Abstract
Context:
There is great interest in the biology of vascular calcification. Wnt/β-catenin signaling is an important mediator of mineralization and may play a role in vascular calcification.
Objective:
We assessed the association between circulating Wnt antagonists and abdominal aortic calcification (AAC) severity in elderly women.
Design:
This was a cross-sectional analysis of the Calcium Intake Fracture Outcome Study.
Setting:
The participants were recruited from the community-dwelling elderly population.
Participants:
We examined 768 women aged over 70 years.
Interventions:
We collected blood samples, and lateral spine images captured during bone density assessment were used to score AAC with a validated 24-point scale.
Main Outcome Measures:
We tested the hypothesis that low Wnt antagonist levels of Dickkopf-1 (DKK1), secreted frizzled related protein 3 (sFRP3), and Wnt inhibitory factor 1 (WIF1) are associated with severe AAC (AAC24 score > 5).
Results:
Severe AAC was present in 146 women (19%). Lower levels of DKK1, but not WIF1 and sFRP3, were associated with higher odds of severe AAC. Per standard deviation decrease in DKK1 was associated with increased multivariable-adjusted odds ratio (OR) of severe AAC [OR, 1.26; 95% confidence interval (CI), 1.04 to 1.52; P = 0.017]. In quartile analyses, the lowest and second-lowest quartiles of DKK1 had increased multivariable-adjusted odds of severe AAC vs the highest quartile (OR, 2.05; 95% CI, 1.18 to 3.56; P = 0.011 and OR, 1.83; 95% CI, 1.05 to 3.19; P = 0.035).
Conclusions:
In elderly women, DKK1, but not sFRP3 or WIF1, is associated with severe AAC. This study supports the concept that Wnt/β-catenin signaling is an important regulator of vascular mineral metabolism and is independent of other risk factors.
Keywords: Dickkopf-1, severe abdominal aortic calcification, secreted frizzled related protein 3, Wnt inhibitory factor 1, elderly women
Précis: Wnt signaling may regulate abdominal aortic calcification. Low levels of the circulating Wnt antagonist DKK1, but not WIF1 and sFRP3, were associated with higher odds of severe AAC.
Unlike coronary artery calcification, which is thought to be predominantly subintimal due to atherosclerosis (1), abdominal aortic calcification (AAC) can be medial or intimal calcification or a mix of both. Medial calcification is more commonly seen in people with diabetes and chronic kidney disease mineral-bone disorder, whereas focal intimal calcification is thought to be due to the healing process after plaque rupture [1, 2]. We and others have shown that severe AAC is associated with the presence and severity of carotid atherosclerosis [3], coronary artery calcification [4] and future cardiovascular events [5]. Emerging evidence suggests that AAC is an actively regulated process with important clinical implications [6]. Although several conditions (e.g., diabetes, chronic kidney disease, and dyslipidaemia) predispose individuals to AAC, the mechanisms remain poorly understood [7].
The Wnt/β-catenin signaling pathway consisting of the canonical β-catenin–dependent and noncanonical β-catenin–independent pathway plays a critical role in the regulation of osteoblast differentiation, proliferation, and survival to maintain bone in a homeostatic state [8] and is also an important modulator of angiogenesis [9]. The Wnt pathways are regulated by multiple families of secreted antagonists or modulators, including secreted frizzled related proteins (sFRP) and dickkopfs (DKK) as well as Wnt inhibitory factor-1 (WIF1). In bone, sFRP3 and WIF1 have been shown to influence both canonical and noncanonical activation by preventing Wnt from binding to its receptors [10–13] whereas DKK1 antagonizes the canonical Wnt pathway by inhibiting Wnt co-receptors, including low-density lipoprotein receptor–related proteins 5 and 6, which are intrinsically involved in regulation of bone remodeling and angiogenesis [9, 14, 15]. However, the role of the Wnt antagonists on vascular calcification remains less clear.
A large observational study by Szulc et al. [16] reported that lower circulating DKK1 concentrations was associated with an increased odds of having severe AAC in elderly men. To date, this relationship has not been examined in large studies of elderly women, and the relationship between other Wnt antagonists and severe AAC has not been examined. Therefore, the aim of this study was to assess the cross-sectional association between 3 circulating Wnt antagonists putatively targeting both the canonical and noncanonical pathways with severe AAC in a large cohort of elderly women.
1. Subjects and Methods
A. Ethics Statement
At baseline, written informed consent was obtained from all participants for the study and follow-up of electronic health records. The Human Ethics Committee of the University of Western Australia approved the study protocol and consent form (approval number 05/06/004/H50). The Human Research Ethics Committee of the Western Australian Department of Health also approved the data linkage study (approval number #2009/24).
