Abstract
AIM
To discover unknown factors associated with carcinoid syndrome (CS) with the goal of earlier diagnosis of CS.
METHODS
In this retrospective case-control study using United States administrative claims, patients (≥ 18 years) newly-diagnosed with gastrointestinal neuroendocrine tumors (GI NETs) without CS (controls) were exactly matched to patients with CS (cases) based on NET diagnosis date at a 3-to-1 ratio. Study index date was first CS diagnosis (controls: same distance from NET diagnosis as cases). The most observed conditions, excluding CS-associated symptoms/diagnoses, during the year before index date were assessed. Forward-stepwise logistic regression models were used to derive predictors, and were validation within another claims database.
RESULTS
In the development database, 1004 patients with GI NETs were identified; 251 (25%) had CS and 753 (75%) were controls. In the validation database, 724 patients with GI NETs were identified; 181 (25%) had CS and 543 (75%) were controls. A total of 33 common diagnoses (excluding conditions already known to be associated with CS) in the development database were entered in forward step-wise logistic regression models. In the final, validated logistic regression model, three factors prior to CS diagnosis were found consistently associated with higher risks for CS, including liver disorder [odds ratio (95%CI): 3.38 (2.07-5.51)], enlargement of lymph nodes [2.13 (1.10-4.11)], and abdominal mass [3.79 (1.87-7.69)].
CONCLUSION
GI NET patients with CS were 2-4 times as likely to have preexisting diagnoses (i.e., liver disorder, enlarged lymph nodes, abdominal mass) than non-CS patients.
Keywords: Carcinoid syndrome, Gastrointestinal neuroendocrine tumors, Predictive factors, Data mining
Core tip: By assessing patients with gastrointestinal neuroendocrine tumors from two independent United States claim databases, this study found that patients with carcinoid syndrome (CS) were 2-4 times as likely to have a preexisting diagnosis of a liver disorder, enlargement of lymph nodes, or abdominal mass than patients without CS.
INTRODUCTION
Neuroendocrine tumors (NETs), the most of common of which are carcinoid tumors[1], are characterized by a relatively indolent rate of growth and an ability to secrete a variety of peptide hormones. These tumors can be functional or non-functional and benign or malignant[1]. Even though NETs can develop anywhere in the body, the majority of them occur in the gastrointestinal (GI) tract (67.5%)[2]. Within the GI tract, sites of origin include the stomach, small intestine, appendix, and colorectum[2]. A 6-fold increase in the age-adjusted incidence of diagnosed NETs has been observed from 1.09 new cases per 100000 individuals in 1973 to 6.98 new cases per 100000 individuals in 2012[3,4].
One of the more common functional NET syndromes is carcinoid syndrome (CS), occurring in 8% to 35% of NET patients[5]. CS occurs when functional carcinoid tumors metastasize to the liver or outside the GI tract, and the vasoactive hormones secreted by metastases, such as serotonin, histamine, or tachykinins, are no longer metabolized and inactivated by the liver and reach the general circulation[6,7]. 5-hydroxyindoleacetic acid (5-HIAA), a product of serotonin metabolism of urinary excretion, is used as a first-line test for biochemical detection of suspected CS, in which 5-HIAA excretion is usually elevated[8]. CS includes an array of signs and symptoms. The classical manifestations of episodic flushing and diarrhea affect more than 80% of patients with CS[6]. Other signs include pellagra, wheezing, abdominal pain, telangiectasia and heart disease (usually right sided valvular regurgitation). The clinical presentation with diarrhea and/or abdominal pain dominating often leads to misdiagnosis of CS as irritable bowel disease or small bowel obstruction[9,10] especially in middle-aged perimenopausal females. Delays in the correct diagnosis are common; reports on median time from onset of symptoms to diagnosis range from 2 to 20 years[9].
Other than the typical signs associated with CS, there is limited knowledge on the existence of any other predictors that may be associated with the risk of developing CS. We conducted the current study in patients with GI NETs, the most common type of NETs, to describe factors associated with CS with the intention of assisting physicians with making an earlier diagnosis of CS.
MATERIALS AND METHODS
Data source
We conducted a matched case-control study using data from two large United States healthcare claims databases - IMS PharMetrics Plus and Truven Health Analytics MarketScan. We used IMS PharMetrics Plus as the development database to derive risk factors for CS and MarketScan databases to validate the factors for CS. We used data from 1/1/2009 to 12/31/2014 from both databases.
