2.8 | 5.8 ± 3.0 | 5.2 ± 2.5 | 0.703 | |
| 2Unable to afford medicines | 19 (20.2) | 1 (2.5) | 12 (36.4) | 6 (28.6) | < 0.001 | |
| 3Education | Nil, Primary, Middle school | 39 (42.4) | 14 (34.1) | 13 (43.3) | 12 (57.1) | 0.215 |
| High school, Trade, University | 53 (57.6) | 27 (65.9) | 17 (56.7) | 9 (42.9) | ||
| 4Employment status | Employed | 18 (19.1) | 9 (22.0) | 6 (18.2) | 3 (14.3) | 0.842 |
| Government welfare | 72 (76.6) | 30 (73.2) | 25 (75.8) | 18 (85.7) | 0.602 | |
| ARIA | Highly accessible | 89 (89.0) | 36 (85.7) | 34 (91.9) | 19 (90.5) | 0.713 |
| Accessible–remote | 11 (11.0) | 6 (14.3) | 3 (8.1) | 2 (9.5) | ||
| IRSD | Most disadvantaged | 32 (32.0) | 16 (38.1) | 7 (18.9) | 9 (42.9) | 0.093 |
| Low–moderate disadvantage | 68 (68.0) | 26 (68.9) | 30 (81.1) | 12 (57.1) | ||
Normally distributed data presented as mean ± SD and analysed using one-way ANOVA. Categorical data presented as proportional percent (%) of column (adherence ranking) and analysed using Pearson’s χ2 or Fisher’s Exact test unless otherwise noted.
Analysed using the Linear-by-Linear Exact Association test for trend;
Excluding 6 patients for whom data was not available (n = 40 High adherers, n = 33 Medium adherers and n = 21 Low adherers);
Excluding 8 patients for whom data was not available (n = 41 High adherers, n = 30 Medium adherers and n = 21 Low adherers);
Excluding 5 patients for whom data was not available (n = 41 High adherers, n = 33 Medium adherers and n = 21 Low adherers). P-values represent “Employed” vs “Unemployed” (data not shown), and “Government Welfare” vs “Not receiving Government Welfare” (data not shown) respectively. Two patients reported concurrent part-time employment and Government Welfare support and are represented twice. “Employed” includes full-time, part-time, casual and self-employment. “Government Welfare” includes disability support, aged pension, carer’s pension, total permanent disability, Newstart allowance. Six patients reported no active income (living off savings or financially supported by family). ALD: Alcoholic liver disease; HCV: Hepatitis C virus ARIA: Accessibility/Remoteness Index of Australia; IRSD: Index for relative socioeconomic disadvantage.
Medication beliefs
Compared to “Low” adherence, there was a non-significant increase in the strength of Necessity beliefs in “High” and “Medium” adherence groups, and a non-significant increase in the strength of Concerns, Harms and Overuse beliefs from “High” to “Low” adherence groups. Compared to patients with “Medium” and “High” medication adherence, patients with “Low” medication adherence reported a lower mean Necessity-Concerns Differential (P = 0.003; Table 2). Three patients had a negative Necessity-Concerns Differential and eight patients had a differential of zero, indicating that their Concerns about their liver disease medicines outweighed or were equal to the perceived Necessity of therapy respectively.
Table 2.
Responses to the beliefs about medications questionnaires in patients with high, medium and low medication adherence
| Medication beliefs domains | All patients (n = 100) |
Medication adherence ranking |
P value | ||
| High | Medium | Low | |||
| (n = 42) | (n = 37) | (n = 21) | |||
| Necessity | 19.3 ± 3.8 | 19.6 ± 3.1 | 19.9 ± 4.1 | 17.6 ± 4.0 | 0.064 |
| Concerns | 12.0 ± 3.6 | 11.2 ± 3.3 | 12.2 ± 3.3 | 13.3 ± 4.2 | 0.074 |
| Necessity-Concerns Differential | 7.3 ± 4.7 | 8.4 ± 4.8 | 7.6 ± 4.2 | 4.3 ± 4.2 | 0.003 |
| Harms | 8.3 ± 2.5 | 7.9 ± 2.8 | 8.3 ± 2.0 | 8.8 ± 2.7 | 0.405 |
| Overuse | 10.3 ± 3.1 | 9.5 ± 3.0 | 10.7 ± 3.0 | 11.3 ± 2.9 | 0.053 |
Data presented as mean ± SD and analysed using one-way ANOVA. Higher scores indicate stronger medication beliefs within a given domain. Harms and Overuse scores range from 5 to 20. Necessity and Concerns scores range from 5 to 25. The Necessity-Concerns Differential (score range 0 to 20) is calculated by subtracting individual patients’ Concern scores from their Necessity scores.
