Natural forest recovery is an effective ecological alternative to tree planting in tropical forests under certain conditions.
Abstract
Is active restoration the best approach to achieve ecological restoration success (the return to a reference condition, that is, old-growth forest) when compared to natural regeneration in tropical forests? Our meta-analysis of 133 studies demonstrated that natural regeneration surpasses active restoration in achieving tropical forest restoration success for all three biodiversity groups (plants, birds, and invertebrates) and five measures of vegetation structure (cover, density, litter, biomass, and height) tested. Restoration success for biodiversity and vegetation structure was 34 to 56% and 19 to 56% higher in natural regeneration than in active restoration systems, respectively, after controlling for key biotic and abiotic factors (forest cover, precipitation, time elapsed since restoration started, and past disturbance). Biodiversity responses were based primarily on ecological metrics of abundance and species richness (74%), both of which take far less time to achieve restoration success than similarity and composition. This finding challenges the widely held notion that natural forest regeneration has limited conservation value and that active restoration should be the default ecological restoration strategy. The proposition that active restoration achieves greater restoration success than natural regeneration may have arisen because previous comparisons lacked controls for biotic and abiotic factors; we also did not find any difference between active restoration and natural regeneration outcomes for vegetation structure when we did not control for these factors. Future policy priorities should align the identified patterns of biophysical and ecological conditions where each or both restoration approaches are more successful, cost-effective, and compatible with socioeconomic incentives for tropical forest restoration.
INTRODUCTION
Restoration of deforested lands is a global priority spurred by ambitious international commitments (1). For example, global initiatives, such as the Bonn Challenge (2) and the New York Declaration on Forests (3), aim to restore 350 million ha of forests on degraded forest and deforested land by 2030. Regional initiatives, such as 20 × 20 (4) and AFR100 (5), aim to restore 20 million and 100 million ha, respectively, in Latin America and Africa. Within these initiatives, activities involving tree planting and soil preparation could cost between US $1,400/ha and US $34,000/ha (6, 7), an enormous barrier for scaling up forest restoration worldwide. Restoration costs vary widely according to the different methods applied, ranging from lower-cost approaches for natural regeneration to higher-cost approaches for active restoration (8–10). Natural forest regeneration is the spontaneous recovery of native tree species that colonize and establish in abandoned fields or natural disturbances; this process can also be assisted through human interventions such as fencing to control livestock grazing, weed control, and fire protection (11, 12). In contrast, active restoration requires planting of nursery-grown seedlings, direct seeding, and/or the manipulation of disturbance regimes (for example, thinning and burning) to speed up the recovery process, often at a high cost to establish structural features of the vegetation (hereafter termed vegetation structure), reassemble local species composition, and/or catalyze ecological succession (10, 13).
In general, practitioners and policymakers prefer more costly active restoration approaches over approaches based on natural regeneration (13, 14). However, few robust comparisons of ecological outcomes of natural regeneration and active restoration have been conducted. The few recent studies conducted at the local scale showed contradictory results—for example, active restoration approaches can have higher (15) or similar plant diversity (16) to natural regeneration approaches. In contrast, natural regeneration has been shown to be the most cost-effective approach for recovering biodiversity, ecological processes, and/or ecosystem services under favorable ecological conditions (8, 13, 14, 17, 18). That is, natural regrowth forests provide higher return on investment in terms of multiple ecological outcomes. However, most of this conclusion is driven by the substantially lower cost of natural regeneration relative to active restoration (6). Given these contradictory results, the limited number of past studies, and the small spatial scale at which these previous investigations were conducted, a useful alternative to quantifying the effectiveness of different restoration approaches is to conduct a global meta-analysis.
A recent global meta-analysis found no difference between active restoration and natural regeneration in terms of biodiversity recovery (19). Nonetheless, selection of the best restoration approach (natural regeneration versus active restoration) must consider a suite of biotic and abiotic factors known to affect the rate of recovery of different forest properties during restoration and regeneration, such as the amount and type of forest cover at the landscape scale, annual precipitation, and intensity of past disturbance or previous land use (10, 13, 14, 17, 18, 20). For example, comparisons of different restoration approaches in separate study areas of varying ages are plagued by confounding factors (19). Active restoration is often favored in areas where natural regeneration is hindered, such as isolated sites with extensive deforestation, low precipitation rates, and long history of intensive disturbances or land uses that led to severe soil degradation, weed infestation, or loss of the seed bank and root sprouts. Thus, understanding the success of ecological restoration (compared to a reference condition; hereafter termed restoration success) for biodiversity and vegetation structure indicators based on different restoration approaches requires that these biotic and abiotic factors be taken into account during the analysis. To fill this knowledge gap, we posed the critical question: Is natural regeneration the most beneficial approach to achieve tropical forest restoration success for biodiversity and vegetation structure?
To answer this question, we conducted, for the first time, a meta-analysis in tropical regions for biodiversity and vegetation structure controlling for biotic and abiotic factors using the most comprehensive global data set on forest restoration to date (21). The data set encompassed studies conducted in different forest ecosystems, with assessments of different taxonomic groups (mammals, birds, herpetofauna, invertebrates, and plants) and/or measures of vegetation structure (density, litter, cover, biomass, and height) in multiple sampling sites of both reference (a benchmark state) and degraded or restored systems. We grouped the large range of restoration methods into two main approaches, natural regeneration or active restoration, because larger sample sizes produce more robust results and, consequently, help find stronger global patterns. Although active restoration has often been favored despite its higher cost, we show that, after controlling for biotic and abiotic factors, natural forest regeneration can be more successful when certain conditions are met.