The PharMetrics Plus database comprises adjudicated medical and pharmacy claims for approximately 150 million patients enrolled in United States health insurance plans, with an annual capture of 40 million. This database is representative of the United States commercially insured population for individuals under age 65 years. The MarketScan Research Database combines 2 separate databases-the Commercial Claims and Encounters database, and the Medicare Supplemental and Coordination of Benefits database. The commercial database contains the inpatient, outpatient, and outpatient prescription drug experience of about 40 million employees and their dependents, who are covered under a variety of fee-for-service and managed care health plans. The Medicare database contains the healthcare experience of about 3 million retirees with Medicare supplemental insurance paid for by employers. Both databases contain detailed cost, use, and outcomes data for inpatient and outpatient healthcare services. The medical claims are linked to outpatient prescription drug claims and person-level enrollment data through the use of unique enrollee identifiers. Both PharMetrics and MarketScan are Health Insurance Portability and Accountability Act compliant[11,12]. The study was exempt from review by an institutional review board
Study population
The study population consisted of patients who were new diagnosed with GI NETs during the identification (ID) period (1/1/2010-12/31/2014). We identified patients with at least one inpatient or two outpatient claims with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for any NET (209.xx.) Among these, patients who had ICD-9-CM codes for GI NET (209.0, 209.1, 209.2, 209.4, 209.5, and 209.6) as the most frequent NET codes were classified as GI NET. To ensure that patients were newly diagnosed, we excluded patients with GI NETs during the 1-year prior to the first diagnosis date. We excluded patients with pancreatic NETs and Merkel cell carcinoma at any time during the study period.
We matched patients with GI NETs without CS (controls) to patients with CS (cases) based on the diagnosis date (month and year) of the first GI NET diagnosis at a 3-to-1 ratio. For cases, the index date was the first CS diagnosis date. For controls, the index date was assigned to have the same distance from the date of first NET diagnosis as the matched case patients. All patients were required to have at least 1-year continuous enrollment prior to the index date (study baseline).
The algorithm used to identify cases was developed based on a series of analyses performed before undertaking the main study. First, we examined overall prevalence of CS and the distribution of all NET locations (e.g., GI, lung, pancreas) in patients with a CS by identifying cases using a diagnosis (ICD-9-CM code 259.2) and found it did not match the expected distribution. Specifically, we found that 6% of patients with pancreatic neuroendocrine tumor (PNET) had at least two claims with a code for the CS, as did 23% of patients with GI NET. We suspected that the diagnosis code was being applied at an early point in the diagnostic process (e.g., as a “rule out”). We tested the application of more stringent criteria, which required two claims with ICD-9-CM code 259.2 and either a urine 24-h 5-HIAA [Current Procedural Terminology (CPT) code: 83497] or a serum serotonin (CPT code: 84260). In addition, the tests must have been ordered in the period 3 mo before or 3 mo after the CS diagnosis. This stricter algorithm resulted in only 1% of patients with PNET and 11% of GI NET being identified as having CS. As a result, we used the 2 CS diagnosis plus testing algorithm to identify cases with CS for this study.
Patient demographic characteristics included age in years on index date, sex, and geographic region (Midwest, Northeast, South, and West). Clinical characteristics included Charlson Comorbidity Index[13,14], and number of chronic condition indicators [15].
The outcome of interest in this study was the presence of CS.
Statistical analysis
We performed descriptive analyses to assess differences between case/control cohorts and patient demographic and clinical characteristics. χ2 tests were used for categorical variables and two sample t-tests were used for continuous variables.
Data mining is a process of selecting, exploring and modeling large amounts of data in order to discover unknown patterns or relationships that provide a clear and useful result[16]. We used data mining to explore unknown factors associated with CS. Specifically, using the development database (PharMetrics Plus), we assessed the top 50 most frequently observed conditions (based on CS patients) other than symptoms/diagnoses known to be associated with CS during the 1 year prior to the index date. We eliminated conditions or symptoms already known to be associated with CS, those related to screening or health maintenance, other GI cancers, and factors occurring in < 10% of both case and control groups. We began the multivariate analysis by forcing demographics and two general comorbidity measures (Charlson Comorbidity Index and number of chronic conditions) into the model. We used forward-stepwise logistic regression to estimate, for each condition in the final model, the extent to which a patient who went on to be diagnosed with CS would have greater odds of having the condition than a patient who went on to be diagnosed with NET, but not CS. In the forward selection, significant factors (P < 0.05) from the list of conditions referred to above were retained. Demographic or comorbidity measures were removed from the final model if they were highly insignificant (P ≥ 0.1). To validate these predictors, we re-ran the model with completely independent data from another administrative claims database (Truven MarketScan).
RESULTS
Descriptive statistics
Of the 23815 NET patients identified from the IMS PharMetrics Plus, the development database, we excluded 23310 patients who did not meet the inclusion criteria, such as enrollment, age, and definition of CS. Of the 505 patients who met the study selection criteria 251 were GI NET patients with CS (cases) (Figure 1). These 251 cases along with the 753 matched controls (GI patients without CS) were included in the final analytic sample in the development database. Case patients were slightly younger than control patients (mean ± SD: 52.8 ± 10.9 years vs 54.4 ± 13.3 years; P = 0.06). The majority of patients were in the 55-64 age range for both cohorts (37.5% in CS cohort; 34.1% in non-CS; P = 0.04). There were no significant differences in sex, region, and number of chronic conditions between the two cohorts (Table 1).