Patients were more likely to have a lower Necessity-Concerns Differential if they were male (6.6 ± 4.3 vs 8.5 ± 5.2, P = 0.046), reported inability to afford medications (4.8 ± 4.6 vs 7.8 ± 4.5, P = 0.011) or had fewer comorbidities (Pearson’s r = 0.263, P = 0.008). Medication beliefs measured using the BMQ scales were not related to age, disease severity, education, sociodemographic status or other clinical and demographic variables.
Illness perceptions
Overall, participants had generally high levels of concern about their liver disease, felt they did not have much personal control over it, and perceived that it would persist for a long duration of time (Table 3). Patients with “Low” medication adherence reported lower perception of how much treatment could help their liver disease (treatment control, P = 0.003) and a lower self-perceived understanding of their liver disease (coherence, P = 0.014).
Table 3.
Illness perceptions in patients with high, medium and low medication adherence
| Brief illness perception questionnaire items | All patients (n = 100) |
Medication adherence ranking |
P value | ||
| High | Medium | Low | |||
| (n = 42) | (n = 37) | (n = 21) | |||
| Consequences | 6.0 ± 3.1 | 6.1 ± 3.0 | 6.0 ± 3.1 | 5.7 ± 3.2 | 0.846 |
| How much does your liver disease affect your life? | |||||
| 0 = No affect; 10 = Severely affects my life | |||||
| Timeline | 10.0 (0-10) | 10 (3-10) | 10 (0-10) | 10 (3-10) | 0.962 |
| How long do you think your liver disease will continue? | |||||
| 0 = Very short time; 10 = Forever | |||||
| Personal Control | 4.8 ± 3.0 | 4.6 ± 3.2 | 4.8 ± 2.9 | 5.0 ± 2.8 | 0.893 |
| How much control do you feel you have over your liver disease? | |||||
| 0 = Absolutely no control; 10 = Extreme amount of control | |||||
| Treatment Control | 8.0 (0-10) | 8 (2-10) | 10 (4-10) | 7 (0-10) | 0.003 |
| How much do you think your treatment can help your liver disease? | |||||
| 0 = Not at all; 10 = Extremely helpful | |||||
| Identity | 5.5 ± 3.0 | 5.6 ± 3.0 | 5.5 ± 3.0 | 5.2 ± 2.9 | 0.905 |
| How much do you experience symptoms from your liver disease? | |||||
| 0 = No symptoms; 10 = Many severe symptoms | |||||
| Concern | 8.0 (0-10) | 8 (0-10) | 9 (0-10) | 8 (0-10) | 0.416 |
| How concerned are you about your liver disease? | |||||
| 0 = Not at all; 10 = Extremely concerned | |||||
| Coherence | 8.0 (0-10) | 8 (3-10) | 8 (0-10) | 7 (2-9) | 0.014 |
| How well do you feel you understand your liver disease? | |||||
| 0 = Don’t understand at all; 10 = Understand very clearly | |||||
| Emotional Representation | 4.9 ± 3.4 | 4.7 ± 3.2 | 5.1 ± 3.9 | 4.7 ± 3.1 | 0.874 |
| How much does your liver disease affect you emotionally? | |||||
| 0 = Not at all; 10 = Extremely affected emotionally | |||||
Normally distributed data presented as mean ± SD and analysed using one-way ANOVA. Non-normally distributed data presented as median group score and (range) and analysed using the Kruskal-Wallis H Test.
Patients with HCV reported experiencing more severe symptoms (identity score 6.5 ± 2.4 vs 4.9 ± 3.1, P = 0.009), greater impact of disease on daily life (consequences score 6.9 ± 2.4 vs 5.5 ± 3.3, P = 0.019) and perceived that their disease would persist for a shorter duration of time (timeline median score 8 [range 0-10] vs 10 [range 2-10], P = 0.010) compared to patients who did not have HCV. Female patients reported greater emotional impact of disease (emotional representation score 5.8 ± 3.3 vs 4.3 ± 3.4, P = 0.041) while patients with higher levels of education (completed high school, formal trade qualification, university degree etc.) perceived greater impact of disease on daily life (consequences score 6.7 ± 2.8 vs 5.3 ± 3.2, P = 0.024) compared to patients educated up to middle school. There was a negative correlation between the total Brief-IPQ and CLDQ scores (r = -0.707, P < 0.001), indicating that stronger threatening perceptions of illness are associated with lower quality of life.