B. Study Population
The participants for this study were a subset of the postmenopausal women recruited from the Calcium Intake Fracture Outcome Study (CAIFOS). The CAIFOS recruited 1460 participants in 1998 for a 5-year prospective, randomized, controlled trial of oral calcium supplements to prevent osteoporotic fractures [17]. An additional 39 participants received oral calcium supplements plus 1000 IU vitamin D2 in a substudy nested within the CAIFOS cohort [18]. Because this was completed prior to the advent of the clinical trials registry, the trial was retrospectively registered in the Australian New Zealand Clinical Trials Registry ACTRN12615000750583. All participants were similar in terms of disease burden and pharmaceutical consumption to the general populations of this age, but they were more likely to be from higher socio-economic groups. Participants had no medical conditions that were likely to influence 5-year survival, and exclusion criteria at baseline (1998) included current use of bone active agents as hormone replacement therapy. In the 5 years of the randomized controlled trial, participants were given 1.2 g of elements of calcium as calcium carbonate on daily basis or a similar placebo. Participants for this study were excluded due to missing data for DKK1, WIF1, and sFRP3 due to serum unavailability (n = 391) or missing or unreadable lateral spine images (n = 341). This resulted in 768 (51%) women from the overall cohort included for this study.
C. Baseline Risk Factors and Disease History
Participants’ medical histories and medications were verified by their general practitioners when possible. Weight was obtained using digital scales with participants wearing light clothes and without shoes. Height was measured using a stadiometer. Body mass index was calculated in kg/m2. Prevalent atherosclerotic vascular disease was determined from the primary discharge diagnosis codes from 1980 to 1998 as described previously and included coronary heart disease, heart failure, cerebrovascular disease, and peripheral arterial disease [19].
D. Biochemistry
Fasting blood samples were collected at baseline in 1998. Serum Wnt-antagonists DKK1, sFRP3, and WIF1 levels were determined using enzyme immunoassay provided by R&D Systems (Minneapolis, MN). Intra- and interassay coefficients of variation were <10% for all assays. Blood samples were analyzed for phosphate using routine methods (BM/Hitachi 747 Analyzer; Boehringer Mannheim GmbH, Mannheim, Germany). Baseline creatinine was measured using an isotope dilution mass spectrometry–traceable Jaffe kinetic assay on a Hitachi 917 analyzer (Roche Diagnostics GmbH, Mannheim Germany). Serum cystatin C was measured on the Siemens Dade Behring Nephelometer (Erlangen, Germany), traceable to the International Federation of Clinical Chemistry Working Group for Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements certified reference materials. The estimated glomerular filtration rate (eGFR) using creatinine and cystatin C was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation derived by Inker et al. [20]. Plasma 25OHD2 and 25OHD3 concentrations were determined using a validated liquid chromatography tandem mass spectrometry method at the RDDT Laboratories (Bundoora, VIC, Australia). Between-run coefficients of variation were 10.1% at a 25(OH)D2 mean concentration of 12 nmol/L and 11.3% at a 25(OH)D3 mean concentration of 60 nmol/L.
E. Lateral Spine Imaging
Digitally enhanced lateral spine images were captured for vertebral fracture assessment from a Hologic 4500A DXA machine (Hologic, Boston, MA) in 1998 (18%) or in 1999 (82%) as described previously [3]. Abdominal aortic calcification imaging was obtained using digitally enhanced lateral spine and aorta image. A single experienced investigator blinded to the outcomes of this study (J.T.S.) assessed all images using the validated 24-point Framingham scale based on the Kauppila scoring system [21–24].
F. Statistical Analysis
The objective of the study was to determine the relationship between 3 circulating Wnt antagonists with prevalent severe AAC (AAC24 score >5) as reported by Szulc et al. [16] in elderly men. The primary outcome of the study was prevalent severe AAC. Data were expressed as mean and standard deviation (SD), median. and interquartile range (IQR) for continuous variables or as number and percentage for categorical variables. Levels of all Wnt antagonists were not normally distributed (Supplemental Fig. 1 (118.8KB, docx) ) and were transformed using the natural logarithm for logistic regression analyses in models meeting goodness-of-fit assumptions. Nonlogarithmically transformed data are presented for a more clear interpretation of results. Spearman’s rho correlation between circulating Wnt antagonists and continuous baseline confounders (age, body mass index, eGFR, and other Wnt antagonists) was undertaken, and Mann-Whitney U tests were used to compare between women with or without atherosclerotic vascular disease (ASVD), diabetes, calcium supplementation, smoking history, statin use, antihypertensive use, or low-dose aspirin use. We used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between prevalent severe AAC and DKK1, WIF1, and sFRP3 levels as either continuous or quartiles analyses. The highest quartile of circulating Wnt antagonist was the referent group for all analyses. Multivariable-adjusted analysis included age, body mass index, history of smoking, prevalent ASVD, treatment code (calcium or placebo), diabetes, estimated glomerular filtration rate, prescription for antihypertensive, statins, and low-dose aspirin. All statistical analysis were performed using IBM SPSS Statistics Version 22 (IBM Corp., Armonk, NY) or STATA (version 13; StataCorp LP, College Station, TX). A 2-tailed P value <0.05 was considered to be significant.
2. Results
Participants with either no measured AAC or no serum to perform assays for the Wnt antagonists (n = 732) (Fig. 1) were on average 0.4 years older and had slightly higher eGFR (both P < 0.05) than those included in the study (n = 768) but were similar for other cardiovascular risk factors (Table 1). The mean age of the study population was 75.0 ± 2.7 years, and mean body mass index was 27.1 ± 4.4 kg/m2. Of the 768 women included in the study, there were 146 (19%) with severe AAC (AAC24 score > 5).