RESULTS
After controlling for amount of forest cover at the landscape scale, total annual precipitation, past disturbance type, and the time elapsed since restoration started, restoration success for biodiversity and vegetation structure was significantly lower in both natural regeneration and active restoration than in reference systems (from −0.39 to −0.07 and from −0.51 to −0.07, respectively) (Fig. 1). However, restoration success for both biodiversity and vegetation structure was significantly higher in natural regeneration than in active restoration systems (Fig. 1). Restoration success also varied among taxonomic groups and measures of vegetation structure. For biodiversity and vegetation structure, restoration success was 34 to 56% and 19 to 56% higher in natural regeneration than in active restoration systems, respectively (table S1). Nonetheless, restoration success was 7 to 17 % and 7 to 32% lower in natural regeneration than in reference systems, respectively (table S1). When these four factors were not controlled for, natural regeneration and active restoration systems did not differ for any measures of vegetation structure in terms of restoration success (fig. S1 and table S2). For each taxonomic group we assessed, restoration success was higher in natural regeneration than in active restoration, except for mammals (fig. S1 and table S2). Note that in our analyses, 74% of our biodiversity data were composed of two ecological metrics only, abundance and species richness.
Fig. 1. Meta-analysis controlling for the biotic and abiotic factors.
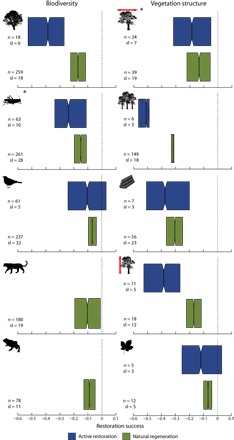
Bootstrapped mean response ratio for biodiversity (plants, invertebrates, birds, mammals, and herpetofauna) and vegetation structure (cover, density, biomass, height, and litter) in natural regeneration or active restoration systems compared with reference systems controlled for the four biotic and abiotic factors (amount of forest cover at the landscape scale, total annual precipitation, past disturbance, and the time elapsed since restoration started). n, total sample size; sl, number of study landscapes (sample size used in each resampling to avoid spatial pseudoreplication). Each box plot shows the median value (central solid line) and first and third quartile ranges (left and right outer borders of the box) of 1000 resampled (with replacement) mean response ratios. Dashed lines indicate a response ratio of 0, that is, no difference to reference systems. Notches in boxes represent 95% confidence intervals, and thus, nonoverlapping notches between boxes imply a significant difference (66). *, not controlled for forest cover (always significantly different between natural regeneration and active restoration systems). For mammals and herpetofauna, restoration success was not estimated in active restoration systems because of the small number of study landscapes.
Overall, restoration success increased with amount of forest cover, the time elapsed since restoration started, and increasing annual precipitation (figs. S2 and S3). These positive relationships occurred in naturally regenerated systems for plants and litter and in active restoration systems for cover and litter. We found few negative relationships for the other taxonomic groups and measures of vegetation structure. For example, the restoration success of vegetation structure in naturally regenerated systems increased with amount of forest cover and precipitation but decreased with the time elapsed since restoration started (fig. S2). Nonetheless, the latter relationship may be correlated with the fact that only a few studies have followed restoration over long time intervals. As expected, restoration success tended to be higher in areas subject to extensive as opposed to intensive past disturbance type (see the definition in Material and Methods) for biodiversity and vegetation structure in both natural regeneration and active restoration (figs. S2 and S3).
DISCUSSION
Our meta-analysis for tropical forests demonstrated, for the first time, that ecological restoration success is higher for natural regeneration relative to active restoration for biodiversity and vegetation structure when controlling for biotic and abiotic factors. This result runs counter to the prevailing view that active restoration should be the preferred approach for accelerating recovery of biodiversity and vegetation structure in tropical regions (15). Natural regeneration is initiated through the colonization of opportunistic and locally adapted species, resulting in a stochastic dynamic process of forest restoration that ultimately leads to higher diversity of native, locally adapted plant species than in tree planting schemes (that is, active restoration) (13, 22). Active restoration also can create a highly diverse habitat through human introduction of up to 6000 seedlings/ha (23), but tree species used in plantings often lack the full range of functional traits found in natural regrowth forests (13). In addition, most tropical forest plantings for restoration or forest plantations use relatively few species (2, 24), that is, these plantations may not be planted primarily for biodiversity outcomes. Thus, the higher plant biodiversity in naturally regenerated systems creates more habitats and resources, which provide additional sources of food, shelter, nesting, and breeding sites, to support higher animal biodiversity (13, 25).
Higher plant biodiversity in naturally regenerating forests also supports higher levels of heterogeneity in vegetation structure (13). In early successional stages, naturally regenerated systems can exhibit a patchy distribution of trees with more variable tree density (26) and slower rates of biomass accumulation per hectare (27, 28) than in active restoration systems. Nonetheless, these differences tend to diminish as ecological succession proceeds (15), especially because the time elapsed since restoration started is the main driver of forest restoration success for a range of measures of vegetation structure (29). In our data set, for vegetation structure, the time elapsed since restoration started varied between 0 and 60 years, and because time was one of the controlled factors in the analyses, the differences became increasingly evident—natural regeneration surpassed active restoration in terms of ecological restoration success for all measures of vegetation structure.