Figure 1.
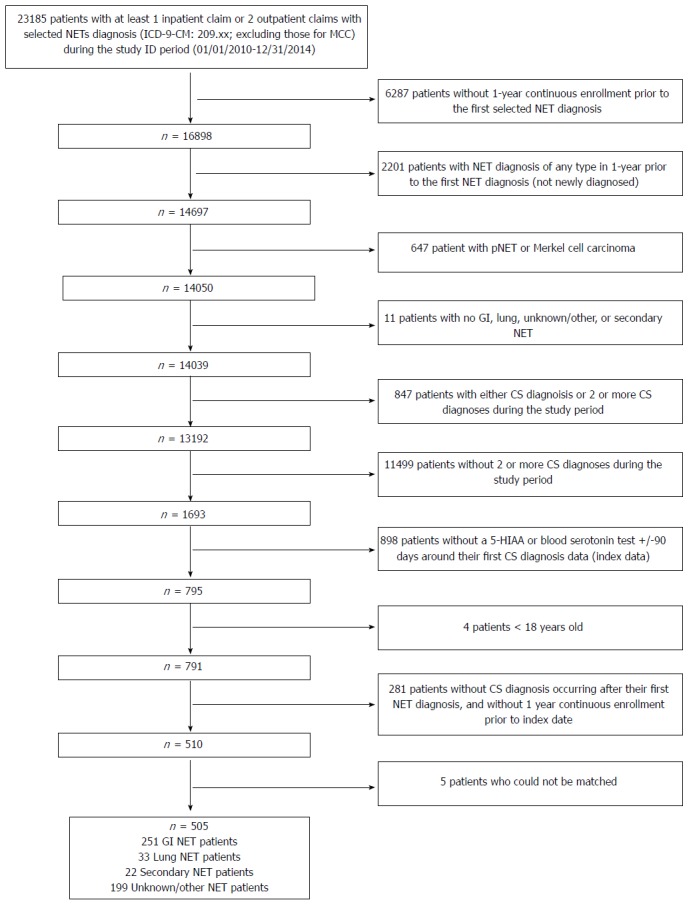
Patient identification. There were 23185 patients with at least one inpatient claim or two outpatient claims with selected NETs diagnosis (ICD-9-CM: 209.xx; excluding those for MCC) during the study ID period (01/01/2010-12/31/2014) in the development database. After excluding patients who were not newly diagnosed and did not have a NET diagnosis of interest; did not have 2 or more CS diagnosis during study period; did not have a 5-HIAA or blood serotonin test +/-90 days around their first CS diagnosis date (index date); were < 18 years old; did not have CS diagnosis occurring after their first NET diagnosis; could not be matched; or were not continuously enrolled in the 1 year pre-index period, there remained 505 NET patients who met the study inclusion criteria. Out of these, 251 were GI NET patients with CS. GI NETs: Gastrointestinal neuroendocrine tumors; CS: Carcinoid syndrome; 5-HIAA: 5-hydroxyindoleacetic acid.
Table 1.
Patient demographics n (%)
|
Development Database |
Validation Database |
|||||||
| CS Cohort | Non-CS Cohort | All | P value | CS Cohort | Non-CS Cohort | All | P value | |
| n | 251 | 753 | 1,004 | 181 | 543 | 724 | ||
| Age (yr) 18-44 | 52.77 ± 10.88 | 54.37 ± 13.28 | 53.97 ± 12.74 | 0.058 | 51.76 ± 9.33 | 51.67 ± 10.03 | 51.69 ± 9.85 | 0.913 |
| 52 (20.7) | 143 (19.0) | 195 (19.4) | 0.034 | 33 (18.2) | 100 (18.4) | 133 (18.4) | 0.637 | |
| 45-54 | 80 (31.9) | 219 (29.1) | 299 (29.8) | 64 (35.4) | 203 (37.4) | 267 (36.9) | ||
| 55-64 | 94 (37.5) | 257 (34.1) | 351 (35.0) | 83 (45.9) | 231 (42.5) | 314 (43.4) | ||
| 65+ | 25 (10.0) | 134 (17.8) | 159 (15.8) | 1 (0.6) | 9 (1.7) | 10 (1.4) | ||
| Female | 133 (53.0) | 407 (54.1) | 540 (53.8) | 0.770 | 109 (60.2) | 297 (54.7) | 406 (56.1) | 0.195 |
| Region | 0.306 | 0.667 | ||||||
| Midwest | 67 (26.7) | 219 (29.1) | 286 (28.5) | 37 (20.4) | 118 (21.7) | 155 (21.4) | ||
| Northeast | 57 (22.7) | 200 (26.6) | 257 (25.6) | 41 (22.7) | 100 (18.4) | 141 (19.5) | ||
| South | 106 (42.2) | 269 (35.7) | 375 (37.4) | 80 (44.2) | 254 (46.8) | 334 (46.1) | ||
| West | 21 (8.4) | 65 (8.6) | 86 (8.6) | 23 (12.7) | 71 (13.1) | 94 (13.0) | ||
In the validation database 724 patients with GI NETs were identified, among whom 181 (25%) had CS and 1158 (75%) were controls (Table 1). The mean (SD) age in the CS cohort was 51.8 (9.33) years, compared to 51.7 (10.03) years in the non-CS cohort. There were no significant differences in age, sex, and region (Table 1). With this approach, we identified a total of 33 most common conditions in both CS and non-CS cohorts (Table 2) of the development database for further assessment. In both cohorts, abdominal pain (66.1% of CS cohort; 51.5% of non-CS cohort), hypertension (50.6%; 52.2%), and dyslipidemia (49.4%; 46.1%) were the most prevalent diagnoses (Table 2).
