Abstract
BACKGROUND
Cardiovascular diseases (CVD) are a set of metabolic disorders affecting heart and blood vessels. Green tea and sour tea (Hibiscus sabdariffa L.) have attracted significant attention recently due to their high popularity, nutrient profile and therapeutic effects. The aim of the present study was to compare the effects of green tea and sour tea supplementation on blood pressure and lipid profile in healthy adult men.
METHODS
This randomized, double-blind, placebo-controlled trial included 54 healthy adult men. The participants were randomly assigned to two intervention groups receiving 450 mg green tea or sour tea and one placebo group which consumed 450 mg placebo (maltodextrin) for 6 weeks. Blood pressure, lipid profile, dietary intake and physical activity were measured pre- and post-intervention and compared.
RESULTS
After 6 weeks of intervention, sour tea supplementation led to a significant decrease in systolic blood pressure (SBP) compared with the placebo group. However, we faild to find any significant difference in SBP between green tea and control groups. Also, no significant changes were observed in diastolic blood pressure (DBP) and lipid profile between the three groups. In comparison with baseline, there was a significant increase in the mean level of serum high-density lipoprotein cholesterol (HDL-C) in green tea and sour tea groups. Also, the interventions resulted in significant decrease in the mean levels of serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) and DBP in the sour tea group compared with the pre-intervention value.
CONCLUSION
On the basis of our findings, sour tea supplementation led to decreased SBP in healthy men compared with the placebo, but there was no significant difference between their effects on DBP and lipid profile.
Keywords: Green Tea, Hibiscus Sabdariffa, Blood Pressure, Adults
Introduction
Cardiovascular diseases (CVD) are a set of metabolic disorders affecting the heart and blood vessels; this chronic disease is a major global cause of death.1 World Health Organization (WHO) estimates that the number of people who die from CVD will rise to more than 23.6 million by 2030.2 Although the clinical emergencies of CVD are mostly displayed in mid-life, early metabolic changes are apparent in youth.3 Hence, effective prevention of CVD is advised to be started in adolescence or adulthood.3 Generally, a combination of various factors such as unhealthy diet, physical inactivity, and smoking could be considered as the leading causes of CVD.4 Dyslipidemia, an imbalance of the plasma lipids, and hypertension are the most important concerns among CVD risk factors.5 Diet plays a notable role in maintaining these risk factors. Moreover, to improve the lipid profile and blood pressure, dietary modifications are safer and more cost-effective than medical strategies.4,6,7 Recent scientific literature emphasizes the therapeutic effects of functional foods, indicating positive applications for optimizing the plasma lipids, blood pressure and subsequently decreasing the risk of CVD.8-10 In this regard, green tea and sour tea (Hibiscus sabdariffa L.) have attracted significant attention recently, both in the scientific and consumer societies, due to their high popularity, nutrient profile and therapeutic effects.11,12 Green tea, obtained from the plant Camellia sinensis, is a common beverage which is used in different cultures around the world.12 Evidence has shown that this wonderful drink can delay the onset or progression of a broad range of diseases such as cancer, cardiovascular disorders, diabetes, liver diseases and hypertension.12,13 The mechanisms underlying the beneficial effects of green tea are related to its phenolic compounds, mainly catechin, epicatechin (EC), epigallocatechin (EGC) and epigallocatechin gallate (EGCG).14 Sour tea is a genus of the Malvaceae family which grows widely in Middle Eastern countries.15 Its calyces are red in color and sour in taste.15 Sour tea is used in many countries as a beverage or medicinal herb.16 Its main biological compounds include polyphenols, anthocyanins (such as hibiscin, gossypicyanin, and anthocyanidin), and flavonoids; these compounds are potentially bioactive with cardiovascular protective effects.16,17 Furthermore, in ancient medical practice, it has been used for the treatment of hypertension, diabetes and metabolic syndrome.17
Although beneficial effects of different kinds of tea have been indicated in several investigations, to the best of our knowledge, there is no study that has compared the effects of these two kinds of tea on CVD risk factors among healthy subjects. Therefore, the current study was carried out to evaluate the effects of green tea and sour tea supplementation on blood pressure and lipid profile in healthy adult men.
