Abstract
Background
Patient-centered healthcare, where we design and deliver care to address the needs and preferences of patients, represents an important paradigm shift. Patient reported outcomes (PROs) are critical to capture the patient voice, understand how illness and treatments affect people, and establish how well services and treatments address what matters most to patients.
Objective
Originally developed for use in research, PROs are now used to monitor individuals and populations, manage care, evaluate services and providers, and inform policy. However, moving PROs beyond research settings incurs considerable methodological, organizational, technological, and ethical considerations. National collaborative networks of researchers, clinicians, patients, and other stakeholders can address these challenges by coordinating development, creating standards for use, sharing costs and delivery platforms, and improving widespread uptake of core sets of measures to better inform healthcare decisions and improve outcomes.
Discussion
We introduce eight papers from researchers, clinicians, patients and decision-makers who participated in deliberations around creating a national network to accelerate the application and harmonized use of PROs in Canada. They offer a snap shot of the strategies pioneers and innovative thinkers are using to integrate the patient voice into comprehensive care, research, and health policy planning of chronic diseases.
Keywords: patient reported outcomes, patient-centered healthcare, healthcare policy
“He who studies medicine without books sails an uncharted sea, but he who studies medicine without patients does not go to sea at all.”
Sir William Osler [1]
1.0 Introduction
We are living at a time of unprecedented efforts to reform healthcare. Patient-centered models, where healthcare is specifically designed and delivered to address the needs, preferences and values of patients, represent a paradigm shift from antiquated systems that evolved over time and focused largely on provider needs. As healthcare costs threaten the economic stability of individuals, families, and society at large, there are growing calls for providers and systems to demonstrate that they improve the health and wellbeing of the populations they serve. Patient-centered models also place wider accountability for not only ensuring the quality of care, but also for the experiences of patients receiving care, and for achieving outcomes that are meaningful to patients.
Multiple events are driving healthcare reform at an unprecedented pace. First, the fundamental recognition of the mismatch between the health people desire and how healthcare is delivered has led to growing dissatisfaction. Whereas traditional metrics of healthcare describe access to and the effectiveness of services (i.e., activities typically captured in administrative datasets), the focus is shifting to ensuring that treatment results in patient-valued outcomes such as quality of life. Yet, as the population ages and people live for longer periods with chronic disease, resources for healthcare services are contracting. Hence, there is growing pressure to demonstrate the cost effectiveness of treatments and services, and to maximize gains achieved on a population level. Reimbursement is rapidly moving toward value-based and data-driven models where providers and systems must demonstrate satisfaction and meaningful outcomes from the perspective of patients. A growing number of regulatory agencies such as the U.S. Food and Drug Administration and the European Medicines Agency require that patient-reported outcomes (PROs) be included when evaluating the benefit of medical treatments and devices. If the overall vision of healthcare reform is to move toward care that truly revolves around the needs of patients, then it is essential that we are able to assess how well our treatments, providers, and delivery systems are aligned with this goal.
While PROs have been used widely in clinical research for decades, their broader use as part of patient management, quality assurance, population monitoring, and policymaking is relatively new. As with all transitions, there have been some early wins, unanticipated challenges, and unforeseen consequences as we use PRO to meet a broader range of stakeholder needs. Collecting a harmonized set of reusable data at the individual level, longitudinally, and across settings is disruptive and costly. Some providers are not yet convinced of the validity or value of PRO data, in part stemming from concerns that simple measures cannot adequately reflect the complex outcomes associated with chronic illness. Another challenge is knowing where to start. The 2015 Institute of Medicine report, Vital Signs: Core Metrics for Health and Healthcare Services, reflects the latest efforts in the U.S. to begin identifying an optimal set of measures from amongst the thousands now available [2]. And while technology can help reduce the burden associated with data collection that falls on already overextended providers and systems, to ensure data are reusable to a broad range of stakeholders, they must be collected in a manner that allows for ongoing aggregation and analysis with results available in real time. Because stakeholders represent diverse groups and disciplines, it is challenging to reach consensus on how to best advance PRO development and use. In short, there is little question that the widespread collection of valid, reliable PRO data that can be reused by stakeholders with diverse needs faces many challenges.