When we did not control for biotic and abiotic factors, we found no difference between active restoration and natural regeneration for all measures of vegetation structure. Meli et al. (19) found a similar result for biodiversity, but our studies differ greatly in four main aspects. First, we used analytical methods that controlled for the variation in biotic and abiotic factors, which can greatly affect the study outcome, as we have shown here. Second, the global meta-analysis completed by Meli et al. (19) comprised both temperate and tropical ecosystems. Here, we focused only on tropical forest ecosystems to reduce noise created by combining studies from these different ecosystems, despite the available data by Crouzeilles et al. (21). Restoration of temperate and tropical forests is affected by different biotic and abiotic factors. Third, we found that differences in restoration success between active restoration and natural regeneration differ among taxonomic groups, whereas Meli et al. (19) focused on the analysis for overall flora and fauna abundance, diversity, and biogeochemical functions. Fourth, our analysis included measures of vegetation structure that were omitted in previous studies. Thus, our findings indicate that amount of forest cover at the landscape scale, total annual precipitation, past disturbance type, and/or the time elapsed since restoration started must be controlled for when comparing restoration approaches to avoid misleading results. For example, the meta-analysis of Bonner et al. (27), which did not control for key factors, showed higher biomass accumulation in active restoration systems, whereas local studies that controlled for past disturbance type showed higher long-term biomass accumulation in natural regeneration (30, 31).
Our general results accord with previous studies that have found a positive relationship between biodiversity or vegetation structure and forest cover (32), precipitation (18), the time elapsed since restoration started (29, 33), and less-intensive past disturbances (20). Nonetheless, these studies did not analyze all these four biotic and abiotic factors together nor did they encompass both natural regeneration and active restoration systems. For example, less than 10% of the studies selected for use in a recent meta-analysis of restoration approaches (19) included comparisons between natural regeneration and active restoration systems within the same study areas. Thus, biodiversity and vegetation structure are related to these four biotic and abiotic factors in both types of restored systems but are affected to different extents.
The recovering assemblages in naturally regenerated and actively restored systems can have complementary values of biodiversity (34, 35). Complementarity of assemblages in adjacent natural regeneration and active restoration systems might be key to reaching similar values of biodiversity to those found in reference systems. This hypothesis remains to be tested because only 11 studies quantified biodiversity in both systems, and only a few of them present information on the species pool for each system. Temporal trends of community assembly during natural regeneration of forests are poorly studied (36, 37), and to date, only four studies have experimentally compared natural regeneration with active restoration (9, 15, 16, 38). Future systematic reviews should also compare biodiversity assemblages of naturally regenerated and active restoration systems using more sensitive ecological metrics of community change than were applied in this investigation, such as similarity indices (39). Note that 74% of our biodiversity data were composed of abundance and richness ecological metrics, both of which take orders of magnitude of time less to achieve restoration success than do metrics of species similarity and composition (29, 39, 40). Thus, biodiversity can be more depleted in natural regeneration and active restoration systems than we report, which highlights the need for restoration practitioners and stakeholders to implement both kinds of restoration and enrich the species pool to improve restoration success within landscapes.
In summary, our study shows that (i) lower-cost natural regeneration surpasses active restoration in achieving tropical forest restoration success for biodiversity and vegetation structure, (ii) the extent of difference in terms of restoration success between active restoration and natural regeneration also differs among taxonomic groups and measures of vegetation structure, and (iii) biotic and abiotic factors must be controlled when comparing restoration approaches to avoid misleading results. Our findings should not be applied uncritically because there will be areas that are unsuitable for natural regeneration and where active restoration is the only suitable approach. In addition, mixing both restoration approaches might be key to attaining a richer species pool. Nonetheless, our findings support the view that restoration strategies should favor natural regeneration approaches when the social and ecological conditions are favorable and when biodiversity conservation is a high restoration priority. The mistaken notion that active restoration hastens biodiversity recovery compared to natural regeneration may have arisen because of a failure to control biotic and abiotic factors in previous analyses and the short-term monitoring period for forest restoration. One factor that was not controlled for in this study was the socioeconomic context where natural regeneration occurred (18). Socioeconomic factors aligned with biotic and abiotic factors determine where natural regeneration occurs (13). One of the major international and national policy priorities for the upcoming years is to align the identified patterns of biophysical and ecological conditions where each or both restoration approaches are more successful, cost-effective, and compatible with socioeconomic incentives and desired outcomes. Both approaches are urgently needed to achieve ambitious global forest restoration targets.
MATERIALS AND METHODS
Many of the materials and methods related to the data set and meta-analysis subsections are similar to those used by Crouzeilles et al. (29).
Data set
We used a global data set on forest restoration (21) to assess the most successful approach (natural regeneration versus active restoration) for restoring biodiversity (plants, invertebrates, birds, mammals, and herpetofauna) and vegetation structure (cover, density, biomass, height, and litter) in tropical forests. In this data set (21), reference systems were defined as old-growth or less-disturbed forests (on the basis of the definition presented in a given selected study). Restored systems were defined as areas completely or partially cleared and that regenerated after disturbance, that is, selectively logged or initial or secondary forests. From this data set, we selected studies conducted in tropical regions that contained quantitative comparisons of biodiversity and/or vegetation structure in multiple sampling sites of both reference and restored systems. Tropical regions harbor the greatest number of restoration projects conducted with different methods (41). We identified a total of 133 studies (table S3), which were spread across 115 study landscapes in five biogeographic tropic realms. In total, they contained 1728 quantitative comparisons between reference and either natural regeneration or active restoration systems. Details on the data collection and extraction can be found in the study by Crouzeilles et al. (21).