Materials and Methods
The present study was a three-arm parallel, randomized, and the double-blind trial conducted in Isfahan University of Medical Sciences, Iran, from October 2015 to December 2016. Fifty-four healthy volunteers were invited to participate in this study by advertising at different schools of Isfahan University of Medical Sciences. To calculate sample size, we used the standard formula suggested for clinical trials by considering a study power of 80%, type I error of 5% (α = 0.05) and type II error of 20% (β = 0.20). According to a previous study,18 we used 1.3 mg/dl as standard deviation (SD) and 0.5 mg/dl as the change in mean (d) of triacylglycerol (TAG) as a main variable. Based on the formula, we needed 15 participants in each group; after considering of 3 dropouts in each group, the final sample size was 18 participants in each group. The inclusion criteria for participation in this study were: men age 18-35 years, body mass index (BMI) 20-25 kg/m2, free of acute or chronic diseases especially diseases affecting blood pressure and plasma lipids including hypothyroidism, thyroid disorders, heart, kidney and inflammatory diseases as well as pancreatitis, not using medications or supplements in the past 2 months, not being substance addict (including alcohol or tobacco products), and not having any special diet. The exclusion criteria included: any allergic reaction to green or sour tea supplements, diagnosis of any illness (such as bacterial or viral infections) during the study, starting medication or supplement therapy during the trial, and irregular use of the tablets (consuming less than 90% of tea supplements delivered to the subjects during the study).
Initially, all subjects signed informed written consent, and the study protocol was approved by Ethics Committee of the Isfahan University of Medical Sciences. The eligible subjects were randomly assigned to two intervention groups, green tea or sour tea and one placebo group by using the random allocation software. Randomization was done by one of the researchers who had no clinical involvement in the trial. Subjects in each group were instructed to take one tablet per day (with lunch meal) for 6 weeks. Tablets were given to the participants weekly. Compliance to tea and placebo tablets was assessed by counting their tablets at the end of use and their results were applied for data analysis if they used more than 90% of their tablets. The participants were advised not change their usual dietary and exercise pattern throughout the study and report any abnormal sensations immediately. The study was registered in clinicaltrials.gov with the record number of NCT02637570.
The calyces of sour tea and green tea leaves were purchased from local market in Isfahan. The calyces and leaves were dried and separately crushed by an electric mixer (Moulinex, Japan). Finally, the powders were delivered to a Barij Essence Pharmaceutical Company, Kashan, Iran, to prepare coated tablets containing 450 mg green tea (containing about 240 mg catechins) or sour tea (containing at least 250 mg of anthocyanin). The placebo tablets with the same size, weight, color, and shape were prepared from maltodextrin powder in collaboration with School of Pharmacy, Isfahan University of Medical Sciences. In order to blind the participants and researchers, tablets were packed in the identical boxes and were labeled with 3 codes by an individual outside this project.
General information including age, height, and weight was evaluated through the interview with the participants. Weight and height were quantified without shoes and minimally clothed using a digital scale (Seca, Hamburg, Germany). BMI was calculated by weight (kg) divided by height in square meters (m2). At the onset and end of the study, to obtain detailed information about the dietary intake and physical activity, participants were asked to complete 3-day food records (one weekend day and two regular days) and International Physical Activity Questionnaire (IPAQ), a valid and reliable self-administered questionnaire that contains 5 activity domains,19 respectively. Physical activity was calculated as a metabolic equivalent task minute per week spent on all activities. Nutritionist IV software (version 7.0, N-Squared Computing, Salem, OR) was used to analyze 3-day food records data. Systolic and diastolic blood pressures (SBP and DBP) were measured two times in every session, and the average was recorded. All measurements were performed in the morning and after a 5-10 minute rest, using a mercury sphygmomanometer.
Participants were required to provide venous blood samples after 10-12 hours overnight fasting (water permitted) at study baseline and after 6-week intervention. A volume of 10 ml of blood samples was obtained from each participant by the laboratory technician. Serums were separated by centrifugation and stored at -70 ºC until analysis. Available commercial kits were used to determine TAG, total cholesterol (TA), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) concentrations (Pars Azmun, Tehran, Iran).
In this study, statistical analysis of data was performed using SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA). Shapiro-Wilk test was used to determine the normality of data distribution. Comparisons within groups and between groups were performed using the paired-sample t-test and analysis of variance (ANOVA), respectively. For the purpose of finding pairwise differences between groups, the Tukey's test was applied. The nonparametric tests (Wilcoxon, Kruskal-Wallis, and Mann-Whitney) were used to analyze the non-normal data. Results were expressed as mean ± SD. P < 0.05 was considered as significant.