Table 2.
Patients with gastrointestinal neuroendocrine tumors: Most frequent diagnoses1 in carcinoid syndrome patients, ordered by prevalence in carcinoid syndrome cohort n (%)
| Diagnosis description |
No. of patients |
Difference in Rate (CS-Non-CS) | Relative Risk (CS vs Non-CS) | |
| CS cohort n = 251 | Non-CS cohort n = 753 | |||
| Abdominal pain2 | 166 (66.14) | 388 (51.53) | 14.61% | 1.28 |
| Hypertension2 | 127 (50.60) | 393 (52.19) | -1.59% | 0.97 |
| Dyslipidemia2 | 124 (49.40) | 347 (46.08) | 3.32% | 1.07 |
| Benign neoplasm large bowel | 79 (31.47) | 241 (32.01) | -0.53% | 0.98 |
| Esophageal reflux | 74 (29.48) | 190 (25.23) | 4.25% | 1.17 |
| Diverticulosis of colon (without mention of hemorrhage) | 68 (27.09) | 159 (21.12) | 5.98% | 1.28 |
| Liver disorder2 | 56 (22.31) | 79 (10.49) | 11.82% | 2.13 |
| Chest pain, unspecified | 53 (21.12) | 147 (19.52) | 1.59% | 1.08 |
| Anemia, unspecified | 51 (20.32) | 141 (18.73) | 1.59% | 1.09 |
| Pain in limb | 41 (16.33) | 117 (15.54) | 0.80% | 1.05 |
| Internal hemorrhoids without mention of complication | 41 (16.33) | 87 (11.55) | 4.78% | 1.41 |
| Neoplasm of uncertain behavior of stomach, intestine, and rectum | 39 (15.54) | 95 (12.62) | 2.92% | 1.23 |
| Nonspecific (abnormal) findings on radiological and other examination of gastrointestinal tract | 38 (15.14) | 66 (8.76) | 6.37% | 1.73 |
| Lumbago | 38 (15.14) | 79 (10.49) | 4.65% | 1.44 |
| Enlargement of lymph nodes | 36 (14.34) | 40 (5.31) | 9.03% | 2.70 |
| Malignant neoplasm of colon, unspecified site | 35 (13.94) | 114 (15.14) | -1.20% | 0.92 |
| Hypothyroidism, unspecified | 34 (13.55) | 106 (14.08) | -0.53% | 0.96 |
| Abdominal or pelvic swelling, mass, or lump, unspecified site (begin 1994) | 34 (13.55) | 60 (7.97) | 5.58% | 1.70 |
| Other and unspecified noninfectious gastroenteritis and colitis | 32 (12.75) | 73 (9.69) | 3.05% | 1.32 |
| Type 2 diabetes mellitus2 | 32 (12.75) | 158 (20.98) | -8.23% | 0.61 |
| Other specified disorders of intestine | 29 (11.55) | 70 (9.30) | 2.26% | 1.24 |
| Obesity, unspecified | 28 (11.16) | 82 (10.89) | 0.27% | 1.02 |
| Diaphragmatic hernia | 28 (11.16) | 65 (8.63) | 2.52% | 1.29 |
| Headache | 28 (11.16) | 74 (9.83) | 1.33% | 1.14 |
| Anxiety state, unspecified | 27 (10.76) | 71 (9.43) | 1.33% | 1.14 |
| Acute appendicitis, unspecified | 26 (10.36) | 91 (12.08) | -1.73% | 0.86 |
| Reflux esophagitis | 26 (10.36) | 41 (5.44) | 4.91% | 1.90 |
| Abdominal or pelvic swelling, mass, or lump, other specified site | 26 (10.36) | 35 (4.65) | 5.71% | 2.23 |
| Dyspepsia and other specified disorders of function of stomach | 26 (10.36) | 26 (3.45) | 6.91% | 3.00 |
| Urinary tract infection, unspecified | 26 (10.36) | 96 (12.75) | -2.39% | 0.81 |
| Pre-operative cardiovascular examination | 25 (9.96) | 102 (13.55) | -3.59% | 0.74 |
| Tobacco use disorder | 24 (9.56) | 77 (10.23) | -0.66% | 0.94 |
| Constipation, unspecified | 24 (9.56) | 79 (10.49) | -0.93% | 0.91 |
1Claims in 1 year prior to the index date, excluding those for CS, NET, or symptoms/conditions known to be associated with CS;
Abdominal Pain (ICD-9-CM 789.0x, 789.6x); Type 2 DM (ICD-9-CM 250.x0, 250.x2); Hypertension (ICD-9-CM 401.xx, 402.xx, 403.xx, 404.xx, 405.xx, 437.2x); Dyslipidemia (ICD-9-CM 272.xx); Liver disorder (ICD-9-CM 573.8x, 573.9x). Variables not shown: Screening (Routine medical examination, Other screening mammogram, Long-term (current) use of other medications, Routine gynecological examination, Vaccination for influenza, Screen for malignant neoplasms of colon, Other specified pre-operative examination; GI cancer (Digestive neoplasm, unspecified); Prevalence < 10% in both CS and non-CS cohort (Screening for malignant neoplasms of prostate, Backache unspecified, Other abnormal blood chemistry, Vitamin D deficiency unspecified, Lumbar or lumbosacral intervertebral disc degeneration, Atrophic gastritis without mention of hemorrhage, Personal history of colonic polyps, Cervicalgia, Iron deficiency anemia unspecified). CS: Carcinoid syndrome; NET: Neuroendocrine tumors.