Quality of life
The greatest overall health-related QoL impacts reported by patients were in the domains of Fatigue and Worry. Impact of QoL on medication adherence is presented in Table 4. “Low” medication adherence was associated with lower QoL in terms of shortness of breath that impacted on daily activity (P = 0.030), greater emotional disturbances (P = 0.050), particularly irritability (P = 0.017) and mood swings (P = 0.031), and greater frequency of abdominal and bodily pain (P = 0.023 and P = 0.037, respectively). Patients with moderate or large ascites at the time of review reported greater impact of abdominal bloating (CLDQ-abdominal bloating score 3.47 ± 2.04 vs 4.69 ± 1.88, P = 0.014) and those with a history of HE reported more frequent irritability (CLDQ-irritability score 4.31 ± 1.57 vs 5.04 ± 1.72, P = 0.029), but these did not translate into an effect on medication adherence.
Table 4.
Health-related quality of life in patients with high, medium and low medication adherence
| Quality of life domains | All patients (n = 100) |
Medication adherence ranking |
P value | ||
| High | Medium | Low | |||
| (n = 42) | (n = 37) | (n = 21) | |||
| Abdominal symptoms | 4.7 ± 1.5 | 4.6 ± 1.4 | 5.1 ± 1.4 | 4.2 ± 1.6 | 0.063 |
| Abdominal bloating | 4.5 ± 2.0 | 4.3 ± 2.0 | 4.9 ± 1.8 | 3.9 ± 2.1 | 0.109 |
| Abdominal pain | 4.9 ± 1.8 | 4.8 ± 1.7 | 5.5 ± 1.5 | 4.1 ± 2.2 | 0.023 |
| Abdominal discomfort | 4.4 ±1.8 | 4.7 ± 1.8 | 4.9 ± 1.7 | 4.6 ± 1.9 | 0.715 |
| Activity | 4.5 ± 1.5 | 4.6 ± 1.6 | 4.5 ± 1.3 | 4.1 ± 1.5 | 0.377 |
| Not been able to eat as much as you would like | 5.1 ± 1.9 | 5.0 ± 2.0 | 5.2 ± 1.6 | 5.0 ± 2.0 | 0.814 |
| Trouble lifting or carrying heavy objects | 3.4 ± 2.1 | 3.8 ± 2.3 | 3.3 ± 1.9 | 2.5 ± 1.9 | 0.067 |
| Bothered by a limitation of your diet | 5.0 ± 1.9 | 5.0 ± 1.9 | 5.0 ± 2.0 | 4.7 ± 2.1 | 0.824 |
| Emotion | 4.3 ± 1.5 | 4.7 ± 1.3 | 4.3 ± 1.7 | 3.7 ± 1.3 | 0.050 |
| Anxiety | 4.4 ± 1.9 | 4.8 ± 1.6 | 4.3 ± 2.1 | 3.8 ± 1.8 | 0.126 |
| Unhappiness | 4.5 ± 1.9 | 4.8 ± 1.9 | 4.6 ± 2.1 | 3.8 ± 1.3 | 0.125 |
| Irritability | 4.7 ± 1.7 | 5.0 ± 1.6 | 4.9 ± 1.8 | 3.8 ± 1.4 | 0.017 |
| Difficulty sleeping at night | 3.6 ± 2.2 | 4.0 ± 2.1 | 3.4 ± 2.3 | 3.1 ± 2.2 | 0.259 |
| Mood swings | 4.7 ± 1.9 | 5.1 ± 1.7 | 4.7 ± 2.1 | 3.8 ± 1.7 | 0.031 |
| Unable to fall asleep at night | 3.9 ± 2.3 | 4.1 ± 2.3 | 3.7 ± 2.3 | 3.7 ± 2.3 | 0.687 |
| Felt depressed | 4.6 ± 1.9 | 5.1 ± 1.8 | 4.5 ± 2.1 | 3.9 ± 1.6 | 0.058 |
| Problems concentrating | 4.3 ± 1.9 | 4.5 ± 2.0 | 4.5 ± 1.9 | 3.7 ± 1.9 | 0.232 |
| Fatigue | 3.2 ± 1.5 | 3.5 ± 1.6 | 3.3 ± 1.5 | 2.9 ± 1.3 | 0.340 |
| Tired or fatigued | 3.0 ± 1.6 | 3.0 ± 1.6 | 3.1 ± 1.7 | 2.7 ± 1.4 | 0.622 |
| Sleepy during the day | 3.2 ± 1.8 | 3.3 ± 1.9 | 3.5 ± 2.0 | 2.7 ± 1.2 | 0.255 |