Results
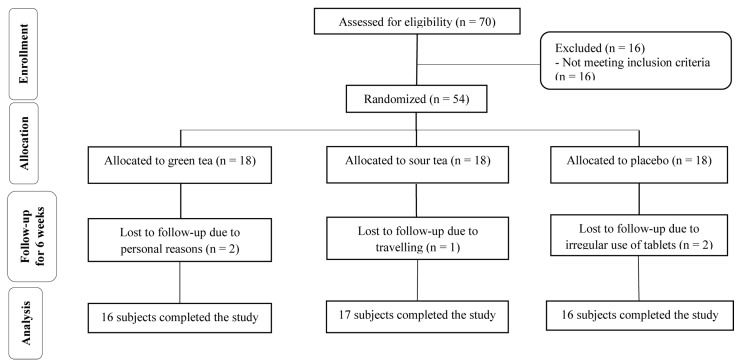
From 70 participants who were assessed for eligibility, 54 subjects were recruited into this 6-weeks trial. Throughout the study, five participants were excluded from the intervention: 2 subjects for personal reasons, 2 for irregular use of tablets and 1 for travel. Finally, 49 participants completed the study (Figure 1).
Figure 1.
Flowchart of participants' recruitment and enrollment in the study
Generally, the rate of compliance in our trial was high, such that almost 95 percent of tablets were taken throughout the study in three groups. At the beginning of the study, no serious side effects were observed from consumption of tablets throughout the intervention.
General characteristics of participants were not significantly different between the three groups (Table 1). Based on 3-day dietary records and physical activity questionnaire, we failed to find any statistically significant difference between the three groups at the beginning and end of the study (Table 2).
Table 1.
Baseline demographic and clinical characteristics of the study participants
| Parameter (unit) | Green tea | Sour tea | Placebo | P* |
|---|---|---|---|---|
| (n = 16) | (n = 17) | (n = 16) | ||
| Age (year) | 20.94 ± 1.43 | 20.71 ± 1.26 | 21.19 ± 2.16 | 0.700 |
| Height (cm) | 180.88 ± 6.06 | 178.24 ± 5.03 | 178.31 ± 7.40 | 0.340 |
| Weight (kg) | 74.12 ± 8.62 | 71.68 ± 7.53 | 72.59 ± 12.67 | 0.770 |
| BMI (kg/m2) | 22.60 ± 1.71 | 22.53 ± 1.85 | 22.82 ± 3.73 | 0.940 |
| TC (mg/dl) | 183.65 ± 29.31 | 196.29 ± 24.92 | 22.87±187.36 | 0.350 |
| LDL-C (mg/dl) | 111.25 ± 26.33 | 117.41 ± 20.14 | 110.37 ± 19.31 | 0.600 |
| HDL-C (mg/dl) | 48.18 ± 6.25 | 51.11 ± 6.65 | 50.56 ± 7.72 | 0.440 |
| TAG (mg/dl) | 121.06 ± 42.40 | 138.82 ± 37.04 | 132.12 ± 37.42 | 0.420 |
| SBP (mmHg) | 123.75 ± 8.06 | 124.41 ± 5.55 | 123.12 ± 8.92 | 0.840† |
| DBP (mmHg) | 83.75 ± 8.06 | 83.23 ± 7.27 | 82.18 ± 8.15 | 0.700† |
All data are means ± standard deviations (SD)
Obtained from ANOVA test for the between group comparisons;
Kruskal-Wallis test was used
BMI: Body mass index; TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; TAG: Triacylglycerol; SBP: Systolic blood pressure; DBP: Diastolic blood pressure
Table 2.