Regarding measures of vegetation structure, density refers to the number of individuals per unit area, litter refers to the amount of leaf litter on the substrate, cover refers to the area covered by vegetation (measured in three forest strata—floor, understory, and canopy), biomass refers to the amount of below- and above-ground biomass produced (for example, basal area), and height refers to the vegetation height above ground (29). We defined natural regeneration as forest regrowth following land abandonment, selective logging (21% of our data set for natural regeneration), or assisted recovery of native tree species through human interventions, such as fencing, to control livestock grazing, weed control, and fire protection (11, 12). Active restoration entailed manipulating disturbance regimes through the use of thinning and burning, the establishment of nursery-grown seedlings, direct seeding, or plantations of tree species (11, 12).
Meta-analysis
We defined ecological restoration as the process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed (42). We compared the restoration success of each taxonomic group and each measure of vegetation structure in natural regeneration and active restoration systems after controlling for four biotic and abiotic factors (amount of forest cover at the landscape scale, total annual precipitation, past disturbance type, and the time elapsed since restoration started). To do so, we used the response ratio [ln(x/y)] to quantify a standardized mean effect size (43) for a comparison of a mean value of a quantified biodiversity or vegetation structure variable between natural regeneration or active restoration (x) and reference (y) systems within the same study (29, 39, 44). Negative effect size or response ratio means lower values of biodiversity or vegetation structure in the natural regeneration/active restoration systems than in the reference system, whereas the opposite holds for a positive effect size. Values around zero are the desired outcome of restoration success, that is, natural regeneration/active restoration systems have reached a benchmark or reference state. The standardized mean effect size is a useful measure to compare two natural groups with respect to some quantitative and normally distributed dependent variable (45). Nearly half of all published meta-analyses in ecology have used the response ratio metric (46, 47). The advantage of response ratio compared to other weighted metrics is that it needs only a raw mean value for the dependent variable for two groups, whereas other weighted metrics also need the variance (or SDs) and sample sizes for two groups (48). Thus, we used the response ratio as the standardized mean effect size because we were interested in obtaining as much information as possible from the available studies to perform separate analyses for each taxonomic group and each measure of vegetation structure. This would not have been possible using a weighted response ratio because many studies did not provide information on variance or SDs.
For each comparison of either a biodiversity response or a measure of vegetation structure within the same assessment, we calculated a standardized mean effect size between reference and restored systems. Measures of either biodiversity or vegetation structure in both restored systems (natural regeneration and active restoration) are usually lower when compared to reference systems. For measures of either biodiversity response or vegetation structure that are a priori expected to be higher than in restored systems, we inverted the sign of data following previous studies (29, 44, 49). The sign of the following measures was inverted: (i) openness, (ii) introduced species, (iii) grasses, (iv) exotic species, (v) herbs, (vi) open-habitat species, (vii) gap species, (viii) trees of diameter at breast height <10 cm, and (ix) bare ground percentage (29).
A study landscape [based on the geographic coordinates reported by the selected studies in the data set of Crouzeilles et al. (21)] could have multiple standardized mean effect sizes due to, for example, multiple studies in the same study landscape or multiple measures for the same taxonomic group (for example, abundance, richness, diversity, and/or similarity) [for further details, see the study by Crouzeilles and Curran (32)]. Thus, to avoid spatial pseudoreplication, we resampled (with replacement) our data set (1000 bootstraps with replacement) by study landscape. We used only one comparison of each taxonomic group or measure of vegetation structure per study landscape at each resample (29, 39, 49). Thus, for each bootstrap procedure, we calculated the mean response ratio of the resampled standardized mean effect sizes, and after the 1000 bootstraps, we generated the median response ratio (that is, restoration success) and 95% confidence interval. Outliers were removed to achieve normally distributed residuals, which were checked by plotting residuals (50).
Meta-analyses may suffer from publication bias, which is the probability that significant results are more likely to be published than nonsignificant results (48). To test for publication bias, data on both study sample size and associated variance (or SDs) are required. As stated above, we selected some studies that did not report variance values, so it was not possible to evaluate publication bias. However, we believe that publication bias is not likely to be a problem in our data set because there are many studies reporting unsuccessful restoration outcomes (51) and approximately 45% of our data came from a review (49) that tested and found a low influence of publication bias.
Control of biotic and abiotic factors
Differences between restoration success in natural regeneration and that in active restoration systems can be driven by the following biotic and abiotic factors and their interaction: (i) amount of forest cover at the landscape scale, (ii) total annual precipitation, (iii) past disturbance type, and (iv) the time elapsed since restoration started (14, 17, 18, 20, 39). These factors are recognized as important drivers of the rate and quality of forest restoration and regeneration (14). The amount of forest cover in the landscape is a key landscape feature associated with forest restoration success (32) because it may facilitate seed dispersal, colonization, and conservation of wildlife populations (52–54). High annual precipitation promotes tree growth and reduces fire frequency (55, 56), which are key to determine rates of aboveground biomass recovery in lowland forests of Latin America (18). Past disturbance can reduce the potential for natural regeneration (57) and strongly influence restoration success (20, 39, 58). The time elapsed since restoration started is a major driver of forest restoration success, especially for measures of vegetation structure (29); hence, older restored systems (natural regeneration and active restoration systems) exhibit levels of biodiversity or vegetation structure more similar to reference systems than to younger restored systems.