| Bothered by having decreased strength | 3.6 ± 2.0 | 3.9 ± 2.0 | 3.4 ± 2.0 | 3.1 ± 2.0 | 0.281 |
| Decreased level of energy | 3.0 ± 1.8 | 3.2 ± 1.7 | 2.9 ± 1.8 | 2.6 ± 1.6 | 0.361 |
| Drowsiness | 3.9 ± 1.7 | 4.1 ± 1.8 | 3.8 ± 1.7 | 3.6 ± 1.6 | 0.466 |
| Systemic symptoms | 4.3 ± 1.3 | 4.3 ± 1.2 | 4.4 ± 1.4 | 4.0 ± 1.5 | 0.519 |
| Bodily pain | 4.2 ± 2.0 | 4.2 ± 1.8 | 4.7 ± 2.2 | 3.3 ± 2.0 | 0.037 |
| Shortness of breath | 4.5 ± 1.9 | 5.0 ± 1.8 | 4.4 ± 1.9 | 3.7 ± 1.8 | 0.030 |
| Muscle cramps | 4.2 ± 1.9 | 4.2 ± 2.0 | 4.1 ± 1.7 | 4.2 ± 2.2 | 0.957 |
| Dry mouth | 3.9 ± 2.0 | 4.0 ± 2.0 | 3.9 ± 2.0 | 3.7 ± 1.9 | 0.833 |
| Itching | 4.5 ± 2.1 | 4.2 ± 2.2 | 4.6 ± 2.0 | 5.0 ± 2.1 | 0.392 |
| Worry | 3.9 ± 1.8 | 4.1 ± 1.8 | 3.8 ± 2.0 | 3.6 ± 1.6 | 0.491 |
| Worry about impact of liver disease has on family/friends | 3.9 ± 2.1 | 4.0 ± 2.0 | 3.9 ± 2.2 | 3.7 ± 2.2 | 0.876 |
| Worried that symptoms will develop into major problems | 3.7 ± 2.1 | 4.0 ± 2.1 | 3.6 ± 2.2 | 3.1 ± 2.0 | 0.357 |
| Worry about condition getting worse | 3.9 ± 2.0 | 4.1 ± 2.0 | 3.7 ± 2.1 | 3.6 ± 2.0 | 0.569 |
| Worry about never feeling any better | 4.2 ± 2.2 | 4.5 ± 2.2 | 4.2 ± 2.3 | 3.8 ± 1.8 | 0.436 |
Normally distributed data presented as mean ± SD and analysed using one-way ANOVA. Item scores range from 1 to 7 where a lower score indicates lower quality of life (i.e., more frequent symptoms). Domain scores (range from 1 to 7) are calculated averages of items within the domain
Patients who reported feeling hassled about sticking to their treatment plan (n = 10) and those that stated they did not take their medicines the preceding day (n = 9) reported lower overall QoL (average CLDQ score 2.96 ± 1.08 vs 4.30 ± 1.17, P = 0.001 and 2.97 ± 0.78 vs 4.28 ± 1.20, P = 0.002 respectively), especially within the domains of Activity, Emotion and Fatigue. Feeling hassled about sticking to the treatment plan was particularly associated with increased irritability (CLDQ-irritability score 3.50 ± 2.01 vs 4.81 ± 1.60, P = 0.019) and mood swings (CLDQ-mood swings score 3.33 ± 1.32 vs 4.81 ± 1.66, P = 0.003 respectively).
Factors associated with low medication adherence
Bivariate analysis indicated that patients with Necessity-Concerns Differential ≤ 5, Brief Illness Perception Questionnaire “treatment control” score ≤ 8 and “coherence” score ≤ 8, or CLDQ score ≤ 3 in the items of bodily pain, abdominal pain, shortness of breath or irritability, had higher odds of reporting “Low” medication adherence (Table 5). However, when included in the regression model having a Necessity-Concerns Differential ≤ 5 (OR = 3.66, 95%CI: 1.18-11.40), Brief IPQ-coherence score ≤ 8 (OR = 8.15, 95%CI: 0.98-67.78) or a CLDQ-shortness of breath score ≤ 3 (OR = 3.87, 95%CI: 1.22-12.25) were the only independent predictors of “Low” medication adherence.
Table 5.
