Abstract
Stem Leydig cells (SLCs), precursors of testicular Leydig cells that secrete testosterone required for male sexual differentiation, spermatogenesis and fertility, were recently identified in rat testes. Various types of stem cells have shown the ability to differentiate into other tissues, but there is no information on the plasticity of adult rat SLCs (rSLCs). This study investigated the ability of rSLCs to transdifferentiate into cell types from all three germ layers—prostatic epithelium (endoderm), uterine epithelium (mesoderm) and epidermis (ectoderm)—under the influence of inductive mesenchyme from fetal and neonatal tissues. To differentiate rSLCs into cells of other lineages, mesenchyme from green fluorescent protein (GFP)-expressing mice was used. Tissue recombinants of urogenital sinus mesenchyme (a potent prostate inducer) and rSLCs grafted into adult male hosts formed ductal structures resembling prostate after 5 weeks. Prostate epithelium was of rSLC origin as determined by absence of GFP expression, and expressed characteristic markers of prostatic epithelium. Similarly, uterine mesenchyme + rSLCs tissue recombinants contained a simple columnar epithelium that was histologically similar to normal uterine epithelium and expressed typical uterine epithelial markers, but was of rSLC origin. In contrast, epidermal tissue was absent in fetal dermis + rSLCs recombinants, suggesting rSLCs did not form skin epithelium. Thus, rSLCs can transdifferentiate into uterine and prostatic epithelium, mesodermal and endodermal derivatives, respectively, but they may have a limited transdifferentiation potential, as shown by their inability to form epidermis, an ectodermal derivative.
Keywords: pluripotent, tissue recombination, mesoderm, endoderm, ectoderm, cell plasticity
Introduction
Leydig cell function is required for male development and fertility (Benton et al., 1995). Leydig cells produce fetal androgens that masculinize the initially ambisexual male fetus and induce differentiation of the Wolffian ducts and urogenital sinus into accessory sex organs (Walters et al., 2010). During puberty, Leydig cell androgen production increases, which drives spermatogenesis and supports male reproductive development and function.
Adult Leydig cells differentiate at puberty and remains functional throughout subsequent life. Once mature ALCs are formed, their number remains fairly constant (Benton et al., 1995). Stem cells for adult Leydig cell populations were first identified and isolated from rats in 2006 (Ge et al., 2006). Rat stem Leydig cells (rSLCs) were isolated from neonatal testis based on their lack of expression of luteinizing hormone receptor (LHR) and expression of platelet-derived growth factor receptor alpha (PDGFRα). These rSLCs can be cultured in undifferentiated states for extended periods, but undergo Leydig cell differentiation when cultured in differentiation-inducing media (Ge et al., 2006). More recently, members of our group identified rSLCs from adult male rats, which could also differentiate into Leydig cells under appropriate culture conditions (Stanley et al., 2012; Li et al., 2016).
Low serum testosterone in men is linked to various reproductive and non-reproductive problems (Huhtaniemi, 2014), as well as increased mortality (Laughlin et al., 2008). Currently, androgen-deficient men are treated with testosterone replacement (Klotz, 2015; Yeap, 2015). However, this does not restore fertility in men who have undergone chemotherapy (Howell and Shalet, 2001) or suffer from primary hypogonadism (Ramasamy et al., 2015) because it does not produce the high intratesticular testosterone concentrations needed for spermatogenesis. Thus, SLCs may offer alternative therapy for age-related testosterone deficiency or other clinical conditions (Jiang et al., 2014), and mouse SLCs have been reported to partially restore serum testosterone when transplanted into mouse testes (Jiang et al., 2014). Adult SLCs might also be a source of cells for organ repair and replacement (Liu et al., 2016), but little information exists on their ability to produce other cell types. One study showed neonatal SLCs could differentiate into multiple lineages (Jiang et al., 2014), but the potential of adult SLCs to differentiate into other cell lineages is unknown, and this was the focus of the current study.
Tissue separation and recombination techniques have been used extensively to study epithelial and mesenchymal interactions; for reviews of mesenchymal/stromal regulation of epithelial differentiation as well as the tissue recombination technique and the insights generated from it, see (Cunha, 2008; Cunha and Baskin, 2016b).