We accounted for the variation in each of these four factors (hereafter termed controlled) by comparing only resampled sub–data sets (that is, at each of 1000 bootstrap procedures) of natural regeneration and active restoration systems that did not differ (P < 0.05) within each of these four biotic and abiotic factors. That is, we first selected two sub–data sets, one for natural regeneration and the other for active restoration, which considered only one data per study landscape. Then, we checked at each resample if there was a significant difference between both sub–data sets (natural regeneration and active restoration) for each of the four biotic and abiotic factors. If there was a significant difference for, at least, one of the four factors, then the resample procedure started again, that is, selecting two new sub–data sets. However, if there was no difference between both sub–data sets for the four biotic and abiotic factors, then we used these sub–data sets to compare the restoration success between natural regeneration and active restoration. We used one-way analysis of variance (ANOVA) for continuous factors (forest cover, the time elapsed since restoration started, and precipitation), and χ2 for categorical factors (past disturbance type). We did not conduct analysis for mammals and herpetofauna because restoration success was not estimated in active restoration systems because of the small number of study landscapes.
To gather information on amount of forest cover, past disturbance, and time elapsed since restoration started within each study landscape, we used the available information from the forest restoration data set of Crouzeilles et al. (21). In this data set, the information for forest cover at the landscape scale provides the percentage of contiguous forest cover (1-km pixels with >60% forest cover only) within three different landscape sizes (that is, buffer sizes of 5-, 10-, and 100-km radius). These landscape sizes are based on a previous global meta-analysis that was conducted to identify the scale of effect (that is, best landscape size) of forest cover on restoration success for biodiversity and vegetation structure to avoid arbitrary decisions in this respect (14). The landscape sizes that most plausibly explained forest restoration success for each taxonomic group and each measure of vegetation structure were 100-km radius for litter, 10-km radius for plants, and 5-km radius for mammals, birds, invertebrates, herpetofauna, biomass, density, cover, and height. See the study by Crouzeilles and Curran (32) for further details about the (i) database used for mapping forest areas (1-km-resolution land cover database) (59), (ii) procedures to calculate the amount of contiguous forest cover within each landscape, and (iii) scale of effect (that is, best landscape size) of continuous forest cover on restoration success for each taxonomic group and measure of vegetation structure. Past disturbance was classified into two types according to previous studies (20, 39, 58): (i) extensive transformation or occupation—areas that were slightly transformed and remained under occupation for a short or long term (for example, partially cleared forests, agroforestry, and shaded plantations)—and (ii) intensive transformation or occupation—areas that were heavily transformed and remained under occupation for a short or long term (for example, clear cut, plantation, pasture, and agriculture). We did not further divide extensive or intensive land use types into other classes that consider the impact of both land occupation and transformation because it would have reduced the sample size for each class. The time elapsed since restoration started was measured in years (29, 39, 60–62), that is, a higher value means a longer restoration time. Because of the lack of information on the total amount of precipitation per year for the selected studies in the forest restoration data set of Crouzeilles et al. (21), we quantified the total annual precipitation within the same landscape size used to measure forest cover (buffer sizes of 5-, 10-, and 100-km radius) for each taxonomic group and measure of vegetation structure. This analysis was carried out in the R. 212 environment (63) using the R package “raster” (64). The total annual precipitation was calculated as the mean value from all pixels that were within the specified landscape size. Precipitation data were downloaded from the WorldClim database (www.worldclim.org/current), generated by the spatial interpolation of climate surfaces measured at weather stations from 1950 to 2000 (65).
Regression and box plot analyses
We quantified the relationship between mean effect size (at each resampled bootstrap) and each biotic or abiotic factor independently, that is, we controlled three factors and quantified the relationship with a specific one. Some of these analyses were not performed for biodiversity and vegetation structure because of either the small number of study landscapes (fig. S2) or the lack of data (figs. S2 and S3). All analyses used in this study were performed in the R. 212 environment (63).
Supplementary Material
Acknowledgments
Funding: Support was provided by the Australian Research Council Laureate Fellowship to D.B.L. R.L.C. was supported by a fellowship from the Coordination for the Improvement of Higher Education Personnel of Brazil for a research grant (#88881.064976/2014-01). This work was also supported by the PARTNERS Research Coordination Network (grant #DEB1313788) from the NSF Coupled Natural and Human Systems Program. Author contributions: R.C. conceived the idea, designed the study, and led the writing. R.C. conducted the analysis, but A.I. collaborated on it. R.C., M.S.F., and L.M. collected the data. All the authors contributed to improve the study design, the idea, and the writing of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The database and R code used in the analyses are available in the Dryad Digital Repository (doi: 10.5061/dryad.5428h). Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/11/e1701345/DC1
fig. S1. Meta-analysis without controlling for the biotic and abiotic factors.
fig. S2. Regression and box plot analyses for biodiversity.
fig. S3. Regression and box plot analyses for vegetation structure.
table S1. Median values of response ratios for active restoration and natural regeneration systems compared with reference systems controlled for the four biotic and abiotic factors (forest cover at the landscape scale, total annual precipitation, past disturbance, and the time elapsed since restoration started) and percentage of enhancement of biodiversity and vegetation structure in natural regeneration with respect to active restoration systems.
table S2. Median values of response ratios for active restoration and natural regeneration systems compared with reference systems without controlling for the four biotic and abiotic factors (forest cover at the landscape scale, total annual precipitation, past disturbance, and the time elapsed since restoration started) and percentage of enhancement of biodiversity and vegetation structure in natural regeneration with respect to active restoration systems.
table S3. Selected papers with data available for the meta-analysis.