Crude and multivariable predictors of low medication adherence in patients with decompensated cirrhosis
|
Crude |
1Multivariable |
P value | ||||
| OR | 95%CI | OR | 95%CI | |||
| Age ≥ 60 yr | 1.20 | 0.46-3.16 | 1.79 | 0.56-5.70 | 0.325 | |
| Gender, male | 1.10 | 0.40-3.04 | 0.74 | 0.21-2.58 | 0.639 | |
| Unable to afford medicines | 1.85 | 0.60-5.66 | 0.88 | 0.25-3.44 | 0.857 | |
| N-C Differential | ≤ 5 | 4.79 | 1.74-13.25 | 3.66 | 1.18-11.40 | 0.025 |
| Overuse | ≥ 13 | 1.69 | 0.59-4.86 | 0.85 | 0.23-3.15 | 0.813 |
| Brief IPQ- treatment control | ≤ 8 | 3.63 | 1.21-10.88 | 3.23 | 0.92-11.39 | 0.068 |
| Brief IPQ- coherence | ≤ 8 | 13.62 | 1.74-106.62 | 8.15 | 0.98-67.78 | 0.052 |
| CLDQ-bodily pain (3) | QoL score ≤ 3 | 2.72 | 1.02-7.27 | 1.69 | 0.54-5.35 | 0.369 |
| CLDQ-abdominal pain (5) | QoL score ≤ 3 | 4.19 | 1.45-12.09 | 1.73 | 0.50-6.05 | 0.389 |
| CLDQ-shortness of breath (6) | QoL score ≤ 3 | 3.93 | 1.44-10.71 | 3.87 | 1.22-12.25 | 0.022 |
| CLDQ-irritability (15) | QoL score ≤ 3 | 3.12 | 1.08-9.04 | 1.70 | 0.47-6.11 | 0.416 |
| CLDQ-mood swings (19) | QoL score ≤ 3 | 2.09 | 0.75-5.82 | 0.93 | 0.24-3.58 | 0.917 |
Odds ratio adjusted for N-C Differential ≤ 5, Brief IPQ-treatment control score ≤ 8, Brief IPQ-coherence score ≤ 8 and CLDQ-shortness of breath score ≤ 3 (indicative of experiencing the symptom “all the time”, “most of the time” or “a good bit of the time”) in the multivariable logistic regression model.
DISCUSSION
In our study, self-reported medication non-adherence in ambulatory patients with decompensated cirrhosis was prevalent, with over one-fifth of patients categorised with “Low” adherence and more than one-third categorised with “Medium” adherence. We have identified that lower levels of medication adherence in this group are associated with stronger patient Concerns about their medication relative to their belief in its Necessity, lower self-perceived understanding of liver disease, and lower QoL.
The relationship between medication beliefs and medication adherence behaviour has been explored in numerous chronic diseases, including asthma, cardiovascular disease and mental health disorders[29]. Perceptions of illness have also been shown to influence medication adherence in asthma, diabetes, hypertension and heart failure[30], though the impact of the different illness perception items on adherence appears to differ between diseases. In the present study, people with decompensated cirrhosis were more likely to have “Low” medication adherence if they had a lower Necessity-Concerns Differential, poorer self-perceived understanding of their hepatic disease (coherence), and had lower perceptions of the benefits of treatment for their hepatic disease (treatment control). It can therefore be inferred that decompensated cirrhosis patients who have a weaker belief in the necessity or helpfulness of their medications, and those who do not understand the consequences of cirrhosis, are less likely to perceive a need to take their medications and may therefore exhibit non-adherent behaviour.
Interestingly, perceptions of symptom frequency/severity (identity), concerns and consequences of disease on daily life were not associated with adherence behaviour. This was further confirmed using the CLDQ which identified that adherence was not influenced by the domains of Activity, Fatigue, Worry, Systemic or Abdominal Symptoms. This may be explained by changes to patients’ priorities in the terminal stages of illness which may affect their decisions for self-care. For example, people with palliative diseases such as cancer and end-stage heart failure may exhibit non-adherent behaviour in an attempt to maintain control, reduce adverse events, or in response to social or financial circumstances[31-33]. Similar issues may affect people with decompensated cirrhosis, as this is a palliative condition for patients who are ineligible for transplantation. Most patients in the present study were aware of the incurable nature of their disease as evidenced by responses to the timeline item. The presence of hepatocellular carcinoma, considered to be an “imminently terminal” occurrence in people with decompensated cirrhosis who are ineligible for liver transplant, was not associated with adherence, which indicates that perceptions of palliation alone may not strongly influence adherence behaviour in this group.