Daily dietary intakes and physical activity before and after the intervention
| Characteristics | Energy | Carbohydrate | FAT | Protein | Fiber | Physical activity |
|---|---|---|---|---|---|---|
| (kcal) | (g/day) | (g/day) | (g/day) | (g/day) | (met-minutes/week) | |
| Green tea (n = 16) | ||||||
| Before | 2298.87 ± 238.60 | 337.46 ± 35.54 | 63.85 ± 6.62 | 95.44 ± 18.49 | 3.54 ± 17.75 | 69.31 ± 214.53 |
| After | 2272.12 ± 189.08 | 333.07 ± 43.45 | 63.13 ± 9.72 | 97.62 ± 18.72 | 4.35 ± 17.25 | 549.56 ± 268.48 |
| P* | 0.401 | 0.558 | 0.637 | 0.678 | 0.626 | 0.682 |
| Sour tea (n = 17) | ||||||
| Before | 2177.52 ± 270.15 | 314.68 ± 70.22 | 62.53 ± 12.37 | 89.30 ± 13.30 | 2.18 ± 18.58 | 549.94 ± 191.01 |
| After | 2157.41 ± 304.47 | 318.19 ± 48.58 | 60.16 ± 8.27 | 85.71 ± 14.12 | 4.39 ± 23.88 | 548.58 ± 228.71 |
| P* | 0.570 | 0.763 | 0.259 | 0.401 | 0.379 | 0.985 |
| Placebo (n = 16) | ||||||
| Before | 2153.81 ± 333.23 | 318.40 ± 47.06 | 60.06 ± 10.98 | 86.80 ± 20.31 | 19.43 ± 2.73 | 555.81 ± 259.76 |
| After | 2151.12 ± 243.71 | 322.78 ± 55.74 | 56.34 ± 9.56 | 88.99 ± 17.72 | 17.40 ± 20.37 | 522.18 ± 208.10 |
| P* | 0.946 | 0.651 | 0.056 | 0.695 | 0.360 | 0.538 |
| P† | 0.885 | 0.782 | 0.482 | 0.631 | 0.497 | 0.925 |
All data are means ± standard deviation (SD)
Obtained from paired t-test for the within-group comparisons;
Obtained from ANOVA test for the between group comparisons
Within-group analysis revealed a significant reduction in mean serum LDL-C and TC levels as well as DBP in the sour tea group at the end of intervention when compared with pre-intervention values (P = 0.009, P = 0.043, and P = 0.007, respectively).
Also, comparing pre- vs. post-intervention, HDL-C concentration was increased in both green tea and sour tea groups (P = 0.005 and P = 0.003, respectively, Table 3).
Table 3.
The effects of interventions on tested parameters after 6 weeks in the study participants
| Parameter (Unit) | TC | LDL-C | HDL-C | TAG | SBP | DBP |
|---|---|---|---|---|---|---|
| (mg/dl) | (mg/dl) | (mg/dl) | (mg/dl) | (mmHg) | (mmHg) | |
| Green tea (n = 16) | ||||||
| Before | 183.65 ± 29.31 | 111.25 ± 26.33 | 48.18 ± 6.25 | 121.06 ± 42.40 | 123.75 ± 8.06 | 83.75 ± 8.06 |
| After | 180.38 ± 25.77 | 102.93 ± 22.10 | 54.12 ± 9.96 | 116.62 ± 38.65 | 118.12 ± 8.34 | 81.25 ± 5.00 |
| P* | 0.643 | 0.242 | 0.005 | 0.623 | 0.020† | 0.285† |
| Sour tea (n = 17) | ||||||
| Before | 196.29 ± 24.92 | 117.41 ± 20.14 | 51.11 ± 6.65 | 138.82 ± 37.04 | 124.41 ± 5.55 | 83.23 ± 7.27 |
| After | 188.63 ± 21.52 | 106.76 ± 17.98 | 56.11 ± 7.38 | 131.58 ± 37.65 | 114.41 ± 7.47 | 75.88 ± 7.95 |
| P* | 0.043 | 0.009 | 0.003 | 0.193 | 0.004† | 0.069† |
| Placebo (n = 16) | ||||||
| Before | 187.36 ± 22.87 | 110.37 ± 19.31 | 50.56 ± 7.72 | 132.12 ± 37.42 | 123.12 ± 8.92 | 82.18 ± 8.15 |
| After | 187.65 ± 26.14 | 109.31 ± 20.18 | 52.62 ± 7.16 | 128.56 ± 41.69 | 120.31 ± 8.84 | 80.93 ± 9.34 |
| P* | 0.945 | 0.815 | 0.192 | 0.501 | 0.359† | 0.618† |
All data are means ± Standard deviation (SDs)
Obtained from paired t-test for the within-group comparisons;
Wilcoxon test was used
TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol, TAG: Triacylglycerol, SBP: Systolic blood pressure; DBP: Diastolic blood pressure
Sour tea supplementation resulted in a significant reduction in SBP (P = 0.004) but not in DBP (P = 0.069) compared with control group. However, we failed to find any significant effect due to green tea consumption on SBP and DBP in comparison with placebo (P = 0.242 and P = 0.758, respectively).