REFERENCES AND NOTES
- 1.Menz M. H. M., Dixon K. W., Hobbs R. J., Hurdles and opportunities for landscape-scale restoration. Science 339, 526–527 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Bonn Challenge, www.bonnchallenge.org.
- 3.D. Conway, P. Keenylside, S. Roe, C. Streck, G. Vargas-Victoria, T. Varns, Progress on the New York declaration on forests – An assessment framework and initial report (2015).
- 4.Initiative 20x20, www.wri.org/our-work/project/initiative-20x20/about-initiative-20x20#project-tabs.
- 5.African Forest Landscape Restoration Initiative (AFR100), www.wri.org/our-work/project/african-restoration-100.
- 6.C. P. Catterall, D. A. Harrison, Rainforest Restoration Activities in Australia’s Tropics and Subtropics (Rainforest CRC, 2006). [Google Scholar]
- 7.FAO/RECOFTC, Forest Landscape Restoration for Asia-Pacific Forests (FAO/RECOFTC, 2016). [Google Scholar]
- 8.Chazdon R. L., Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 320, 1458–1460 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Birch J. C., Newton A. C., Aquino C. A., Cantarello E., Echeverría C., Kitzberger T., Schiappacasse I., Garavito N. T., Cost-effectiveness of dryland forest restoration evaluated by spatial analysis of ecosystem services. Proc. Natl. Acad. Sci. U.S.A. 107, 21925–21930 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holl K. D., Aide T. M., When and where to actively restore ecosystems? For. Ecol. Manag. 261, 1558–1563 (2011). [Google Scholar]
- 11.Shono K., Cadaweng E. A., Durst P. B., Application of assisted natural regeneration to restore degraded tropical forestlands. Restor. Ecol. 15, 620–626 (2007). [Google Scholar]
- 12.Zahawi R. A., Reid J. L., Holl K. D., Hidden costs of passive restoration. Restor. Ecol. 22, 284–287 (2014). [Google Scholar]
- 13.Chazdon R. L., Guariguata M. R., Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 48, 716–730 (2016). [Google Scholar]
- 14.R. L. Chazdon, Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation (University of Chicago Press, 2014). [Google Scholar]
- 15.Shoo L. P., Freebody K., Kanowski J., Catterall C. P., Slow recovery of tropical old-field rainforest regrowth and the value and limitations of active restoration. Conserv. Biol. 30, 121–132 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Gilman A. C., Letcher S. G., Fincher R. M., Perez A. I., Madell T. W., Finkelstein A. L., Corrales-Araya F., Recovery of floristic diversity and basal area in natural forest regeneration and planted plots in a Costa Rican wet forest. Biotropica 48, 798–808 (2016). [Google Scholar]
- 17.D. Lamb, Large-scale Forest Restoration (Routledge, 2014). [Google Scholar]
- 18.Poorter L., Bongers F., Aide T. M., Almeyda Zambrano A. M., Balvanera P., Becknell J. M., Boukili V., Brancalion P. H. S., Broadbent E. N., Chazdon R. L., Craven D., de Almeida-Cortez J. S., Cabral G. A. L., de Jong B. H. J., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Espírito-Santo M. M., Fandino M. C., César R. G., Hall J. S., Hernandez-Stefanoni J. L., Jakovac C. C., Junqueira A. B., Kennard D., Letcher S. G., Licona J.-C., Lohbeck M., Marín-Spiotta E., Martínez-Ramos M., Massoca P., Meave J. A., Mesquita R., Mora F., Muñoz R., Muscarella R., Nunes Y. R. F., Ochoa-Gaona S., de Oliveira A. A., Orihuela-Belmonte E., Peña-Claros M., Pérez-García E. A., Piotto D., Powers J. S., Rodríguez-Velázquez J., Romero-Pérez I. E., Ruíz J., Saldarriaga J. G., Sanchez-Azofeifa A., Schwartz N. B., Steininger M. K., Swenson N. G., Toledo M., Uriarte M., van Breugel M., van der Wal H., Veloso M. D. M., Vester H. F. M., Vicentini A., Vieira I. C. G., Bentos T. V., Williamson G. B., Rozendaal D. M. A., Biomass resilience of Neotropical secondary forests. Nature 530, 211–214 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Meli P., Holl K. D., Rey Benayas J. M., Jones H. P., Jones P. C., Montoya D., Moreno Mateos D., A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLOS ONE 12, e0171368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latawiec A. E., Crouzeilles R., Brancalion P. H. S., Rodrigues R. R., Sansevero J. B., Santos J. S., Mills M., Nave A. G., Strassburg B. B., Natural regeneration and biodiversity: A global meta-analysis and implications for spatial planning. Biotropica 48, 844–855 (2016). [Google Scholar]
- 21.Crouzeilles R., Ferreira M. S., Curran M., Forest restoration: A global dataset for biodiversity and vegetation structure. Ecology 97, 2167 (2016). [DOI] [PubMed] [Google Scholar]
- 22.R. L. Chazdon, in Tropical Forest Succession, W. Carson, S. A. Schnitzer, Eds. (Wiley-Blackwell Publishing, 2008), pp. 384–408. [Google Scholar]
- 23.Rodrigues A. S. L., Ewers R. M., Parry L., Souza C. Jr, Veríssimo A., Balmford A., Boom-and-bust development patterns across the Amazon deforestation frontier. Science 324, 1435–1437 (2009). [DOI] [PubMed] [Google Scholar]
- 24.S. Minnemeyer, L. Laestadius, N. Sizer, C. Saint-Laurent, P. Potapov, A world of opportunity (World Resource Institute, 2011); www.wri.org/sites/default/files/world_of_opportunity_brochure_2011-09.pdf.