Mesenchyme/stroma regulates epithelial function and instructively induces epithelial differentiation (Cunha, 2008). This inductive capacity of mesenchyme/stroma can be exploited to induce transdifferentiation of epithelium or other tissues by recombining mesenchyme from one organ with epithelium or other cell types from another organ, and then grafting these tissue recombinants under the renal capsule of hosts, where they vascularize and grow extensively (Cunha and Baskin, 2016b). For example, bladder epithelium forms prostatic epithelium following recombination with urogenital sinus mesenchyme (UGM) and growth in vivo (Cunha et al., 1980). Similarly, uterine mesenchyme (UtM) instructively induces differentiation of neonatal vaginal epithelium into uterine epithelium (UtE) in tissue recombinations in vivo (Kurita et al., 2001).
In this study, using tissue separation/recombination and in vivo grafting, we tested the hypothesis that adult rSLCs can form epithelia of tissues from differing embryonic germ layers when recombined with instructive mesenchyme from fetal and neonatal organs. Our results indicate that adult rSLCs can form endodermal and mesodermal derivatives such as prostatic and uterine epithelium, respectively, but not epidermis, an ectodermal derivative.
Materials and methods
Animals
All protocols and procedures were approved by the Institutional Animals Care and Use Committee of the University of Florida and Johns Hopkins University, and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Adult male Brown Norway rats (3- to 4-month-old; Harlan Sprague Dawley, Indianapolis, IN) used for SLC isolation were acclimated at the Johns Hopkins School of Public Health before use. Transgenic C57BL/6 mice expressing enhanced green fluorescent protein (GFP) under the control of a chicken β-actin promoter and cytomegalovirus enhancer and adult athymic nude male and female mice used as graft hosts were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained at the University of Florida. Male GFP+ mice were bred to wild-type females on a mixed C57BL/6 and 129SvEv genetic background. Vaginal plug detection was considered day 0 of pregnancy, and pregnant mice were used for fetal tissue collection at gestational days 14–16. Animals were housed in polycarbonate or polysulfone cages, provided food and water ad libitum, and maintained at 23°C and 42% humidity with 12h light/dark cycles.
Stem Leydig cell isolation and culture
Adult SLCs were isolated as described previously (Stanley et al., 2012) from 3- to 4-month-old Brown Norway rats. Briefly, rats were injected intraperitoneally with 75 mg/kg bw ethane-1,2-dimethyl sulphonate (EDS), a Leydig cell-specific toxicant. Four days later, testes were harvested, de-capsulated and their cells dispersed using specific steps that minimize peritubular myoid cells (e.g., collagenase-D exposure). Interstitial cells were separated, re-suspended and centrifuged. Precursor cells were purified by Percoll density gradient centrifugation followed by BSA gradients. PDGFRα positive cells were isolated after plating the cells into cell culture plates coated with PDGFRα antibody (Ge et al., 2006). Isolated cells were washed, dispersed with trypsin, and plated on fibronectin-coated plates in expansion media, as described previously (Stanley et al., 2012). Following plating, undifferentiated rSLCs were expanded and maintained in SLC media until combined with inductive mesenchymes. Purity of the rSLCs was evaluated by 1) PDGFRα expression and 2) their ability to differentiate (become CYP11A1-positive). Essentially all the SLCs expressed PDGFRα, but this marker is shared by peritubular cells that can also express PDGFRα. However, essentially 100% of the SLC preparations were able to differentiate and express markers indicative of Leydig cells (become CYP11A1-positive), indicating that purity of the SLC preparations was high even though these preparations may also contain some cells that are not SLCs.
Tissue separation and recombination
To determine whether adult rSLCs can differentiate into cells of endodermal, mesodermal and ectodermal origin, rSLCs were combined with various inductive fetal or neonatal mouse mesenchymes, and grafted and grown for 5 weeks under the renal capsule of nude mouse hosts. To allow cell lineage identification, mesenchymes from GFP+ mice were used with rSLCs lacking GFP. Presence or absence of GFP expression in constituent cells of tissue recombinants was used to identify cell origin.