Figure 1.
Overview of the 768 women included in the study. *Thirty-nine participants received calcium supplements with vitamin D. LSI, lateral spine imaging using a bone densitometer.
Table 1.
Characteristics of Subjects Included in the Study and Those With Missing Data Not Included in the Study
| Variable | Included in the Study | Missing Data |
|---|---|---|
| Number | 768 | 732 |
| Age, ya | 75.00 ± 2.65 | 75.39 ± 2.76a |
| Body mass index, kg/m2 | 27.06 ± 4.43 | 27.40 ± 5.06 |
| History of smoking, yes (%) | 273 (35.7) | 281 (38.7) |
| Diabetes, yes (%) | 43 (5.6) | 52 (7.1) |
| Prevalent ASVD, yes (%) | 91 (11.8) | 91 (12.4) |
| eGFR, mL/min/1.73 m2 | 66 ± 13 | 65 ± 13a |
| 25OHD, nmol/L | 67 ± 28 | 66 ± 30 |
| Phosphate, mg/L | 3.63 ± 0.42 | 3.64 ± 0.43 |
| Medications | ||
| Low-dose aspirin, yes (%) | 159 (20.7) | 154 (21.0) |
| Antihypertensive, yes (%) | 321 (41.9) | 331 (45.2) |
| Statin therapy, yes (%) | 141 (18.4) | 141 (19.3) |
| Treatment code | ||
| Calcium, yes (%) | 393 (51.2) | 376 (51.4) |
Data expressed as mean ± SD or as number and percentage.
P < 0.05 by Student t test and χ2 test where appropriate.
A. Wnt Antagonists
The median values of the untransformed circulating DKK1, sFRP3, and WIF1 were 0.45 (IQR, 0.27 to 0.71), 2.16 (IQR, 1.65 to 3.01), and 0.17 (IQR, 0.10 to 0.34) ng/mL, respectively. Using Spearman’s rank correlation with all continuous variables in Table 1, serum DKK1 concentrations were weakly positively correlated with age (rs = 0.092; P = 0.011), phosphate (rs = 0.109; P = 0.003), and sFRP3 (rs = 0.140; P < 0.001) but not WIF1 (rs = −0.047; P = 0.193). sFRP3 was positively correlated with WIF1 concentrations (rs = 0.655; P < 0.001). Women who had been prescribed statins had significantly lower concentrations of WIF1 (P = 0.001) and sFRP3 (P = 0.005), and women with diabetes or ASVD had significantly lower concentrations of WIF1 (P = 0.001 and P = 0.026, respectively).
B. Association Between Wnt Antagonists and Severe AAC
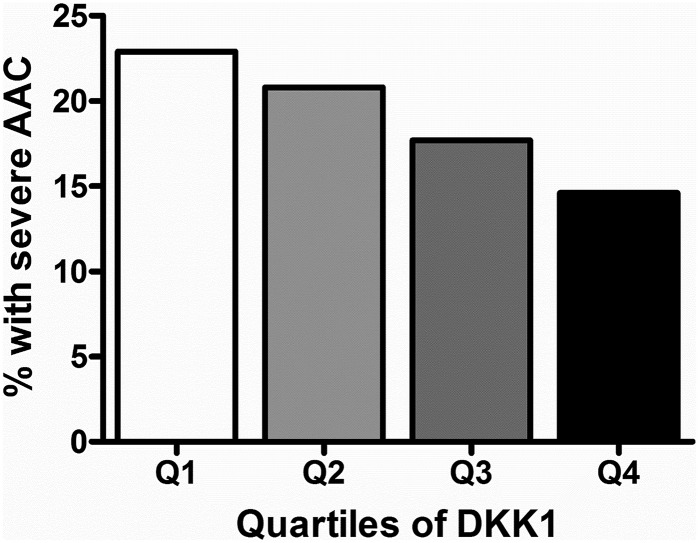
In continuous analyses, the per SD decrease of ln-DKK1 was associated with an increased odds of severe AAC in unadjusted (OR, 1.20; 95% CI, 1.00 to 1.44; P = 0.042), age-adjusted (OR, 1.23; 95% CI, 1.03 to 1.48; P = 0.024), and multivariable-adjusted logistic regression (OR, 1.26; 95% CI, 1.04 to 1.52; P = 0.017). When women were categorized by quartiles of DKK1 (Table 2), women in the lowest quartile had the highest prevalence of severe AAC compared with low and moderate AAC (Fig. 2), with twice the odds of having severe AAC compared with the highest quartile in age-adjusted and multivariable-adjusted models (Table 3). WIF1 was not associated with severe AAC in unadjusted, age-adjusted, or multivariable-adjusted logistic regression as either continuous (per SD decrease: OR, 1.05; 95% CI, 0.87 to 1.26; P = 0.598; OR, 1.04; 95% CI, 0.87 to 1.25; P = 0.640; and OR, 1.01; 95% CI, 0.84 to 1.23; P = 0.903, respectively) or quartile analyses (Table 3). Similarly, sFRP3 was not associated with severe AAC in unadjusted, age-adjusted, or multivariable-adjusted logistic regression as either continuous (per SD decrease: OR, 1.13; 95% CI, 0.94 to 1.36; P = 0.199; OR, 1.13; 95% CI, 0.94 to 1.36; P = 0.190; and OR, 1.11; 95% CI, 0.92 to 1.35; P = 0.275, respectively) or quartile analyses (Table 2).