- 25.Chazdon R. L., Peres C. A., Dent D., Sheil D., Lugo A. E., Lamb D., Stork N. E., Miller S. E., The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Scervino R. P., Torezan J. M. D., Factors affecting the genesis of vegetation patches in anthropogenic pastures in the Atlantic forest domain in Brazil. Plant Ecol. Divers. 8, 475–482 (2015). [Google Scholar]
- 27.Bonner M. T. L., Schmidt S., Shoo L. P., A meta-analytical global comparison of aboveground biomass accumulation between tropical secondary forests and monoculture plantations. For. Ecol. Manage. 291, 73–86 (2013). [Google Scholar]
- 28.Holl K. D., Zahawi R. A., Factors explaining variability in woody above-ground biomass accumulation in restored tropical forest. For. Ecol. Manage. 319, 36–43 (2014). [Google Scholar]
- 29.Crouzeilles R., Curran M., Ferreira M. S., Lindenmayer D. B., Grelle C. E. V., Rey Benayas J. M., A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 7, 11666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan C. F., Farnworth E. G., Natural vs. plantation forest: A case study of land reclamation strategies for the humid tropics. Environ. Manage. 6, 485–492 (1982). [Google Scholar]
- 31.Han S. R., Woo S. Y., Lee D. K., Carbon storage and flux in aboveground vegetation and soil of sixty-year old secondary natural forest and large leafed mahogany (Swietenia macrophylla King) plantation in Mt. Makiling, Philippines. Asia Life Sci. 19, 357–372 (2010). [Google Scholar]
- 32.Crouzeilles R., Curran M., Which landscape size best predicts the influence of forest cover on restoration success? A global meta-analysis on the scale of effect. J. Appl. Ecol. 53, 440–448 (2016). [Google Scholar]
- 33.Arroyo-Rodríguez V., Melo F. P. L., Martínez-Ramos M., Bongers F., Chazdon R. L., Meave J. A., Norden N., Santos B. A., Leal I. R., Tabarelli M., Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 92, 326–340 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Cunningham R. B., Lindenmayer D. B., Crane M., Michael D., MacGregor C., Reptile and arboreal marsupial response to replanted vegetation in agricultural landscapes. Ecol. Appl. 17, 609–619 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Lindenmayer D. B., Northrop-Mackie A. R., Montague-Drake R., Crane M., Michael D., Okada S., Gibbons P., Not all kinds of revegetation are created equal: Revegetation type influences bird assemblages in threatened australian woodland ecosystems. PLOS ONE 7, e34527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norden N., Chazdon R. L., Chao A., Jiang Y.-H., Vílchez-Alvarado B., Resilience of tropical rain forests: Tree community reassembly in secondary forests. Ecol. Lett. 12, 385–394 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Norden N., Angarita H. A., Bongers F., Martínez-Ramos M., Granzow-de la Cerda I., van Breugel M., Lebrija-Trejos E., Meave J. A., Vandermeer J., Williamson G. B., Finegan B., Mesquita R., Chazdon R. L., Successional dynamics in Neotropical forests are as uncertain as they are predictable. Proc. Natl. Acad. Sci. U.S.A. 112, 8013–8018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans M. C., Carwardine J., Fensham R. J., Butler D. W., Wilson K. A., Possingham H. P., Martin T. G., Carbon farming via assisted natural regeneration as a cost-effective mechanism for restoring biodiversity in agricultural landscapes. Environ. Sci. Policy 50, 114–129 (2015). [Google Scholar]
- 39.Curran M., Hellweg S., Beck J., Is there any empirical support for biodiversity offset policy? Ecol. Appl. 24, 617–632 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Voldoire A., Sanchez-Gomez E., Salas y Mélia D., Decharme B., Cassou C., Sénési S., Valcke S., Beau I., Alias A., Chevallier M., Déqué M., Deshayes J., Douville H., Fernandez E., Madec G., Maisonnave E., Moine M.-P., Planton S., Saint-Martin D., Szopa S., Tyteca S., Alkama R., Belamari S., Braun A., Coquart L., Chauvin F., The CNRM-CM5.1 global climate model: Description and basic evaluation. Clim. Dyn. 40, 2091–2121 (2013). [Google Scholar]
- 41.Rodrigues R. R., Gandolfi S., Nave A. G., Aronson J., Barreto T. E., Vidal C. Y., Brancalion P. H. S., Large-scale ecological restoration of high-diversity tropical forests in SE Brazil. For. Ecol. Manage. 261, 1605–1613 (2011). [Google Scholar]
- 42.A. Clewell, J. Aronson, K. Winterhalder, The SER International Primer on Ecological Restoration (Society for Ecological Restoration International Science & Policy Working Group, 2004). [Google Scholar]
- 43.Hedges L. V., Gurevitch J., Curtis P. S., The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999). [Google Scholar]
- 44.Rey Benayas J. M., Newton A. C., Diaz A., Bullock J. M., Enhancement of biodiversity and ecosystem services by ecological restoration: A meta-analysis. Science 325, 1121–1124 (2009). [DOI] [PubMed] [Google Scholar]