Also, no significant difference was found between three groups in terms of TG, TC, LDL-C, and HDL-C (Table 4).
Table 4.
The comparison on changes of lipid profile and blood pressure measurements between three groups
| Parameter (Unit) | Green tea (n = 16) | Sour tea (n = 17) | Placebo (n = 16) | P* |
|---|---|---|---|---|
| TC (mg/dl) | -3.26 ± 27.59 | -7.65 ± 14.39 | 0.28 ± 16.38 | 0.540 |
| LDL-C (mg/dl) | -8.31 ± 27.27 | -10.64 ± 14.78 | -1.06 ± 17.80 | 0.524 |
| HDL-C (mg/dl) | 5.93 ± 7.31 | 5.00 ± 6.01 | 2.06 ± 6.03 | 0.426 |
| TAG (mg/dl) | -4.43 ± 29.47 | -7.23 ± 36.94 | -3.56 ± 29.77 | 0.920 |
| SBP (mm Hg) | -5.62 ± 8.13 | -10.00 ± 5.59† | -2.81 ± 10.16 | 0.016† |
| DBP (mm Hg) | -2.50 ± 9.30 | -7.35 ± 9.37 | -1.25 ± 9.74 | 0.151£ |
All data are means ± Standard deviation (SDs);
Obtained from ANCOVA test; adjustment was made for baseline values, dietary intake and BMI;
Kruskal-Wallis test was used
Those in comparison of sour tea group with placebo group was significant
TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; TAG: Triacylglycerol; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; BMI: Body mass index
Discussion
In the current study, the effects of green tea and sour tea on blood pressure and plasma lipids in healthy men were examined. Our results showed consumption of sour tea and green tea had a significantly positive effect on SBP after 6 weeks, also a significant difference in SBP was observed between groups. Post hoc analysis revealed that sour tea supplementation led to a significant reduction in SBP compared with the placebo group. Nevertheless, we failed to find any significant effect on DBP between groups. There are various studies that assessed the beneficial effects of green tea and sour tea on human health status; however, to the best of our knowledge, this study is the first clinical trial which investigated the effect of green tea and sour tea supplements simultaneously on blood pressure and lipid profile in healthy adult men.
Dyslipidemia and hypertension are the most common risk factors in the pathogenesis of CVD.20 Regarding the key role of dyslipidemia in CVD, management of hyperlipidemia is an important therapeutic way against CVD.21,22 Earlier investigations in humans and animals have shown that medicinal plants are able to alleviate the cardiovascular risk factors.21-24 Tea has been proven to be an effective herbal therapy to improve blood pressure and lipid profile, due to the high concentrations of phenolic compounds.25-27 The results of our study are similar to previous studies. In a trial, no significant change in blood pressure was seen following the green tea consumption (714 mg/day) for 3 weeks in healthy men.28 Furthermore, in a meta-analysis, it was indicated that sour tea supplementation significantly reduced SBP and DBP.11 Previous studies reported that sour tea due to its specific ingredients such as anthocyanin and quercetin can be used as an antihypertensive drug.29,30 Accurate mechanisms responsible for the antihypertensive effect of sour tea are not completely understood.
The probable mechanism by which sour tea could decrease blood pressure may be attributed to an increase in nitric oxide (NO) release from the endothelium of blood vessels and a reduction in calcium influx into vascular smooth muscle cells.11,31 Furthermore, anthocyanin, main flavonoid of sour tea, decreases the angiotensin-converting enzyme (ACE) activity, which ultimately leads to reduced blood pressure.11,32 Nevertheless, findings of a clinical trial did not demonstrate a significant effect of sour tea on blood pressure.33 Also, in a similar study, Nantz et al. showed that moderate green tea supplementation for 3 weeks reduced DBP and SBP in healthy adults.34 Noteworthy, based on the results of systematic review studies, we have concluded that green tea had a greater effect on blood pressure when the subjects were in a high blood pressure status.35,36
Another important finding was that the green tea and sour tea supplementation had beneficial effects on some lipid profiles in comparison with the baseline but there were no significant differences between groups. Overall, few studies have assessed the impacts of sour tea administration on serum lipids. Our findings are in agreement with the systematic review and meta-analysis that investigated the impact of sour tea supplementation on serum lipids.16 No significant reduction in plasma lipids was observed in subjects who consumed sour tea compared with those who consumed placebo. Besides, Frank et al.28 showed that 3-weeks green tea extract supplementation caused no significant changes in serum lipids of healthy men. In contrast, the majority of previous studies have reported that green tea supplementation can markedly reduce the higher serum concentrations of TC and LDL-C.34,35 Furthermore, a significant decrease in serum cholesterol level was observed after the intake of sour tea for 4 weeks in healthy adults.27 It seems that the possible cause for this diversity in findings might be explained by different study designs, different form and dosages of tea used and discrepancy in participants or duration of the studies.