It was against this backdrop that we saw an opportunity to bring together innovative thinkers with common interests to create a national collaboration to advance PRO use and development. Within such a network, members could work together to help bring the patient perspective to the center of healthcare reform where it belongs. However, we recognized in order for a network to thrive in a country as geographically and culturally diverse as Canada, it was essential to engage stakeholders from the beginning to identify and prioritize needs, build relationships and partnerships, and create a realistic, feasible action plan to accelerate PRO development and use in Canada. While previous work has presented pockets of success and challenges, this forum provided a unique opportunity to engage an inclusive and representative group of stakeholders to identify, together, promising solutions to accelerate the application of PROs. National collaborative networks of researchers, clinicians, patients, and other stakeholders can address these challenges by coordinating the development of PROs, creating standards for use, sharing costs and delivery platforms, and improving widespread uptake of core sets of measures to better inform healthcare decisions and improve outcomes. Building on the current best evidence of PRO science, chronic disease management, e-health technology, organizational and behavioral change theories, and modern approaches to address ethical issues, participants created a roadmap for advancing the application of PROs highlighting the required conditions for success. Core aspects of this roadmap are presented in the eight papers in this special issue.
2.0 Montreal Accord to Accelerate and Harmonize Patient-Reported Outcomes Use
In November 2013, we brought key stakeholders together in Montreal, Quebec to deliberate on needs, priorities, strategic partnerships and resources necessary to create a sustainable national PRO collaborative network. Over 50 patients, patient advocates, researchers, clinicians, policymakers, research funders, and regulatory and public health representatives participated in the workshop over 1.5 days. We began with expert presentations from PRO methodologists and users on past, current, and future directions of PRO development, applications, and research. Presenters provided rich examples from their work and that of others where PRO data were used to better manage patients, identify strengths and gaps in existing services, improve the quality of care, monitor public health, and aid in policymaking. Following the presentations, interactive panel discussions were used to facilitate discussions with all participants.
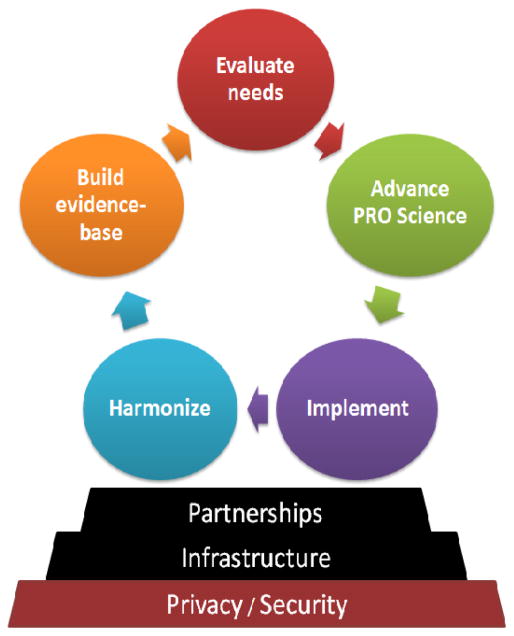
In the afternoon and following morning, small group break-out sessions were held using the nominal group consensus approach. Our goal was to stimulate discussion around: 1) end-users and their needs to successfully apply PROs to meet their objectives; 2) PRO implementation methods across multiple users, platforms, settings and regions; 3) strategic national and international partnerships and existing infrastructure; and 4) core network activities to support research and implementation of PROs. Within the small groups, a SWOT (strengths, weaknesses, opportunities and threats) analysis was conducted to identify priorities, challenges, potential solutions, and to generate goals to move a national collaboration forward. Priorities were then ranked using dotmocracy and results were reported back to the larger group for further deliberation. As a result, as shown in Figure 1, key activities and considerations, along with potential resources and partnerships that could create the necessary infrastructure, address critical privacy and security concerns, and ensure long-term sustainability of a national PRO network.