- 45.M. Borenstein, L. V. Hedges, J. P. T. Higgins, H. R. Rothstein, Introduction to Meta-Analysis (John Wiley & Sons Ltd, 2009), vol. 1999. [Google Scholar]
- 46.Nakagawa S., Santos E. S. A., Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274 (2012). [Google Scholar]
- 47.Koricheva J., Gurevitch J., Uses and misuses of meta-analysis in plant ecology. J. Ecol. 102, 828–844 (2014). [Google Scholar]
- 48.Viechtbauer W., Conducting meta-analysis in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010). [Google Scholar]
- 49.Gibson L., Lee T. M., Koh L. P., Brook B. W., Gardner T. A., Barlow J., Peres C. A., Bradshaw C. J. A., Laurance W. F., Lovejoy T. E., Sodhi N. S., Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 50.M. J. Crawley, The R Book (John Wiley & Sons Ltd, 2007). [Google Scholar]
- 51.Suding K. N., Toward an era of restoration in ecology: Successes, failures, and opportunities ahead. Annu. Rev. Ecol. Evol. Syst. 42, 465–487 (2011). [Google Scholar]
- 52.Thomlinson J. R., Serrano M. I., Lopez T. M., Aide T. M., Zimmerman J. K., Land-use dynamics in a post-agricultural Puerto Rican landscape (1936–1988). Biotropica 28, 525–536 (1996). [Google Scholar]
- 53.Helmer E. H., Brandeis T. J., Lugo A. E., Kennaway T., Factors influencing spatial pattern in tropical forest clearance and stand age: Implications for carbon storage and species diversity. J. Geophys. Res. 113, G02S04 (2008). [Google Scholar]
- 54.Crk T., Uriarte M., Corsi F., Flynn D., Forest recovery in a tropical landscape: What is the relative importance of biophysical, socioeconomic, and landscape variables? Landsc. Ecol. 24, 629–642 (2009). [Google Scholar]
- 55.Daly C., Helmer E. H., Quiñones M., Mapping the climate of Puerto Rico, Vieques and Culebra. Int. J. Climatol. 23, 1359–1381 (2003). [Google Scholar]
- 56.T. J. Brandeis, E. H. Helmer, S. N. Oswalt, The Status of Puerto Rico’s Forests, 2003 (U.S. Department of Agriculture Forest Service, Southern Research Station, 2007). [Google Scholar]
- 57.R. B. Waide, A. E. Lugo, A research perspective on disturbance and recovery of a tropical montane forest, in Tropical Forests in Transition, J. G. Goldammer, Ed. (Birkhäuser, 1992), pp. 173–189. [Google Scholar]
- 58.Dent D. H., Wright S. J., The future of tropical species in secondary forests: A quantitative review. Biol. Conserv. 142, 2833–2843 (2009). [Google Scholar]
- 59.Tuanmu M.-N., Jetz W., A global 1-km consensus land-cover product for biodiversity and ecosystem modelling. Glob. Ecol. Biogeogr. 23, 1031–1045 (2014). [Google Scholar]
- 60.Dunn R. R., Recovery of faunal communities during tropical forest regeneration. Conserv. Biol. 18, 302–309 (2004). [Google Scholar]
- 61.Martin P. A., Newton A. C., Bullock J. M., Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. R. Soc. B 280, 20132236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole L. E. S., Bhagwat S. A., Willis K. J., Recovery and resilience of tropical forests after disturbance. Nat. Commun. 5, 3906 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.R Core Team, R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2010).
- 64.R. J. Hijmans, J. van Etten, J. Cheng, M. Mattiuzzi, M. Sumner, J. A. Greenberg, O. P. Lamigueiro, A. Bevan, E. B. Racine, A. Shortridge, Package “raster” (2016); http://cran.r-project.org/package=raster.
- 65.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 66.Krzywinski M., Altman N., Visualizing samples with box plots. Nat. Methods 11, 119–120 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/11/e1701345/DC1
fig. S1. Meta-analysis without controlling for the biotic and abiotic factors.
fig. S2. Regression and box plot analyses for biodiversity.
fig. S3. Regression and box plot analyses for vegetation structure.
table S1. Median values of response ratios for active restoration and natural regeneration systems compared with reference systems controlled for the four biotic and abiotic factors (forest cover at the landscape scale, total annual precipitation, past disturbance, and the time elapsed since restoration started) and percentage of enhancement of biodiversity and vegetation structure in natural regeneration with respect to active restoration systems.
table S2. Median values of response ratios for active restoration and natural regeneration systems compared with reference systems without controlling for the four biotic and abiotic factors (forest cover at the landscape scale, total annual precipitation, past disturbance, and the time elapsed since restoration started) and percentage of enhancement of biodiversity and vegetation structure in natural regeneration with respect to active restoration systems.
table S3. Selected papers with data available for the meta-analysis.