Multivariable analysis
Eight of the 33 common diagnoses tested remained significant in the forward step-wise logistic regression models and were further validated using the validation database (Table 3). In the final, validated logistic regression model, three factors were associated with higher risks for CS, including liver disorder [odds ratio (95%CI): 3.38 (2.07-5.51)], enlargement of lymph nodes [2.13 (1.10-4.11)], and abdominal mass [3.79 (1.87-7.69)] (Table 4).
Table 3.
Patients with gastrointestinal neuroendocrine tumors: Selected measures n (%)
|
Development database |
Validation database |
|||||||
| CS Cohort | Non-CS Cohort | All | P value | CS Cohort | Non-CS Cohort | All | P value | |
| n | 251 | 753 | 1004 | 181 | 543 | 724 | ||
| Abdominal pain | 166 (66.1) | 388 (51.5) | 554 (55.2) | < 0.001 | 104 (57.5) | 253 (46.6) | 357 (49.3) | 0.011 |
| Dyslipidemia | 124 (49.4) | 347 (46.1) | 471 (46.9) | 0.361 | 67 (37.0) | 209 (38.5) | 276 (38.1) | 0.724 |
| Diverticulosis of colon | 68 (27.1) | 159 (21.1) | 227 (22.6) | 0.050 | 37 (20.4) | 93 (17.1) | 130 (18.0) | 0.314 |
| Liver disorder | 56 (22.3) | 79 (10.5) | 135 (13.4) | < 0.001 | 50 (27.6) | 44 (8.1) | 94 (13.0) | < 0.001 |
| Enlarged lymph nodes | 36 (14.3) | 40 (5.3) | 76 (7.6) | < 0.001 | 23 (12.7) | 25 (4.6) | 48 (6.6) | < 0.001 |
| Type 2 diabetes | 32 (12.7) | 158 (21.0) | 190 (18.9) | 0.004 | 30 (16.6) | 91 (16.8) | 121 (16.7) | 0.954 |
| Abdominal mass | 26 (10.4) | 35 (4.6) | 61 (6.1) | 0.010 | 23 (12.7) | 16 (2.9) | 39 (5.4) | < 0.001 |
| Dyspepsia and other specified disorders of function of stomach | 26 (10.4) | 26 (3.5) | 52 (5.2) | < 0.001 | 8 (4.4) | 35 (6.4) | 43 (5.9) | 0.318 |
CS: Carcinoid syndrome
Table 4.
Patients with gastrointestinal neuroendocrine tumors: Results of final model
| Independent variable1 |
Development database |
Validation database |
||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age group | ||||
| 18-44 vs 65+ | 2.30 (1.26-4.23) | 0.007 | 4.88 (0.56-42.11) | 0.150 |
| 45-54 vs 65+ | 1.94 (1.14-3.31) | 0.015 | 3.63 (0.43-30.41) | 0.235 |
| 55-64 vs 65+ | 2.01 (1.20-3.38) | 0.008 | 3.85 (0.46-31.93) | 0.212 |
| Number of chronic conditions | 0.94 (0.87-1.01) | 0.093 | 1.13 (1.02-1.24) | 0.014 |
| Abdominal pain | 1.50 (1.08-2.09) | 0.016 | 1.22 (0.83-1.77) | 0.309 |
| Dyslipidemia | 1.52 (1.08-2.15) | 0.016 | 0.83 (0.55-1.25) | 0.373 |
| Diverticulosis of colon | 1.55 (1.08-2.24) | 0.019 | 1.16 (0.72-1.86) | 0.537 |
| Liver disorder2 | 2.12 (1.40-3.20) | < 0.001 | 3.38 (2.07-5.51) | < 0.001 |
| Enlarged lymph nodes | 2.33 (1.38-3.92) | 0.001 | 2.13 (1.10-4.11) | 0.025 |
| Type 2 diabetes | 0.59 (0.38-0.92) | 0.021 | 0.89 (0.54-1.48) | 0.653 |
| Abdominal mass | 1.89 (1.06-3.37) | 0.031 | 3.79 (1.87-7.69) | < 0.001 |
| Dyspepsia and other specified disorders of function of stomach | 2.95 (1.60-5.43) | < 0.001 | 0.54 (0.23-1.26) | 0.154 |
Variables found consistently significantly associated with risk of CS are bolded;
Liver disorder includes other specified disorders of liver (ICD-9-CM 573.8x, 573.9x). CS: Carcinoid syndrome.