Table 2.
Characteristics of Study Population Means Stratified by Quartiles of DKK1
| Variable | Quartile 1 (<0.27) | Quartile 2 (0.27 to 0.45) | Quartile 3 (0.45 to 0.71) | Quartile 4 (≥0.71) | P Valuea |
|---|---|---|---|---|---|
| Number | 192 | 192 | 192 | 192 | |
| Age, y | 74.8 ± 2.6 | 75.0 ± 2.5 | 74.8 ± 2.6 | 75.4 ± 2.8 | 0.076 |
| Randomized to calcium, yes (%) | 96 (50.0) | 101 (52.6) | 101 (52.6) | 95 (49.5) | 0.923 |
| Body mass index, kg/m2 | 27.3 ± 4.5 | 27.4 ± 4.2 | 26.5 ± 4.4 | 27. 1 ± 4.6 | 0.207 |
| sFRP-3, ng/mL | 1.96 (1.36 to 3.00) | 2.17 (1.70 to 2.86) | 2.19 (1.79 to 3.16) | 2.25 (1.76 to 3.13) | <0.001 |
| WIF1, ng/mL | 0.19 (0.11 to 0.41) | 0.16 (0.09 to 0.30) | 0.16 (0.10 to 0.31) | 0.16 (0.09 to 0.34) | 0.353 |
| eGFR, mL/min/1.73 m2b | 66 ± 13 | 67 ± 14 | 67 ± 12 | 65 ± 13 | 0.540 |
| 25OHD, nmol/L | 66 ± 27 | 68 ± 28 | 70 ± 30 | 65 ± 27 | 0.314 |
| Phosphate, mg/L | 3.57 ± 0.41 | 3.64 ± 0.43 | 3.63 ± 0.42 | 3.70 ± 0.39 | 0.027 |
| History of smoking, yes (%) | 70 (36.6) | 69 (35.9) | 61 (32.1) | 73 (38.0) | 0.983 |
| Diabetes, yes (%) | 10 (5.2) | 11 (5.7) | 9 (4.7) | 13 (6.8) | 0.623 |
| Prevalent ASVD, yes (%) | 23 (12.0) | 23 (12.0) | 17 (8.9) | 28 (14.6) | 0.653 |
| Medications | |||||
| Low-dose aspirin, yes (%) | 42 (21.9) | 43 (22.4) | 20 (10.4) | 58 (28.1) | 0.605 |
| Antihypertensive therapy, yes (%) | 89 (46.4) | 75 (39.1) | 72 (37.5) | 85 (44.3) | 0.624 |
| Statin therapy, yes (%) | 40 (20.8) | 37 (19.3) | 26 (13.5) | 38 (19.8) | 0.479 |
Data expressed as mean ±SD, median (interquartile range), or number and percentage.
Significant at P < 0.05 by ANOVA or the Mantel-Haenszel test for trend test where appropriate.
Estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation based on serum creatinine and cystatin C.
Figure 2.
Quartile of circulating DKK1 and severe AAC in the elderly women. P < 0.05 by Mantel-Haenszel trend test.
Table 3.
Odds of Having Severe AAC (n = 146) According to Wnt Antagonist Concentrations in Elderly Women Aged Over 70 y (n = 768)
| Age-Adjusted OR (95% CI) | Multivariable-Adjusteda OR (95% CI) | |
|---|---|---|
| Circulating DKK1 levels, ng/mL | ||
| Quartile 1 (<0.27) | 1.83 (1.08–3.09)b | 2.05 (1.18– 3.56)b |
| Quartile 2 (0.27–0.45) | 1.59 (0.93–2.71) | 1.83 (1.05–3.19)b |
| Quartile 3 (0.45–0.71) | 1.32 (0.76– 2.29) | 1.64 (0.91–2.94) |
| Quartile 4 (≥0.71) | Referent | Referent |
| P value for trendc | 0.020 | 0.008 |
| Circulating sFRP3 levels, ng/mL | ||
| Quartile 1 (<1.64) | 1.35 (0.80–2.27) | 1.33 (0.78–2.29) |
| Quartile 2 (1.64–2.18) | 1.32 (0.78–2.22) | 1.19 (0.68–2.06) |
| Quartile 3 (2.18–3.01) | 1.17 (0.69–1.98) | 1.16 (0.67–2.00) |
| Quartile 4 (≥3.01) | Referent | Referent |
| P value for trendc | 0.219 | 0.310 |
| Circulating WIF1 levels, ng/mL | ||
| Quartile 1 (<0.10) | 1.06 (0.45–1.80) | 0.96 (0.54–1.71) |
| Quartile 2 (0.10–0.17) | 1.51 (0.90–2.54) | 1.33 (0.78–2.28) |
| Quartile 3 (0.17–0.34) | 1.50 (0.89–2.52) | 1.48 (0.87–2.54) |
| Quartile 4 (≥ 0.34) | Referent | Referent |
| P value for trendc | 0.310 | 0.538 |
Multivariate adjustments include: age, body mass index, treatment code, prevalent diabetes, smoking history, prescription of statins, prescription of antihypertensive medication, estimated glomerular filtration rate, low-dose aspirin use, and prevalent AVSD.