Tissue recombination of mouse UGM + rSLCs
Timed-pregnant mice at gestational days 14–16 were used to obtain fetal urogenital sinus (UGS), as previously described (Cunha and Donjacour, 1987; Cunha and Baskin, 2016a). The UGS tissue was incubated in 1% trypsin (#215240, BD Diagnostic Systems, Sparks, MD) in Hanks CMF for 90 min at 4°C to digest the basement membrane between the UGM and urogenital sinus epithelium (UGE), then washed in Hanks CMF and placed into 20% fetal calf serum/Hanks CMF to halt trypsinization. Tissues were washed, and placed in Hanks CMF containing DNase (#DN25, Sigma-Aldrich).
Using a von Graefe knife and fine forceps, UGM and UGE were separated. The UGM fragments were combined with 5–10 ×104 SLCs in 0.5 ml basal Eagles medium (#B9638, Sigma-Aldrich) with FCS, L-glutamine, sodium bicarbonate, antibiotic and antimycotic solution (#A-9909, Sigma-Aldrich) in 1.5 ml Eppendorf tubes, then centrifuged at 400g for 5 mins to pellet the rSLCs and UGM together. Resultant tissue recombinants were incubated overnight at 37°C and 5% CO2. The following day, tissue recombinants were grafted under the kidney capsule of nude male hosts (Cunha and Baskin, 2016a). As controls, GFP−-UGM alone or GFP−-UGM + GFP+-UtE were also grafted under the kidney capsule of nude mouse hosts. Buprenorphine was administered for two days for pain management. After five weeks, grafts and host prostate were collected and fixed in 10% neutral buffered formalin for 48–72h at room temperature.
Tissue recombination of UtM + rSLCs
The UtM and UtE were separated as described (Cooke et al., 1986) from 2- to 4-day-old wild-type and GFP+ uteri. Briefly, uterine horns were cut into 1–2 mm pieces and trypsinized. Two to three pieces of UtM were combined with 5–10 × 104 SCLs and incubated overnight as described above. Grafts of GFP−-UtM alone or tissue recombinants of GFP−-UtM and GFP+-UtE were prepared as negative and positive controls, respectively. The following day, tissue recombinants were grafted under kidney capsules of nude female hosts, then grafts and host uteri were collected and processed as above.
Tissue recombination of fetal dermis (FD) + rSLCs
Back skin (1 mm2) was obtained from gestational day 14–16 fetuses and grafts prepared as described previously (Simon et al., 2009; Simon et al., 2013; Cunha and Baskin, 2016a). The FD and epidermis was separated after trypsinization. The grafts containing FD from GFP+ fetuses and rSLCs, and positive or negative control grafts of GFP−-FD alone or GFP−-FD and GFP+-fetal skin epithelium (FSE) were implanted under the kidney of either male or female nude mouse hosts and grown 5 weeks. For each tissue type, all recombination procedures were carried out at least 4 times, with 3–5 individual test and control grafts implanted each time.
Immunohistochemistry
Grafts were processed as described (Simon et al., 2009) and 6 µm sections were placed on slides, deparaffinized and subjected to antigen retrieval using citrate buffer (Nanjappa et al., 2015), followed by 20 mins in 0.9% hydrogen peroxide in methanol. Sections were washed in 0.05% tween-20 phosphate buffered saline (PBST) and incubated with blocking solution (goat serum solution provided in VECTASTAIN Elite ABC HRP kit [#PK6101, Vector Laboratories]) before overnight incubation at 4°C with primary antibodies in blocking buffer. Antibodies for cytokeratin (#Z0622, Dako North America, Carpinteria, CA), MKI67 (#ab16667, Abcam, Cambridge, MA), complement component 3 (C3; sc-20137; Santa Cruz Biotechnologies, Santa Cruz, CA) and NKX3.1 (#AB5983, EMD Millipore Corporation, Billerica, MA) were used at 1:100 dilutions according to the manufacturer’s instructions. The following day, sections were washed and incubated with biotinylated secondary goat anti-rabbit IgG at room temperature for 60 min. Sections were washed, and incubated with Avidin/Biotinylated enzyme complex (ABC) for 30 min followed by DAB (SK-4100, Vector Laboratories) for 1–2 min. The DAB reaction was stopped by rinsing in tap water, and sections were counter-stained with hematoxylin, and dehydrated, mounted and images captured using light microscopy. Negative controls without primary antibody were run in parallel.