DISCUSSION
By assessing patients from two independent United States claim databases, this study suggested that in patients with proven GI NETs, patients with CS were 2-4 times as likely to have a preexisting diagnosis of a liver disorder, enlargement of lymph nodes, or abdominal mass than patients without CS.
Among patients with NETs, preexisting enlarged lymph nodes, abdominal mass lesions, and a liver disorder seem to jointly indicate increasing tumor burden, which implies that tumor progression may be associated with a higher risk of developing CS. Although there is no obvious clinical explanation for these findings, we propose the following two considerations: first, patients with CS have a larger systemic tumor bulk at diagnosis than patients who do not manifest CS, implying that increasing tumor burden correlates with increasing tumor products, which in turn led to the syndrome; and second, that patients with CS have more aggressive tumors than those without CS, which leads to more rapid growth of tumor and a larger burden of disease when diagnosed. The first consideration appears less likely since patients with small strategically located tumors (e.g., the ovary or lung) commonly present with CS prior to large bulky liver disease developing. On the other hand, it is well known that patients with CS have a poorer quality of life and outcome than those without CS suggesting that the latter explanation is more likely. To the best of our knowledge, this is only the second study assessing distinctions between CS and non-CS patients. The only other study we could identify in the existing literature is a recent study by Halperin and colleagues[17] that used SEER-Medicare data to assess the clinical factors that may be associated with CS specifically. They found that their CS cohort included more patients with regional and distant metastases, while their non-CS cohort included more patients with local disease; for each distinct site of tumor origin, the percentage of CS increased with tumor progression. In the aforementioned study, CS was also associated with shorter survival compared without patients without CS, further supporting the second explanation for our findings[17]. Of note, our findings extend Halperin’s data in that we have now identified three specific predictors of the CS that can be used to direct physicians to specifically consider CS over a more general diagnosis of NET, and to monitor those patients more closely by 5-HIAA or serum serotonin testing In our study, we matched the CS and non-CS cohorts on duration of disease as carefully as possible, knowing that one of the limitations of using claims databases for research is that the precise diagnosis date is not the true date of tumorgenesis. Consequently the duration between the first NET diagnosis and first CS diagnosis was the best proxy for disease duration that we could use. Conditional on the reliability of this proxy, our findings suggest that patients who were eventually diagnosed with CS tended to have additional diagnoses indicating tumor advancement, compared with the non-CS patients who had the same duration of disease.
In general, early diagnosis of cancer can improve quality of life and survival[18]. Patients with CS have a significantly worse quality of life than patients with NETs but without CS[19]. Therefore, reducing delays in diagnosis may help improve quality of life in CS. In addition, our findings indicate that the poorer quality of life among patients with CS may also be a composite result of both tumor progression and CS symptoms, as the CS patients also tended to have more advanced tumor. Therefore, therapy for CS patients should address both symptom control as well as tumor progression to maintain quality of life.
In addition, we find it rather curious that there were no gender differences in our study. Since flushing is a common symptom of the CS, and since flushing is often confused with the hot flashes of menopause, one might have expected males with CS to be diagnosed earlier than females if the propensity is to misdiagnose CS until the disease is well established (with a known liver disorder, abdominal mass or lymphadenopathy). On the other hand, the delay in identifying CS in NET patients may simply be because flushing is not often sought out by physicians when interviewing patients. Indirectly then, these data suggest that educating physicians about the unusual manifestation of CS may help lead to an earlier diagnosis. The gender equivalence in our study may also be evidence that our algorithm for identifying CS, which required the presence of 5-HIAA or serotonin test around the diagnosis date, was reasonably specific for the condition, so that we minimized the misdiagnosed CS in our CS cohort by requiring this criterion for diagnosis.