Significantly different from the highest quartile of DKK1: P < 0.05.
Test for trend conducted using the median value for each quartile of Wnt antagonists.
C. Further Analyses
To assess whether these results were independent of other Wnt antagonists, all 3 antagonists were added to fully adjusted models, which did not attenuate the association between DKK1 and severe AAC (Supplemental Table 1 (118.8KB, docx) ). We performed 2 further adjusted models, including the potential confounding factors serum phosphate and 25OHD, with similar findings observed in the continuous models multivariable-adjusted logistic regression (OR, 1.27; 95% CI, 1.05 to 1.53; P = 0.016 and OR, 1.25; 95% CI, 1.03 to 1.51; P = 0.022, respectively) and quartiles analyses (Supplemental Table 2 (118.8KB, docx) ). Because 82% of the lateral spine images were taken at year 1 of a randomized controlled trial of calcium supplements, we tested whether there was an interaction between DKK1 and treatment group (P = 0.681) and performed sensitivity analyses in the placebo group and calcium-supplemented group separately. Similar results were seen for both groups (multivariable-adjusted OR, 1.29; 95% CI, 1.00 to 1.67; P = 0.052 and OR, 1.21; 95% CI, 0.91 to 1.60; P = 0.183, respectively).
3. Discussion
Abdominal aortic calcification is an actively regulated process [6] that is related to the presence and severity atherosclerosis and calcification at other vascular beds [3, 4] and predicts risk of future cardiovascular events [5]. In this study we found that DKK1, but not WIF1 or sFRP3, was robustly associated with the severity of AAC in elderly white women. We sought to investigate the association between Wnt antagonists putatively targeting both the canonical and the noncanonical signaling pathways with AAC severity identified on lateral spine images captured during bone density assessment. These findings provide important new information on the likely role of Wnt signaling with age-related severe AAC and extend upon on previous studies investigating DKK1 in elderly men and younger women. Because elderly women in the lowest and second-lowest quartiles of DKK1 had nearly twice the odds of having severe AAC than women in the highest quartile, assessing DKK1 may be a useful strategy to identify individuals with severe AAC, which is an established risk factor for long-term cardiovascular disease risk [5]. However, further studies are needed to determine whether DKK1 improves identification of individuals with severe AAC and predicts future cardiovascular disease events and prognosis.
No association between WIF1 and sFRP3 levels with AAC severity was observed. In bone, WIF1 is known to influence both canonical and noncanonical Wnt signaling, whereas sFRP3 is thought to act primarily on the noncanonical pathways [25]. The lack of an association with these antagonists for severe AAC may suggest that in vascular tissues the noncanonical β-catenin-independent pathway plays less of a role in the progression of AAC severity than the canonical β-catenin signaling that is inhibited by DKK1. Supporting this concept are the findings from a warfarin-induced rat model of medial vascular calcification that observed DKK1, but not WIF1, inhibiting β-catenin induced vascular smooth muscle cell transdifferentiation and vascular calcification [26]. However, the null findings may also be due to a lack of power to identify weaker associations between these antagonists and severe AAC. If these null findings for WIF1 and sFRP3 with vascular calcification are confirmed in further studies, this highlights important differences between skeletal and nonskeletal regulation of calcification, potentially leading to tissue-specific therapeutic interventions.
Regarding the mechanism in animal studies, Wnt signaling and DKK1 have been shown to enhance endothelial-mesenchymal transition in bovine aortic endothelial cells [27] whereas animal and cell studies have demonstrated that Wnt signaling and DKK1 are regulators of vascular calcification. Awan et al. [28] reported increased Wnt and low-density lipoprotein receptor–related proteins 5 expression in the aortic wall and severe AAC in LDLR−/− mice on high fat chow, whereas Martinez-Moreno et al. [29] found that the addition of DKK1 to human vascular smooth muscle cells, stimulated with calcifying media, attenuated the calcification. Similarly Beazley et al. [26] demonstrated that in rats, DKK1, but not WIF1, prevented warfarin-induced activation canonical Wnt signaling and osteogenic transdifferentiation in vascular smooth muscle cells, leading to vascular calcification. Conversely, a recent study of diabetic mice subjected to renal injury to induce CKD displayed higher DKK1 and increased vascular calcification compared with non-CKD diabetic control mice, and this calcification was reversed by a monoclonal antibody neutralizing DKK1 and phosphate control [30]. As such, there is still uncertainty over the exact role of DKK1 in the development and progression of AAC.
There is now great interest in neutralizing antibodies targeting the Wnt signaling pathways to improve bone health and treat some malignant conditions. The most advanced of these is romosozumab, which is an antisclerostin antibody that, in a phase 2 randomized control trial in 419 women over 12 months, was shown to increase bone density and to reduce bone resorption in women with low bone turnover [31]. In this trial there was not an increased risk of adverse clinical cardiovascular side effect. Although these findings are reassuring, the nature of vascular calcification means that increases in cardiovascular events would be unlikely to be seen over such a short period of time. More recently, a bispecific antibody that inhibits both sclerostin and DKK1 has been developed and tested in nonprimate and primate animal models [32]. Given our findings, it would be prudent to evaluate and monitor long-term changes in AAC in patients randomized to neutralizing antibodies to sclerostin and/or DKK1 to rule out any off-target effects, similar to what has been done previously in the large 3-year randomized controlled trial of anti-RANKL antibody (denosumab) that demonstrated no effect on AAC [33].