Notably, people with HCV perceived that their liver disease would last for a shorter duration of time compared to people with other aetiologies of cirrhosis, possibly related to availability of new HCV direct-acting antiviral therapies. Unfortunately, this may be the result of false hope in this group as it is possible that many patients with HCV cirrhosis will have persisting complications despite a HCV “cure”. People with HCV also perceived greater impact of symptom frequency/severity (identity) and consequences of disease on their daily lives compared to other aetiologies, but this was not associated with adherence behaviour. Where improved control over debilitating symptoms may be an incentive for better adherence for some patients, others could perceive side effects of therapy to outweigh benefits. This may be evidenced by the increased likelihood for “Low” adherence in patients who had stronger Concerns (i.e., side effects) relative to Necessity (i.e., benefits) beliefs, and those with less understanding about cirrhosis (coherence) and the role of medicines in disease and symptom management (treatment control). Alternatively, studies in other chronic diseases with a heavy symptom burden like decompensated cirrhosis, such as asthma and heart failure, have suggested that many patients with good adherence report a more benign perception of illness identity, concerns and consequences[34,35]. This may be attributed to improved disease control or fewer episodes of exacerbation because of good adherence with treatment, and may partially explain the heterogeneity of responses to these Brief-IPQ items among people with decompensated cirrhosis.
A previous investigation by Polis et al[18] in cirrhosis patients has identified that medication adherence, defined as “never missing medications”, was associated with having less abdominal symptoms and increased emotional well-being[18]. Similarly, we have identified that decompensated patients with “Low” medication adherence reported more frequent emotional disturbances, greater abdominal and bodily pain, and activity-limiting shortness of breath. Rather than a cause, these symptoms are postulated to be an effect of non-adherence. Conversely, irritability and mood swings were associated with patients reporting they felt “hassled about sticking to their treatment plan” and failure to take medications the day preceding the survey. Irritability and mood swings are thus postulated to impact on adherence in this group. While emotional disturbances are often associated with hepatic encephalopathy, irritability and mood swings were not necessarily more common in people with a history of HE, and these symptoms were not found to relate to non-adherence in patients prescribed lactulose. While the effect of adverse drug events experienced by individual patients on medication adherence was not specifically explored, the BMQ items designed to capture negative beliefs potentially related to adverse effects were not strongly related to nonadherence. Interestingly, disease severity measured using the Child-Turcotte Pugh classification was not related to QoL at either the domain or item level. This is contrary to other studies in cirrhosis patients[36,37], however by the nature of this study we aimed to recruit patients with a diverse decompensation history, which may explain the heterogeneity of responses to the CLDQ.
Strengths and limitations
The single study site, the General Hepatology Clinic at the Princess Alexandra Hospital, is one of the largest hepatology centres in Australia. The Clinic delivers ambulatory care to a substantial proportion of south-east Queensland hepatology patients, in addition to regional, remote and interstate patients who travel to access specialist services. Despite the broad representation of people with chronic liver diseases at our site, an element of selection bias may exist in the present study due to recruitment constraints. If two or more eligible patients were scheduled for hepatology review simultaneously (parallel hepatology clinics), patients who were taking more medicines for decompensation events were selectively approached. Thus, the patients included may represent the more severe end of the disease spectrum. Furthermore, participants could only be recruited if they were scheduled to attend clinic on a day the principal researcher (KH) was present and as such a convenience sample of patients was recruited.
As far as we are aware, this is the first study to use the BMQ and Brief-IPQ to measure beliefs and perceptions about medications and illness in people with decompensated cirrhosis. While these tools have not been previously validated in this cohort, our findings are comparable with studies in other chronic diseases. Non-equal questioning methodology (due to patients’ preference, need for carer/family member involvement, researcher assistance and feasibility) may have introduced an element of bias, however this was difficult to avoid given the study group of interest often requires assistance completing complex tasks and reflects a “real-world” clinic setting. To minimise potential bias, the study coordinator read survey questions aloud verbatim and did not actively seek additional information beyond what patients volunteered.
In conclusion, a large proportion of ambulatory patients with decompensated cirrhosis are non-adherent with prescribed medications. The association between “Low” medication adherence and patients having strong concerns or doubting the necessity of their medications should be explored further given the potential clinical relevance. Interventions that promote positive reinforcement of the value and necessity of medications in addition to education about disease and medication management tailored to individual patient needs may improve adherence.
COMMENTS
Background
Non-adherence and medication-mismanagement in people with decompensated cirrhosis have been associated with substantial potentially-preventable harm. The current study describes the relationship between patients’ self-reported adherence and medication beliefs, illness perceptions and quality of life. The study was conducted in a cohort of patients receiving ambulatory care at a large tertiary government hospital.
Research frontiers
Existing data on medication-nonadherence behaviour in people with decompensated cirrhosis is largely qualitative. The present study intended to quantitatively measure these variables using validated tools, to correlate potentially-modifiable beliefs, perceptions and quality of life with medication adherence.
Innovations and breakthroughs
In this study, the authors have identified that patients with lower levels of medication adherence were more likely to have lower perception of treatment helpfulness, higher concerns relative to necessity beliefs surrounding their medicines, and poorer quality of life.