Figure 1.
Key activities, considerations and resources needed to support development of a national patient reported outcomes collaborative network.
The research presented during the workshop, views and reactions expressed, and the emerging themes from the consensus voting were subsequently developed by the authors into eight manuscripts (box). The manuscripts were submitted by the authors for review by the editorial team and external experts. Below, we offer a summary of the papers.
3.0 A new era of PRO users and applications
Mayo et al. opened the workshop by reviewing how measurement and the taxonomy used to classify health outcomes have evolved over time. They suggest a framework for identifying optimal sources of information, and methods to combine PROs with clinician judgements, performance observations and other technology-based results to comprehensively assess health outcomes [3].
Next, the focus moves to the use of PROs for clinical and patient decision-making, providing insight into both the incentives and obstacles encountered when collecting PROs from individuals. Noonan and colleagues begin with the perspective of the ultimate stakeholders of healthcare – the individuals or as they are increasingly described in Canada, the consumers of healthcare.[4] Several examples illustrate how PROs can be used to empower patients and help them better manage their health, improve communication with providers, and ensure treatments align with patient needs, preferences, and values. Noonan et al. note that both theory and research are needed to address current gaps and generate evidence to demonstrate how PROs contribute to efforts to improve quality of care and outcomes. Opportunities to accelerate broader uptake and use of PROs from a patient perspective are outlined.
Bingham et al. offer experiences and findings from clinicians who currently use PROs to screen, diagnose, and monitor health as part of routine care visits [5]. There is little doubt that PRO data can facilitate shared decision-making and identify unmet needs in individuals, while also contributing to ongoing quality improvement. Recommendations are offered to help ensure that selected PROs are indeed “fit for purpose”, are collected with minimal disruption to work flow, and yield valid data with actionable results. However, widespread uptake will depend in part on the extent to which PRO data are perceived as relevant, meaningful and actionable to those who will have to invest the time and effort to collect it. They caution also of the need for full transparency as to how PRO data will be utilized by patients, providers and administrators.
Mamiya et al. move us from individuals to society, illustrating how PRO data can be used to monitor population health, and identify disease patterns and the emergence of epidemics [6]. They review recent methodological advancements to enhance the validity and reproducibility of PROs, standardize measures and data collection across organizations, link multiple data sources, and establish norms that will be needed to effectively use PRO data for health surveillance and research at the population level.
4.0 Building and maintaining a national collaborative PRO network
The remaining papers offer a pragmatic focus on what is needed to implement a sustainable national collaborative PRO network. Because successful networks can serve as a framework for new initiatives, we invited leaders from the National Institutes of Health PROMIS® initiative to share their experiences with us. This initiative, which began in 2004, represents the largest investment of its kind to advance PRO science, and has resulted in more than 60 state-of-the-science measures for adults and 50 measures for children that are freely available and readily accessible online. PROMIS offers an example of how to work across distances to form essential partnerships, create a common vision, and leverage cutting edge technology to accelerate the development and testing of “universal” PROs that are broadly applicable across health conditions. Bartlett and colleagues describe how, in the United States, researchers, clinicians, patients, and other stakeholders came together to develop standards, coordinate efforts, and share resources to create universal measures of physical, social and emotional health that could be used for chronic disease research and clinical care [7]. They begin with a historical overview of the Patient Reported Outcomes Measurement Information System (PROMIS) and end by describing ongoing efforts including the international consortium which is coordinating translation and cultural validation efforts needed to harmonize use of these measures globally.