In a recent study that used a similar cross-sectional study design, Szulc et al. [16] reported that in 803 men over 60 years of age, but not in 336 younger men (20–59 years of age), lower serum DKK1 was associated with the severe AAC. Similar to our study, Szulc et al. [16] used lateral spine images obtained by a Hologic DXA machine to detect the presence of AAC, the same Kauppila 24-point scoring system [24] and the same definition of severe AAC. In both the current study in elderly women and in the study of Szulc et al. [16] in elderly men, the association between DKK1 remained significant after adjusting for conventional cardiovascular disease risk factors and in the current study remained significant after adjusting for renal function, circulating 25-hydroxyvitamin D, and phosphate. A previous smaller study [34] of 113 younger postmenopausal women (mean age, 62 years) with lateral spine images obtained on a Hologic DXA machine using the Kauppila scoring system also reported an inverse association between DKK1 and less severe AAC (AAC24 score >1); however, few women in this study had detectable AAC, and no results were presented for severe AAC. Taken together, our findings confirm previous studies demonstrating an inverse association between circulating DKK1 and AAC severity that is independent of vascular risk factors, estimated renal function, circulating 25-hydroxyvitamin D, and phosphate.
In 2 of 3 published studies, data from patients with advanced CKD also support an inverse relationship between DKK1 and severe AAC. In a younger cohort consisting of 77 patients with stage 3B and stage 4 CKD, DKK1 levels were inversely associated with arterial stiffness index [35] which is strongly related to AAC [36]. In a study of 177 stable patients undergoing hemodialysis [37], DKK1 was inversely associated with the severity of AAC using the same scoring methodology (AAC24 scores) from standard radiographs. In contrast, a study of 125 patients on hemodialysis that assessed aortic arch calcification from standard radiographs found that sclerostin, but not DKK1 concentrations, were associated with aortic calcification assessed using a different scoring methodology [38].
Studies investigating the association between DKK1 and calcification in other vascular beds, such as coronary artery, have been conflicting. A study of African American patients with type II diabetes [39] found that DKK1 was inversely associated with atherosclerotic calcified plaque at the coronary and aortoiliac arterial segments in fully adjusted models. However, in a study of patients presenting with chest pain not due to myocardial infarction, Kim et al. [40] found that DKK1 levels were positively associated with coronary artery calcification. As such, the relationship between DKK1 and coronary artery calcification remains unclear. A potential explanation for these findings is that in addition to being implicated in vascular calcification development and progression, DKK1 has also been shown to be expressed at high levels by macrophages and endothelial cells in atherosclerotic plaques, promoting enhanced release of inflammatory cytokines [41]. Therefore, reported differences are likely to be due to differences in the cohort characteristics reflecting different expression site and disease pathologies. However, further study on the role of DKK1 on the development and progression of atherosclerosis and/or vascular calcification is needed.
There are a number of limitations that must be considered in the interpretation of the findings from this study. First, because this was a cross-sectional observational study, causality cannot be determined, and we cannot exclude the possibility of bias. This is especially true because we did not have complete data on all participants in this cohort and cannot exclude the possibility of selection bias affecting our results, even though women without measures of Wnt antagonists or AAC were similar to those with measures. Second, because we only had 1 measurement of the Wnt antagonists and severe AAC, we cannot determine the true nature of the relationship between these antagonists and progression of AAC. Third, AAC is actively regulated by many proteins with numerous inducers and inhibitors of calcification [6]. Other Wnt antagonists and modulators, such as sclerostin, were not measured in our study and could also be associated with severe AAC. This is important because several cross-sectional studies have shown that, in patients with impaired renal function, sclerostin levels are positivity related to vascular calcification [42, 43]. However, other studies have found no association [44] or inverse associations [37], and 1 study in transplant recipients even demonstrated a positive association in unadjusted analyses and an inverse association in multivariable-adjusted analyses [45]. These conflicting findings are likely due to increased circulating levels of sclerostin in patients with CKD [37] despite increased urinary excretion [46]. This suggests that sclerostin may also be produced at the site of vascular calcification as a compensatory mechanism [37, 43]. Finally, because this was a cohort of elderly white women, these findings may not extend to other ethnicities or to younger women. As such, further large prospective cohort studies with repeated measures of DKK1 and AAC are needed to determine the true nature of the relationship and to confirm these findings.
Strengths of our study include measurement of 3 circulating Wnt antagonists, which are likely to affect both canonical Wnt signaling (DKK1), noncanonical Wnt signaling (sFRP3), and both Wnt signaling pathways (WIF1). Second, a single highly experienced investigator (J.T.S.) blinded to clinical study data assessed the AAC phenotypes. Third, we had detailed information on vascular risk factors, medications, and disease history as well as glomerular filtration rate estimated using both creatinine and cystatin C, which is a closer measure of true glomerular filtration rate than creatinine alone [20]. Finally, we had detailed biochemical measures in this study allowing adjustment for phosphate and 25-hydroxvitamin D measures using liquid chromatography tandem mass spectrometry.