Androgen receptor (AR) and secretory DLP protein were localized by immunohistochemistry in tissues as previously described (Prins et al., 1991). Sections were heat treated in a Decloaker pressure cooker (Beicard Medical, Walnut Creek, CA) in 0.1M citrate buffer, pH 6.0. All sections were blocked with Superblock (Vector Laboratories, Burlingame, CA) and incubated overnight at 4°C with 2 mg/ml rabbit anti-AR (PG-21) (Prins et al., 1991) or rabbit anti-mouse DLP antibody (1:250 dilution, kindly provided by Drs. Gerald Cunha and Annemarie Donjacour, University of California-San Francisco). Sections were reacted with biotinylated goat anti-rabbit-IgG (1:200 dilution; Vector Laboratories, Inc.) and detected with avidin-biotin peroxidase (ABC-Elite, RTU kit, Vector Laboratories) using diaminobenzidine tetrachloride as the chromagen. For controls, normal rabbit IgG was substituted for primary antibody.
Immunofluorescence for GFP
To visualize GFP, tissue sections were incubated with primary anti-GFP antibody (#2956, Cell Signaling Technology, Danvers, MA; 1:200 dilution) in a blocking solution overnight at 4°C, then washed three times with PBST and incubated with secondary fluorescein goat anti-rabbit IgG (#FI-1000, Vector Laboratories, Burlingame, CA) in blocking solution (1:200 dilution, 60 min). Slides were re-washed and 1–2 drops of Vectashield with DAPI (H-1200, Vector Laboratories) was applied before mounting. Negative controls lacking primary antibody were run in parallel. Images were captured with an Olympus BX51 fluorescence microscope (Olympus, Melville, NY) using fluorescence excitation and emission filters.
Results
Transdifferentiation of rSLCs into prostatic epithelium
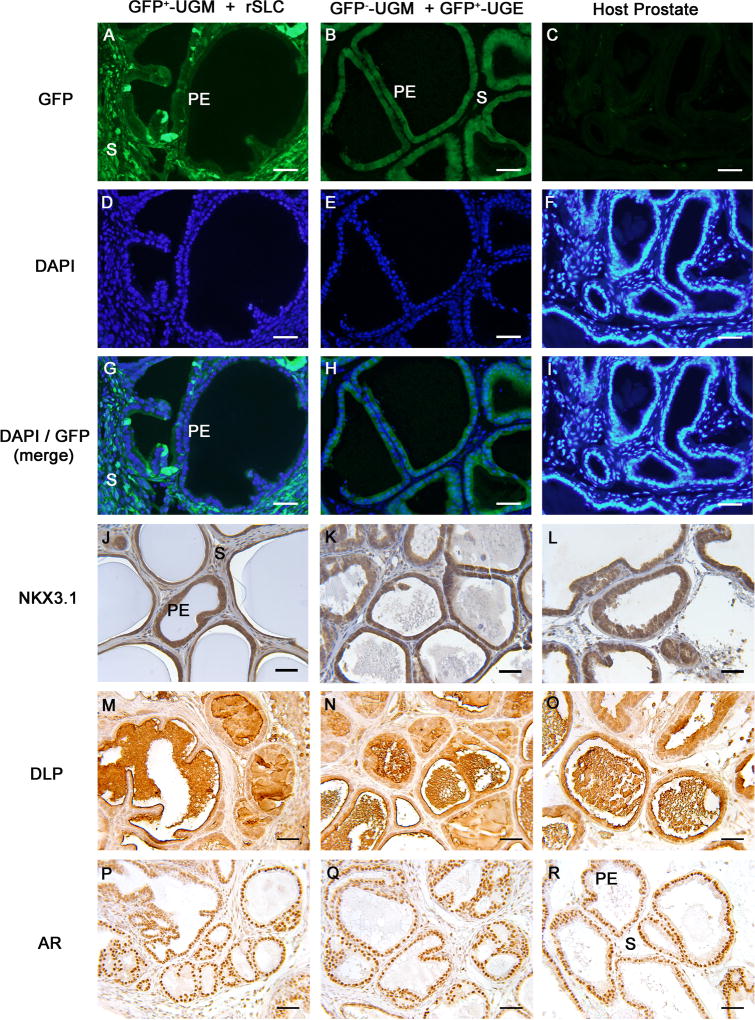
To determine whether rSLCs can differentiate into prostatic epithelium, an endodermal derivative, GFP+-UGM was combined with rSLCs and grown in male hosts. Tissue recombinants formed structures that histologically resembled prostate (Figure 1A, D, G, J, M, P) with tubular structures containing a GFP+ stroma lined by cuboidal GFP− epithelium (Figure 1A, G), indicating that rSLCs transdifferentiated into prostate epithelium. Controls prepared with GFP−-UGM + GFP+-UGE (Figure 1B, H) formed prostate with GFP− stroma and GFP+ epithelium and host prostate was GFP− (Figure 1C), as expected. The epithelium of GFP+-UGM + rSLC and GFP−-UGM + GFP+-UGE tissue recombinants, as well as host prostate, robustly expressed NKX3.1 (Figure 1J–L), a prostate marker that drives prostatic differentiation and morphogenesis during