Our study had limitations. First, GI NET and CS diagnoses were identified from healthcare claims coded for reimbursement, not research, and misclassification was possible. Errors in coding could bias our analysis. Specifically, patients with CS who have less severe symptoms may never be coded as having the syndrome. Nevertheless, health insurance claims data remain a valuable source of information as they constitute a fairly valid, large sample of patient characteristics and outcomes in a real-world setting. A strength of this study was that it was drawn from two very large underlying databases covering nearly 200 million patients enrolled in United States health insurance plans. The rate of CS among GI-NET patients in our study was higher than in some prior studies[20], and it was lower than at least one other[17]. The criteria we used to identify CS were more restrictive than the study by Halperin et al[17]. Current recommendations for diagnosing CS include measuring 5-HIAA[1,21]. We incorporated that recommendation into our identification algorithm, requiring two claims with an ICD-9-CM code for CS and a claim for either a urine 24-hour 5-HIAA or a serum serotonin in the period surrounding that diagnosis, whereas the prior study required two claims for CS, diarrhea, or flushing. Second, because ICD-9-CM codes may be inaccurate, we were not able to identify the specific anatomic location (e.g., portion of large or small bowel) of the GI NET in our study. This could lead to confounding. For instance, small bowl NETs are more commonly associated with CS than large bowel tumors, and previous studies have suggested that hepatic and lymph node metastasis are usually present at time of small bowel NET diagnosis[22]. If our CS cohort systematically includes more small bowel patients than the non-CS cohorts, and the small bowel patients tend to be more advanced at diagnosis, this could explain why we see that CS patients were more likely to have this diagnosis. However, due to the lack of specificity of ICD-9-CM codes, we cannot confirm the anatomic section of the GI tract in which the tumor was identified. Therefore, we could not validate if small bowel NETs may present at a more advanced stage than other NETs based only on the data from a claims database. A study in a data source where the exact location of the tumor could be identified with confidence would be very useful to confirm our findings. Third, our results are reflective of a commercially-insured population, but may not be generalizable to patient populations with other insurance types.
In summary, our study suggests that a liver disorder, enlargement of lymph nodes, or an abdominal mass is associated with higher risk of a future CS diagnosis. There are two aspects with regards how this finding may be applicable to a real world clinical setting. First, CS develops insidiously, and patients who grow accustomed to their syndromic features such as flushing may not think of reporting these symptoms, even when prompted. If physicians are not aware of the presence of CS symptoms in their patients, our findings offer additional signs to prompt them to inquire specifically. Second, patients with a liver disorder, enlarged nodes, or abdominal mass may also not yet have clinically detectable CS symptoms, but may be destined to develop them in the future. In this context, our data suggest that in a patient with NET, even in the absence of typical symptoms, if one of these three findings is noted, consideration should be given to ordering a 5-HIAA or serotonin level. Although validation studies using patients’ medical charts are warranted, such a practice could potentially aid physicians in speeding the diagnosis of CS.
ATRICLE HIGHLIGHTS
Research background
Functional neuroendocrine tumors (NETs) have the ability to secrete hormones that may cause carcinoid syndrome (CS), the most common symptoms of which include flushing and diarrhea. Carcinoid syndrome occurs in 8% to 35% of NET patients. Delays in diagnosis of CS are common, ranging from 2 to 20 years. Little is known about predictors that might be related the risk of developing CS.
Research motivation
Patients with CS often face delays in getting the correct diagnosis. Their symptoms, such as diarrhea, are often mistaken for other diseases, such as irritable bowel disease. Finding risk factors that are associated with CS might help physicians with making an earlier diagnosis of CS.
Research objectives
The objective was to identify risk factors that are associated with a future CS diagnosis.
Research methods
The authors conducted the study using data from two large United States health insurance claims databases. These databases contain healthcare cost, use, and outcomes data for covering nearly 200 million insured Americans. We first identified patients newly diagnosed with gastrointestinal NETs between 2010 and 2014 and then compared patients with CS to those without CS. We performed statistical analyses to identify the risk factors associated with CS from one databased and then validated the results using the other database.
Research results
The authors identified 251 patients with CS and 753 without CS in one database, and 386 patients with CS and 1158 patients without CS in the other database. There were no significant differences in age, sex, and region between patients with CS and those without CS. In both databases, we found that CS patients were 2-4 times more likely than non-CS patients to have a diagnosis of liver disorder, enlargement of lymph nodes, or abdominal mass within 1 year prior to their CS diagnosis.
Research conclusions
This study suggests that diagnosis codes of liver disorder, enlargement of lymph nodes, or an abdominal mass are associated with higher risk of a future CS diagnosis. In a patient with NETs, even in the absence of typical symptoms, if one of these three conditions is noted, consideration should be given to ordering a test for CS. Such a practice could potentially aid physicians in speeding the diagnosis of CS.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States.
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Novartis Pharmaceuticals, One Health Plaza, East Hanover, NJ 07936-1080, United States.
Institutional review board statement: We conducted a retrospective case-control study using the Truven Health Analytics MarketScan Database and the IMS Health PharMetrics Database, both commercial health insurance claims database for employer-insured beneficiaries in the United States. The databases are fully compliant with the Health Insurance Portability and Accountability Act and meet the criteria for a limited-use dataset. Since the patient and provider data included in this analysis were fully de-identified, this study was exempt from the Institutional Review Board review.