Applications
The authors findings have important implications for evolving clinical practice, as medication-management and disease education interventions can be developed to target these potentially-modifiable patient variables.
Terminology
Levels of adherence (High, Medium and Low) were assessed using a validated adherence tool, the Morisky Medication Adherence Scale-8 (MMAS-8). Patients’ beliefs and perceptions towards their medicines were elicited using previously validated questionnaires (Beliefs about Medicines Questionnaire and Brief Illness Perception Questionnaire), which have been correlated with adherence behaviour. Quality of Life was measured using the Chronic Liver Disease Questionnaire.
Peer-review
This is a very interesting article dealing with the adherence to therapy by decompensated cirrhotic patients.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was conducted in accordance with the National Statement on Ethical Conduct in Human Research 2007 (Updated May 2015) and was approved by the Human Ethics Committees of Metro South Hospital and Health Service HREC/15/QPAH/688 and the University of Queensland, UQ2016000032.
Informed consent statement: Written informed consent was obtained from participants or, where they lacked capacity, assent was obtained from a personal or nominated consultee.
Conflict-of-interest statement: None.
Data sharing statement: Raw data and materials have not been made publically available as participants in the present study did not consent to release of the same. Scientists wishing to access raw data for non-commercial purposes may contact the corresponding author directly.
Peer-review started: May 12, 2017
First decision: June 5, 2017
Article in press: September 5, 2017
P- Reviewer: Grattagliano I, Tarantino G, Li ZF S- Editor: Wei LJL- Editor: A E- Editor: Ma YJ
Contributor Information
Kelly L Hayward, Pharmacy Department, Princess Alexandra Hospital, The Centre for Liver Disease Research, Translational Research Institute, The University of Queensland, Woolloongabba, Queensland 4102, Australia.
Patricia C Valery, Cancer and Chronic Disease Research Group, QIMR Berghofer Medical Research Institute, Herston, Queensland 4006, Australia.
Jennifer H Martin, School of Medicine and Public Health, The University of Newcastle, Callaghan, New South Wales 2308, Australia.
Antara Karmakar, The Centre for Liver Disease Research, Translational Research Institute, The University of Queensland, Woolloongabba, Queensland 4102, Australia.
Preya J Patel, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, The Centre for Liver Disease Research, Translational Research Institute, The University of Queensland, Woolloongabba, Queensland 4102, Australia.
Leigh U Horsfall, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, The Centre for Liver Disease Research, Translational Research Institute, The University of Queensland, Woolloongabba, Queensland 4102, Australia.
Caroline J Tallis, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Woolloongabba, Queensland 4102, Australia.
Katherine A Stuart, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Woolloongabba, Queensland 4102, Australia.
Penny L Wright, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Woolloongabba, Queensland 4102, Australia.
David D Smith, Statistics Unit, QIMR Berghofer Medical Research Institute, Herston, Queensland 4006, Australia.
Katharine M Irvine, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, The Centre for Liver Disease Research, Translational Research Institute, The University of Queensland, Woolloongabba, Queensland 4102, Australia.
Elizabeth E Powell, Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, The Centre for Liver Disease Research, Translational Research Institute, The University of Queensland, Woolloongabba, Queensland 4102, Australia.
W Neil Cottrell, School of Pharmacy, The University of Queensland, Woolloongabba, Queensland 4102, Australia. n.cottrell@uq.edu.au.