Another advantage of collaborative networks is the ability to define and standardize methods to develop, test, and administer PROs across settings. Sawatzky and colleagues illustrate how careful attention to PRO methodology, standards, and measurement curation can foster the meaningful and consistent development, use and interpretation of PRO data [8]. Directly incorporating patient reports of health and experiences in the electronic health record (EHR) is critical to accelerating widespread use of PRO data. While e-health technology can reduce the burden of data collection considerably (e.g., with patient portals tethered to EHRs), Ahmed et al. note that there are still considerable methodological and technical challenges that must be addressed to ensure the right information is available in the right format and accessible through the right channel at the right time [9]. A national network can leverage expertise across the country to jointly develop flexible methods and standards for the collection, storage, and sharing of PRO and clinical results as EHRs are widely implemented in healthcare settings.
Finally, in this era where health records have become a target for hackers worldwide, Arbuckle and colleagues explore ways to mitigate the considerable ethical and security vulnerabilities that can occur when collecting and sharing individual level health information [10]. They also explore how to standardize collection to support aggregation and integration of PRO data with administrative and other databases. Sharing of data across platforms is a prerequisite to being able to conduct the kinds of ongoing analyses in real time required to leverage PRO data for decision making. They also underscore the importance of establishing well-developed organizational protocols and policies to oversee access to PRO data.
5.0 Conclusion
PROs are emerging as critical tools that uniquely represent the viewpoint of healthcare consumers, and are essential to telling us whether our considerable investment in healthcare in fact is resulting in better health and wellness. PROs are currently being used to monitor health, manage care, evaluate and improve health service delivery, and inform policy. However, there are considerable methodological challenges and unanswered questions about how to best extend PROs data collection and use in these newer applications. This series highlights presentations and structured deliberations from thought leaders and strategists attending a recent workshop aimed at creating a sustainable national PRO collaboration. Our desire is that readers can benefit from the ideas and collective experiences shared by stakeholders representing multiple perspectives, and we can all learn how to best leverage PROs to help better inform healthcare decisions and improve patient outcomes world-wide.
References
- 1.Osler W. The Quotable Osler. Philadelphia, PA: American College of Physicians; 2008. Revised paperback ed. [Google Scholar]
- 2.Institute of Medicine. Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 3.Mayo N, Figueiredo S, Ahmed S, Bartlett SJ. Terminology proposed to measure what matters in health: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.013. in press. [DOI] [PubMed] [Google Scholar]
- 4.Noonan VK, Lyddiatt A, Ware P, Jaglal SB, Riopelle RJ, Bingham CO, III, et al. Patient reported outcomes (PRO) can facilitate shared decision-making and guide self-management: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.017. in press. [DOI] [PubMed] [Google Scholar]
- 5.Bingham CO, III, Noonan V, Auger C, Feldman D, Ahmed S, Bartlett SJ. Patient reported outcomes (PRO) can inform clinical decision-making in chronic care: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamiya H, Lix L, WG, Ahmed S, Bartlett SJ, Buckeridge D. Patient reported outcomes (PRO) can be linked to epidemiologic measures to monitor populations and inform public health decisions: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.018. in press. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett SJ, Witter J, Cella D, Ahmed S. Creating national initiatives to support patient reported outcomes (PRO) development and use—the PROMIS example: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.015. in press. [DOI] [PubMed] [Google Scholar]
- 8.Sawatzky R, Chan EKH, Zumbo BD, Ahmed S, Bartlett SJ, Bingham CO, III, et al. Modern Perspectives of Measurement Validation Emphasize Justification of Inferences: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed S, Ware P, Gardner W, Witter J, Bingham CO, III, Khairy D, et al. Patient reported outcomes (PRO) in electronic health records can inform clinical and policy decisions: Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.011. in press. [DOI] [PubMed] [Google Scholar]
- 10.Arbuckle L, Moher E, Ahmed S, Bartlett SJ, El Emam K. Anonymization and ethics considerations for capturing and sharing patient reported outcomes (PRO): Proceedings from the Montreal Accord to Accelerate and Harmonize PRO Use. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.04.016. in press. [DOI] [PubMed] [Google Scholar]