Informed consent statement: This study involved analyses of Health Insurance Portability and Accountability Act-compliant secondary databases, MarketScan and PharMetrics, thus no informed consent was feasible or necessary.
Conflict-of-interest statement: Cai is an employee of Novartis Pharmaceuticals Corporation. Broder, Chang, and Yan are employees of the Partnership for Health Analytic Research, LLC, which received funding from Novartis to conduct the researchdescribed in this manuscript. Metz is an employee of Northwestern University and was paid by Novartis to consult as a subject matter expert. Metz is Chair of the North American Neuroendocrine Tumor Society (NANETS) and also a consultant for Ipsen. Metz has received commercial research grants from Lexicon and Advanced Accelerator Applications (AAA), and is a consultant/on the advisory board for AAA.
Data sharing statement: The study statistician, Eunice Chang, conducted all statistical analysis for this study using Health Insurance Portability and Accountability Act-compliant commercial-insurance secondary databases MarketScan and PharMetrics.
Peer-review started: June 30, 2017
First decision: July 27, 2017
Article in press: September 13, 2017
P- Reviewer: Caboclo JLF, Tarnawski AS, Treeprasertsuk S S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ
Contributor Information
Beilei Cai, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936, United States. beilei.cai@novartis.com.
Michael S Broder, Partnership for Health Analytic Research, LLC, 280 S. Beverly Dr., Beverly Hills, CA 90212, United States.
Eunice Chang, Partnership for Health Analytic Research, LLC, 280 S. Beverly Dr., Beverly Hills, CA 90212, United States.
Tingjian Yan, Partnership for Health Analytic Research, LLC, 280 S. Beverly Dr., Beverly Hills, CA 90212, United States.
David C Metz, Division of Gastroenterology, University of Pennsylvania Health System, 3400 Civic Center Boulevard, Perelman Center for Advanced Medicine, Philadelphia, PA 19104, United States.
References
- 1.National Comprehensive Cancer Network. 2016. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Neuroendocrine Tumors Version 2. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151–160. doi: 10.1002/jso.20179. [DOI] [PubMed] [Google Scholar]
- 6.McCormick D. Carcinoid tumors and syndrome. Gastroenterol Nurs. 2002;25:105–111. doi: 10.1097/00001610-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rupp AB, Ahmadjee A, Morshedzadeh JH, Ranjan R. Carcinoid Syndrome-Induced Ventricular Tachycardia. Case Rep Cardiol. 2016;2016:9142598. doi: 10.1155/2016/9142598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman JM. Urinary serotonin in the diagnosis of carcinoid tumors. Clin Chem. 1986;32:840–844. [PubMed] [Google Scholar]
- 9.Toth-Fejel S, Pommier RF. Relationships among delay of diagnosis, extent of disease, and survival in patients with abdominal carcinoid tumors. Am J Surg. 2004;187:575–579. doi: 10.1016/j.amjsurg.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, Nutting C, Bushnell DL, Caplin ME, Yao JC; North American Neuroendocrine Tumor Society (NANETS) The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39:753–766. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]
- 11.Ellis LA, Lafeuille MH, Gozalo L, Pilon D, Lefebvre P, McKenzie S. Treatment Sequences and Pharmacy Costs of 2 New Therapies for Metastatic Castration-Resistant Prostate Cancer. Am Health Drug Benefits. 2015;8:185–195. [PMC free article] [PubMed] [Google Scholar]
- 12.Cepeda MS, Fife D, Denarié M, Bradford D, Roy S, Yuan Y. Quantification of missing prescriptions in commercial claims databases: results of a cohort study. Pharmacoepidemiol Drug Saf. 2017;26:386–392. doi: 10.1002/pds.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. HCUP Chronic Condition Indicator [Internet]. Healthc. Cost Util. Proj. HCUP2015; Available from: www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp.
- 16.Bellazzi R, Zupan B. Predictive data mining in clinical medicine: current issues and guidelines. Int J Med Inform. 2008;77:81–97. doi: 10.1016/j.ijmedinf.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, Shih YT, Yao JC. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18:525–534. doi: 10.1016/S1470-2045(17)30110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Guide to cancer early diagnosis. [Google Scholar]
- 19.Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41:461–466. doi: 10.1097/MPA.0b013e3182328045. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Asa SL, Dey C, Kennecke H, Laidley D, Law C, Asmis T, Chan D, Ezzat S, Goodwin R, et al. Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence-based Canadian consensus. Cancer Treat Rev. 2016;47:32–45. doi: 10.1016/j.ctrv.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Watzka FM, Fottner C, Miederer M, Weber MM, Schad A, Lang H, Musholt TJ. Surgical Treatment of NEN of Small Bowel: A Retrospective Analysis. World J Surg. 2016;40:749–758. doi: 10.1007/s00268-016-3432-2. [DOI] [PubMed] [Google Scholar]