References
- 1.De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2:323. doi: 10.1016/S1474-5151(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 2.Hayward KL, Valery PC, Cottrell WN, Irvine KM, Horsfall LU, Tallis CJ, Chachay VS, Ruffin BJ, Martin JH, Powell EE. Prevalence of medication discrepancies in patients with cirrhosis: a pilot study. BMC Gastroenterol. 2016;16:114. doi: 10.1186/s12876-016-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins-Jones S, Rubin RR, Burton CM, Evans M. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35:1279–1284. doi: 10.2337/dc11-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal K, Kumar P, Markert R, Agrawal S. Risk Factors for 30-Day Readmissions of Individuals with Decompensated Cirrhosis. South Med J. 2015;108:682–687. doi: 10.14423/SMJ.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 7.Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737–741. doi: 10.1007/s10620-006-9442-4. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Sanyal AJ, Bell D, Gilles H, Heuman DM. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31:1012–1017. doi: 10.1111/j.1365-2036.2010.04257.x. [DOI] [PubMed] [Google Scholar]
- 9.Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–1041.e1. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–895. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M, Bell DE, Sterling RK, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz M, Krueger K, Schuessel K, Friedland K, Laufs U, Mueller WE, Ude M. Medication adherence and persistence according to different antihypertensive drug classes: A retrospective cohort study of 255,500 patients. Int J Cardiol. 2016;220:668–676. doi: 10.1016/j.ijcard.2016.06.263. [DOI] [PubMed] [Google Scholar]
- 13.Viana M, Laszczynska O, Mendes S, Friões F, Lourenço P, Bettencourt P, Lunet N, Azevedo A. Medication adherence to specific drug classes in chronic heart failure. J Manag Care Spec Pharm. 2014;20:1018–1026. doi: 10.18553/jmcp.2014.20.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch-Moverman Y, Shrestha-Kuwahara R, Bethel J, Blumberg HM, Venkatappa TK, Horsburgh CR, Colson PW; Tuberculosis Epidemiologic Studies Consortium (TBESC) Latent tuberculous infection in the United States and Canada: who completes treatment and why? Int J Tuberc Lung Dis. 2015;19:31–38. doi: 10.5588/ijtld.14.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen KB, Pressler T, Mortensen LH, Jarden M, Skov M, Quittner AL, Katzenstein T, Boisen KA. Associations between adherence, depressive symptoms and health-related quality of life in young adults with cystic fibrosis. Springerplus. 2016;5:1216. doi: 10.1186/s40064-016-2862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polis S, Zang L, Mainali B, Pons R, Pavendranathan G, Zekry A, Fernandez R. Factors associated with medication adherence in patients living with cirrhosis. J Clin Nurs. 2016;25:204–212. doi: 10.1111/jocn.13083. [DOI] [PubMed] [Google Scholar]
- 19.Lau-Walker M, Presky J, Webzell I, Murrells T, Heaton N. Patients with alcohol-related liver disease--beliefs about their illness and factors that influence their self-management. J Adv Nurs. 2016;72:173–185. doi: 10.1111/jan.12826. [DOI] [PubMed] [Google Scholar]
- 20.Vaughn-Sandler V, Sherman C, Aronsohn A, Volk ML. Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci. 2014;59:681–686. doi: 10.1007/s10620-013-2942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2011 [Google Scholar]
- 22.Australian Institute of Health and Welfare. Rural, regional and remote health: A guide to remoteness classifications. Canberra: AIHW Cat. No. PHE 2004, 53 [Google Scholar]
- 23.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 24.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64:255–257; discussion 258-263. doi: 10.1016/j.jclinepi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. [Google Scholar]
- 27.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foot H, La Caze A, Gujral G, Cottrell N. The necessity-concerns framework predicts adherence to medication in multiple illness conditions: A meta-analysis. Patient Educ Couns. 2016;99:706–717. doi: 10.1016/j.pec.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Kucukarslan SN. A review of published studies of patients' illness perceptions and medication adherence: lessons learned and future directions. Res Social Adm Pharm. 2012;8:371–382. doi: 10.1016/j.sapharm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Sand AM, Harris J, Rosland JH. Living with advanced cancer and short life expectancy: patients' experiences with managing medication. J Palliat Care. 2009;25:85–91. [PubMed] [Google Scholar]
- 32.Riegel B, Carlson B. Facilitators and barriers to heart failure self-care. Patient Educ Couns. 2002;46:287–295. doi: 10.1016/s0738-3991(01)00165-3. [DOI] [PubMed] [Google Scholar]
- 33.Bestvina CM, Zullig LL, Rushing C, Chino F, Samsa GP, Altomare I, Tulsky J, Ubel P, Schrag D, Nicolla J, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10:162–167. doi: 10.1200/JOP.2014.001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horne R, Weinman J. Self-regulation and selfmanagement in asthma: exploring the role of illness perceptions and treatment beliefs in explaining nonadherence to preventer medication. Psychol Health. 2002;17:17–32. [Google Scholar]
- 35.Molloy GJ, Gao C, Johnston DW, Johnston M, Witham MD, Struthers AD, McMurdo ME. Adherence to angiotensin-converting-enzyme inhibitors and illness beliefs in older heart failure patients. Eur J Heart Fail. 2009;11:715–720. doi: 10.1093/eurjhf/hfp059. [DOI] [PubMed] [Google Scholar]
- 36.Les I, Doval E, Flavià M, Jacas C, Cárdenas G, Esteban R, Guardia J, Córdoba J. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol. 2010;22:221–227. doi: 10.1097/MEG.0b013e3283319975. [DOI] [PubMed] [Google Scholar]
- 37.Sumskiene J, Sumskas L, Petrauskas D, Kupcinskas L. Disease-specific health-related quality of life and its determinants in liver cirrhosis patients in Lithuania. World J Gastroenterol. 2006;12:7792–7797. doi: 10.3748/wjg.v12.i48.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]