In conclusion, we did not find an association between the Wnt antagonists WIF-1 and sFRP3 with severe AAC in elderly women. In addition, our findings add further support to the concept that canonical Wnt signaling regulates vascular calcification and that DKK1 may inhibit severe AAC in elderly women, extending previous findings in elderly men and patients with advanced chronic kidney disease.
Acknowledgments
The authors thank the staff at the Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for providing data for this study.
Acknowledgments
This work was supported by Kidney Health Australia Grant S07 10; Healthway Health Promotion Foundation of Western Australia; Sir Charles Gairdner Hospital Research Advisory Committee Grants; and by project grants 254627, 303169, and 572604 from the National Health and Medical Research Council of Australia. The salary of Dr. Lewis is supported by a National Health and Medical Research Council of Australia Career Development Fellowship (ID: 1107474). Dr. Rivadeneira is partially supported by the Netherlands Scientific Organization (Project: NWO/ZONMW-VIDI-016-136-367). Dr. Kiel’s time was supported by a grant from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR 41398). None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author contributions: T.U., J.B., J.T.S., P.L.T., G.W., W.H.L., R.L.P., and J.R.L. collected the data; W.A.T., F.R., R.L.P., and J.R.L. analyzed the data; W.A.T., T.U., F.R., R.L.P., and J.R.L. interpreted the data. W.A.T., R.L.P., and J.R.L. drafted the manuscript. All authors revised the manuscript. All authors the final version of manuscript. W.A.T. and J.R.L. take responsibility for the integrity of the data analysis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAC
- abdominal aortic calcification
- ASVD
- atherosclerotic vascular disease
- CAIFOS
- Calcium Intake Fracture Outcome Study
- CI
- confidence interval
- DKK1
- Dickkopf-1
- eGFR
- estimated glomerular filtration rate
- IQR
- interquartile range
- OR
- odds ratio
- SD
- standard deviation
- sFRP3
- secreted frizzled related protein 3
- WIF1
- Wnt inhibitory factor 1.
References and Notes
- 1.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56(20):1613–1622. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JR, Schousboe JT, Lim WH, Wong G, Zhu K, Lim EM, Wilson KE, Thompson PL, Kiel DP, Prince RL. Abdominal aortic calcification identified on lateral spine images from bone densitometers are a marker of generalized atherosclerosis in elderly women. Arterioscler Thromb Vasc Biol. 2016;36(1):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oei HH, Vliegenthart R, Hak AE, Iglesias del Sol A, Hofman A, Oudkerk M, Witteman JC. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39(11):1745–1751. [DOI] [PubMed] [Google Scholar]
- 5.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98(13):988–994. [DOI] [PubMed] [Google Scholar]
- 6.Szulc P. Abdominal aortic calcification: A reappraisal of epidemiological and pathophysiological data. Bone. 2016;84:25–37. [DOI] [PubMed] [Google Scholar]
- 7.Jayalath RW, Mangan SH, Golledge J. Aortic calcification. Eur J Vasc Endovasc Surg. 2005;30(5):476–488. [DOI] [PubMed] [Google Scholar]
- 8.Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: a union made for bone. J Bone Miner Res. 2004;19:1749–1757. [DOI] [PubMed]
- 9.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107(8):943–952. [DOI] [PubMed] [Google Scholar]
- 10.Surmann-Schmitt C, Widmann N, Dietz U, Saeger B, Eitzinger N, Nakamura Y, Rattel M, Latham R, Hartmann C, von der Mark H, Schett G, von der Mark K, Stock M. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci. 2009;122(Pt 20):3627–3637. [DOI] [PubMed] [Google Scholar]
- 11.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88(6):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin K, Wang S, Julius MA, Kitajewski J, Moos M Jr, Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA. 1997;94(21):11196–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88(6):757–766. [DOI] [PubMed] [Google Scholar]
- 14.Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–961. [DOI] [PubMed] [Google Scholar]
- 15.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. [DOI] [PubMed] [Google Scholar]
- 16.Szulc P, Schoppet M, Rachner TD, Chapurlat R, Hofbauer LC. Severe abdominal aortic calcification in older men is negatively associated with DKK1 serum levels: the STRAMBO study. J Clin Endocrinol Metab. 2014;99(2):617–624. [DOI] [PubMed] [Google Scholar]
- 17.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166(8):869–875. [DOI] [PubMed] [Google Scholar]
- 18.Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(3):743–749. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JR, Zhu K, Thompson PL, Prince RL. The effects of 3 years of calcium supplementation on common carotid artery intimal medial thickness and carotid atherosclerosis in older women: an ancillary study of the CAIFOS randomized controlled trial. J Bone Miner Res. 2014;29:534–541. [DOI] [PubMed]
- 20.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schousboe JT, Wilson KE, Kiel DP. Detection of abdominal aortic calcification with lateral spine imaging using DXA. J Clin Densitom. 2006;9:302–308. [DOI] [PubMed]