development (Tanaka et al., 2000). Epithelium and luminal secretions of the GFP+-UGM + rSLC tissue recombinants also immunostained with DLP (Figure 1M), an antibody that recognizes the major secretory proteins produced by the dorsolateral region of the mouse and rat prostate (Donjacour et al., 1990; Lopes et al., 1996); DLP expression in GFP−-UGM + GFP+-UGE tissue recombinants and host prostate was similar (Figure 1N, O). Androgen receptor expression was also similar in GFP+-UGM + rSLC and GFP−-UGM + GFP+-UGE tissue recombinants and host prostate (Fig. 1P–R). Three out of total 6 tissue separation/recombination experiments (50%) with GFP+-UGM + rSLCs yielded tissue containing rSLC-derived prostatic epithelium, as indicated by lack of GFP expression. All positive control grafts consisting of GFP−-UGM + GFP+-UGE (6/6) formed prostate with GFP+-UGE, while negative control GFP−-UGM grafts (5/5) formed fibromuscular tissue devoid of epithelium (not shown).
Figure 1. Rat stem Leydig cells (rSLCs) can transdifferentiate into prostatic epithelium (PE).
After 5 weeks of growth in vivo, GFP+-urogenital sinus mesenchyme (UGM) + rSLCs tissue recombinants developed into structures histologically resembling prostate and consisted of tubular structures lined by simple cuboidal epithelium supported by fibromuscular stroma (A, D, G, J, M, P). Critically, in GFP+-UGM + rSLCs grafts, the cuboidal epithelium (PE) was GFP−, while the stroma (S) was GFP+ (A, G), indicating that the epithelium was of rSLC origin. This contrasts with control GFP−-UGM + GFP+-urogenital sinus epithelium (UGE) tissue recombinants (B, E, H, K, N, Q) and host prostate (C, F, I, L, O, R), where GFP expression occurs only in epithelium (B, H) but not the stroma, or is seen in neither epithelium or stroma (C, I), respectively. In addition, the presumptive prostatic epithelium of rSLC origin robustly expressed a prostate epithelial-specific marker, NKX3.1 (J) that was also seen in GFP−-UGM + GFP+-UGE tissue recombinants (K) and host prostate (L). In the epithelium of GFP+-UGM + rSLCs grafts, dorsolateral prostate secretory protein (DLP) production was seen in the epithelium and lumens of the ducts (M), while androgen receptor (AR) was expressed in all cells of these grafts (P). Both the secretory protein (DLP) expression and AR expression in these grafts was identical to control GFP−-UGM + GFP+-UGE tissue recombinants (N, Q) and host prostate (O, R). Magnification bars in all panels are 50 µm. GFP, green fluorescent protein; DAPI, 4′,6-diamidino-2-phenylindole.