Generally, in interpreting the present findings several limitations should be considered. First of all, this study was conducted on healthy men. Therefore, the findings cannot easily be extrapolated to women and patients. Second, in the present trial we used the tea powders without extraction which led to receiving lower doses of the plant material. Although high concentrations of blood inflammatory markers such as interleukin-6 and tumor necrosis factor alpha have been reported as remarkable risk factors for CVD, due to budget limitations we could not evaluate these biomarkers in the present study.
Conclusion
In conclusion, daily consumption of 450 mg sour tea can decreased SBP in healthy adults compared with placebo, but there was no significant difference between their effects on DBP and lipid profile. Therefore, given the long reputation of sour tea in humans, it can be applied as an ideal plant supplement in the prevention of hypertension in general population. However, this information can be used to develop targeted interventions with higher doses, longer duration, and larger sample size.
Acknowledgments
The authors would like to thank the Isfahan University of Medical Sciences for financial support. Special gratitude should go to the students who participated in this study.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29(4):611–8. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Alwan A. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999-2008. Pediatrics. 2012;129(6):1035–41. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 4.Khosravi-Boroujeni H, Sarrafzadegan N, Mohammadifard N, Sajjadi F, Maghroun M, Asgari S, et al. White rice consumption and CVD risk factors among Iranian population. J Health Popul Nutr. 2013;31(2):252–61. doi: 10.3329/jhpn.v31i2.16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelishadi R, Hashemipour M, Sheikh-Heidar A, Ghatreh-Samani S. Changes in serum lipid profile of obese or overweight children and adolescents following a lifestyle modification course. ARYA Atheroscler. 2012;8(3):143–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Sahebkar A, Simental-Mendia LE, Giorgini P, Ferri C, Grassi D. Lipid profile changes after pomegranate consumption: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2016;23(11):1103–12. doi: 10.1016/j.phymed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Kafeshani O, Sarrafzadegan N, Nouri F, Mohammadifard N. Major dietary patterns in Iranian adolescents: Isfahan Healthy Heart Program, Iran. ARYA Atheroscler. 2015;11(Suppl 1):61–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Hasler CM, Kundrat S, Wool D. Functional foods and cardiovascular disease. Curr Atheroscler Rep. 2000;2(6):467–75. doi: 10.1007/s11883-000-0045-9. [DOI] [PubMed] [Google Scholar]
- 9.Johnston C. Functional foods as modifiers of cardiovascular disease. Am J Lifestyle Med. 2009;3(1 Suppl):39S–43S. doi: 10.1177/1559827609332320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CD, Wei GX. Tea as a functional food for oral health. Nutrition. 2002;18(5):443–4. doi: 10.1016/s0899-9007(02)00763-3. [DOI] [PubMed] [Google Scholar]
- 11.Serban C, Sahebkar A, Ursoniu S, Andrica F, Banach M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2015;33(6):1119–27. doi: 10.1097/HJH.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 12.Serban C, Sahebkar A, Antal D, Ursoniu S, Banach M. Effects of supplementation with green tea catechins on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Nutrition. 2015;31(9):1061–71. doi: 10.1016/j.nut.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Pezeshki A, Safi S, Feizi A, Askari G, Karami F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int J Prev Med. 2016;7:28. doi: 10.4103/2008-7802.173051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffari-Khosravi H, Ahadi Z, Fallah Tafti M. The effect of green tea versus sour tea on insulin resistance, lipids profiles and oxidative stress in patients with type 2 diabetes mellitus: A Randomized Clinical Trial. Iran J Med Sci. 2014;39(5):424–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Asgary S, Soltani R, Zolghadr M, Keshvari M, Sarrafzadegan N. Evaluation of the effects of roselle (Hibiscus sabdariffa L.) on oxidative stress and serum levels of lipids, insulin and hs-CRP in adult patients with metabolic syndrome: A double-blind placebo-controlled clinical trial. J Complement Integr Med. 2016;13(2):175–80. doi: 10.1515/jcim-2015-0030. [DOI] [PubMed] [Google Scholar]