- 22.Schousboe JT, Wilson KE, Hangartner TN. Detection of aortic calcification during vertebral fracture assessment (VFA) compared to digital radiography. PLoS One. 2007;2(8):e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schousboe JT, Taylor BC, Kiel DP, Ensrud KE, Wilson KE, McCloskey EV. Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J Bone Moner Res. 2008;23:409–416. [DOI] [PubMed]
- 24.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PWF. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–250. [DOI] [PubMed] [Google Scholar]
- 25.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–2634. [DOI] [PubMed] [Google Scholar]
- 26.Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-mediated activation of β-catenin signaling has a critical role in warfarin-induced vascular calcification. Arterioscler Thromb Vasc Biol. 2012;32(1):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awan Z, Denis M, Bailey D, Giaid A, Prat A, Goltzman D, Seidah NG, Genest J. The LDLR deficient mouse as a model for aortic calcification and quantification by micro-computed tomography. Atherosclerosis. 2011;219(2):455–462. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Moreno JM, Muñoz-Castañeda JR, Herencia C, Oca AM, Estepa JC, Canalejo R, Rodríguez-Ortiz ME, Perez-Martinez P, Aguilera-Tejero E, Canalejo A, Rodríguez M, Almadén Y. In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/β-catenin activation. Am J Physiol Renal Physiol. 2012;303(8):F1136–F1144. [DOI] [PubMed] [Google Scholar]
- 30.Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC, Freedman BI, Monier-Faugere MC, Malluche H, Hruska KA. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol. 2014;25(8):1760–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. [DOI] [PubMed] [Google Scholar]
- 32.Florio M, Gunasekaran K, Stolina M, Li X, Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li C, Sun B, Tan HL, Zhang L, Han CY, Case R, Duguay AN, Grisanti M, Stevens J, Pretorius JK, Pacheco E, Jones H, Chen Q, Soriano BD, Wen J, Heron B, Jacobsen FW, Brisan E, Richards WG, Ke HZ, Ominsky MS. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samelson EJ, Miller PD, Christiansen C, Daizadeh NS, Grazette L, Anthony MS, Egbuna O, Wang A, Siddhanti SR, Cheung AM, Franchimont N, Kiel DP. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014;29:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampson G, Edwards S, Conroy S, Blake GM, Fogelman I, Frost ML. The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone. 2013;56(1):42–47. [DOI] [PubMed] [Google Scholar]
- 35.Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, Hampson G. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int. 2012;90(6):473–480. [DOI] [PubMed] [Google Scholar]
- 36.McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki-Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB; Anglo-Cardiff Collaboration Trial Investigators . Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53(3):524–531. [DOI] [PubMed] [Google Scholar]
- 37.Lee YT, Ng HY, Chiu TT, Li LC, Pei SN, Kuo WH, Lee CT. Association of bone-derived biomarkers with vascular calcification in chronic hemodialysis patients. Clin Chim Acta. 2016;452:38–43. [DOI] [PubMed] [Google Scholar]
- 38.Yang CY, Chang ZF, Chau YP, Chen A, Yang WC, Yang AH, Lee OK. Circulating Wnt/β-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol Dial Transplant. 2015;30(8):1356–1363. [DOI] [PubMed] [Google Scholar]
- 39.Register TC, Hruska KA, Divers J, Bowden DW, Palmer ND, Carr JJ, Wagenknecht LE, Hightower RC, Xu J, Smith SC, Dietzen DJ, Langefeld CD, Freedman BI. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab. 2013;98(1):E60–E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KI, Park KU, Chun EJ, Choi SI, Cho YS, Youn TJ, Cho GY, Chae IH, Song J, Choi DJ, Kim CH. A novel biomarker of coronary atherosclerosis: serum DKK1 concentration correlates with coronary artery calcification and atherosclerotic plaques. J Korean Med Sci. 2011;26(9):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(8):1228–1234. [DOI] [PubMed] [Google Scholar]
- 42.Wang XR, Yuan L, Zhang JJ, Hao L, Wang DG. Serum sclerostin values are associated with abdominal aortic calcification and predict cardiovascular events in patients with chronic kidney disease stages 3-5D [published online ahead of print May 10, 2016]. Nephrology (Carlton). doi:10.1111/nep.12813 [DOI] [PubMed]
- 43.Brandenburg VM, Kramann R, Koos R, Krüger T, Schurgers L, Mühlenbruch G, Hübner S, Gladziwa U, Drechsler C, Ketteler M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2013;14:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desjardins L, Liabeuf S, Oliveira RB, Louvet L, Kamel S, Lemke HD, Vanholder R, Choukroun G, Massy ZA; European Uremic Toxin (EUTox) Work Group . Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol Ther. 2014;10(6):463–470. [DOI] [PubMed] [Google Scholar]
- 45.Evenepoel P, Goffin E, Meijers B, Kanaan N, Bammens B, Coche E, Claes K, Jadoul M. Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients. J Clin Endocrinol Metab. 2015;100(12):4669–4676. [DOI] [PubMed] [Google Scholar]
- 46.Cejka D, Marculescu R, Kozakowski N, Plischke M, Reiter T, Gessl A, Haas M. Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab. 2014;99(1):248–255. [DOI] [PubMed] [Google Scholar]