- 16.Aziz Z, Wong SY, Chong NJ. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J Ethnopharmacol. 2013;150(2):442–50. doi: 10.1016/j.jep.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Hadi A, Pourmasoumi M, Kafeshani M, Karimian J, Maracy MR, Entezari MH. The Effect of Green Tea and Sour Tea (Hibiscus sabdariffa L.) Supplementation on Oxidative Stress and Muscle Damage in Athletes. J Diet Suppl. 2017;14(3):346–57. doi: 10.1080/19390211.2016.1237400. [DOI] [PubMed] [Google Scholar]
- 18.Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem. 2005;16(3):144–9. doi: 10.1016/j.jnutbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Hashemipour M, Soghrati M, Malek AM, Soghrati M. Anthropometric indices associated with dyslipidemia in obese children and adolescents: A retrospective study in Isfahan. ARYA Atheroscler. 2011;7(1):31–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong X, Borrelli F, de Sa Ferreira A, Ashfaq T, Feng B. Herbal medicines for cardiovascular diseases. Evid Based Complement Alternat Med. 2014;2014:809741. doi: 10.1155/2014/809741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson MH. Combination therapy for dyslipidemia: Safety and regulatory considerations. Am J Cardiol. 2002;90(10B):50K–60K. doi: 10.1016/s0002-9149(02)02970-3. [DOI] [PubMed] [Google Scholar]
- 23.Benzie IF, Wachtel-Galor S. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd. Boca Raton, FL: CRC Press; 2011. [PubMed] [Google Scholar]
- 24.Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009;15(25):3073–85. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarmolinsky J, Gon G, Edwards P. Effect of tea on blood pressure for secondary prevention of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73(4):236–46. doi: 10.1093/nutrit/nuv001. [DOI] [PubMed] [Google Scholar]
- 26.Babu PV, Liu D. Green tea catechins and cardiovascular health: An update. Curr Med Chem. 2008;15(18):1840–50. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TL, Lin HH, Chen CC, Lin MC, Chou MC, Wang CJ. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr Res. 2007;27(3):140–5. [Google Scholar]
- 28.Frank J, George TW, Lodge JK, Rodriguez-Mateos AM, Spencer JP, Minihane AM, et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2009;139(1):58–62. doi: 10.3945/jn.108.096412. [DOI] [PubMed] [Google Scholar]
- 29.Herrera-Arellano A, Flores-Romero S, Chavez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine. 2004;11(5):375–82. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140(2):298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffari-Khosravi H, Ahadi Z, Barzegar K. The effect of green tea and sour tea on blood pressure of patients with type 2 diabetes: A randomized clinical trial. J Diet Suppl. 2013;10(2):105–15. doi: 10.3109/19390211.2013.790333. [DOI] [PubMed] [Google Scholar]
- 32.Sarr M, Ngom S, Kane MO, Wele A, Diop D, Sarr B, et al. In vitro vasorelaxation mechanisms of bioactive compounds extracted from Hibiscus sabdariffa on rat thoracic aorta. Nutr Metab (Lond) 2009;6:45. doi: 10.1186/1743-7075-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurrola-Diaz CM, Garcia-Lopez PM, Sanchez-Enriquez S, Troyo-Sanroman R, Andrade-Gonzalez I, Gomez-Leyva JF. Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine. 2010;17(7):500–5. doi: 10.1016/j.phymed.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Nantz MP, Rowe CA, Bukowski JF, Percival SS. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition. 2009;25(2):147–54. doi: 10.1016/j.nut.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: A systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24(8):823–36. doi: 10.1016/j.numecd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H. Green tea catechins and blood pressure: A systematic review and meta-analysis of randomised controlled trials. Eur J Nutr. 2014;53(6):1299–311. doi: 10.1007/s00394-014-0720-1. [DOI] [PubMed] [Google Scholar]