Transdifferentiation of rSLCs into uterine epithelium
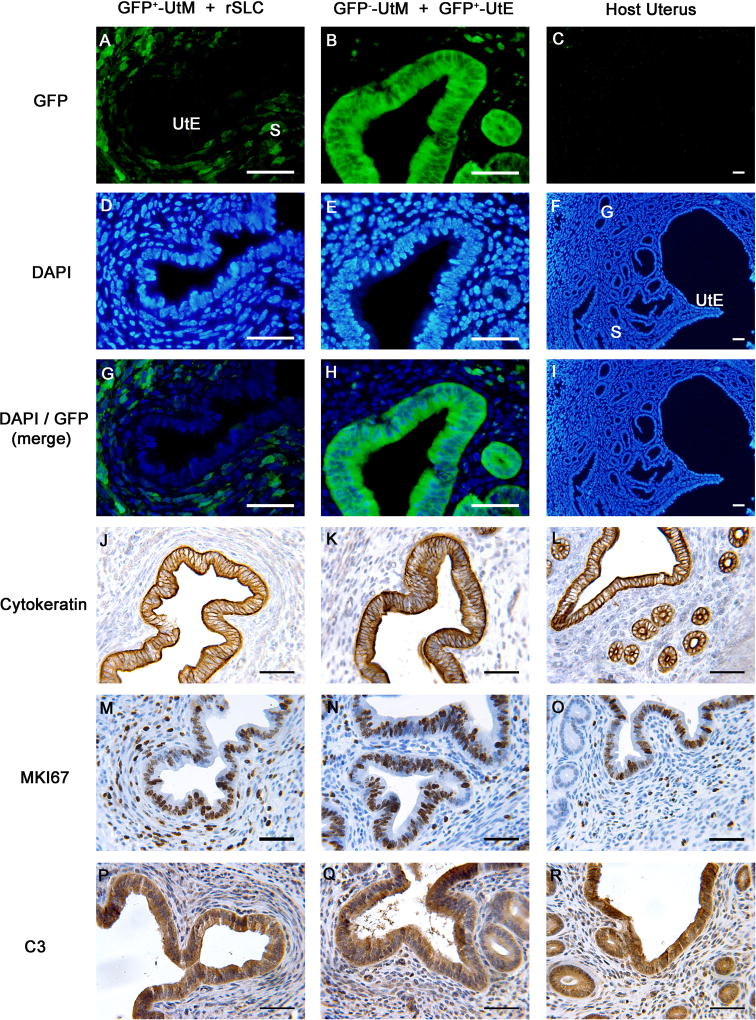
To determine whether rSLCs can differentiate into uterine epithelium, a mesodermal derivative, we used tissue recombination of neonatal GFP+-UtM + rSLCs. Tissue recombinants of GFP+-UtM + rSLCs (Figure 2A, D, G, J, M, P) contained stroma and cuboidal/columnar epithelium resembling UtE of GFP−-UtM + GFP+-UtE tissue recombinants that functioned as positive controls (Figure 2B, E, H, K, N, O) as well as host uterus (Figure 2C, F, I, L, O, R). Importantly, UtE were GFP− (Figure 2A, G), consistent with an rSLC origin. The DAPI-stained GFP− epithelium in these grafts (Figure 2D) showed homogenous nuclear staining, indicative of rat cells (Lipschutz et al., 1996; Li et al., 2000), rather than the more punctate nuclear staining pattern of mouse stromal cells, confirming that the UtE was of rat, and therefore rSLC, origin. This contrasted to control GFP−-UtM + UtE tissue recombinants (Figure 2E), in which both stroma and epithelium were of mouse origin, with characteristic mouse nuclear punctate patterns. Furthermore, epithelium in GFP+-UtM + rSLCs tissue recombinants expressed cytokeratin (Figure 2J), an epithelial marker not expressed in Leydig cells but expressed in GFP−-UtM + GFP+-UtE tissue recombinants and host uterus (Figure 2K, L). Epithelium in GFP+-UtM + rSLCs tissue recombinants showed a proliferative pattern similar to control GFP−-UtM + GFP+-UtE tissue recombinants and host uterus (Figure 2M, N, O), suggesting that epithelium of both graft types and the host uterus respond similarly to endogenous ovarian steroids. Finally, epithelium in GFP+-UtM + rSLCs and GFP−-UtM + GFP+-UtE tissue recombinants, as well as host uterus, expressed C3, an estrogen-dependent uterine epithelial secretory protein (Figure 2P, Q, R). Six of the 8 total (75%) GFP+-UtM + rSLCs tissue recombinants produced grafts containing GFP−-UtE, indicating transdifferentiation of the GFP− rSLCs. Control tissue recombinants of GFP−-UtM + GFP+-UtE all (4/4) produced grafts containing UtE, while grafts of GFP−-UtM alone (5/5) produced fibromuscular tissue lacking epithelium (not shown). Critically, no tissue recombinants containing GFP+-UtM + rSLCs produced grafts containing GFP+-UtE. This indicates that initial UtM/UtE separation in neonatal uteri was complete, as GFP+-UtE would result from residual UtE not completely separated initially from the UtM.
Figure 2. Rat stem Leydig cells (rSLCs) can transdifferentiate into uterine epithelium (UtE).
Tissue recombinants consisting of GFP+-uterine mesenchyme (UtM) + rSLCs (A, D, G, J, M, P) resembled control GFP−-UtM + GFP+-UtE tissue recombinants (B, E, H, K, N, Q) that functioned as positive controls, as well as host uterus (C, F, I, L, O, R). Critically, in GFP+-UtM + rSLC grafts, the UtE was GFP− (A, G), indicating rSLC origins. This contrasts with GFP−-UtM + GFP+-UtE tissue recombinants and host uterus, where GFP is expressed only in epithelium (B, H), or not expressed (C, I), respectively. In addition, UtE derived from rSLCs expressed cytokeratin (J) and uterine secretory protein C3 (P), proteins that are typical of normal uterine epithelium, as shown by their expression in GFP−-UtM + GFP+-UtE tissue recombinants (K, Q) and host uterus (L, R). Finally, GFP+-UtM + rSLC grafts (M) showed cell proliferation similar to GFP−-UtM + GFP+-UtE tissue recombinants (N) and host uterus (O), as indicated by MKI67 immunostaining. Magnification bars of all panels are 50 µm. GFP, green fluorescent protein; DAPI, 4′,6-diamidino-2-phenylindole; S, stroma; G, glands.
Transdifferentiation of rSLCs into skin epithelium
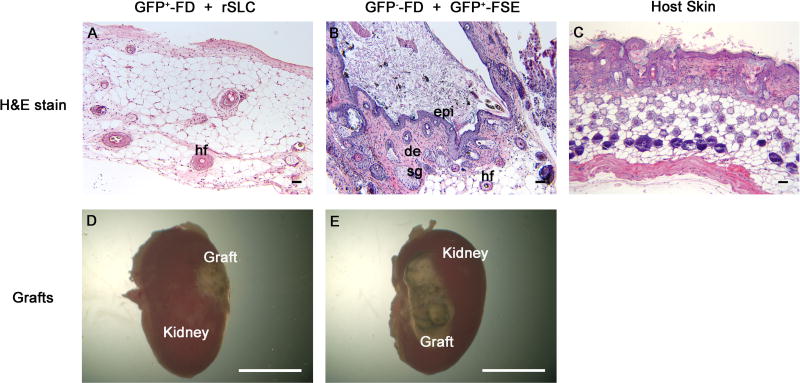
To investigate whether rSLCs can differentiate into skin epidermis, an ectodermal derivative, we utilized GFP+-FD + rSLCs tissue recombinants. These grafts were strikingly smaller (Figure 3A, D) than control GFP−-FD + GFP+-FSE tissue recombinants (Figure 3B, E). Grafts of GFP+-FD + rSLCs (Figure 3A) lacked organized skin structures and consisted of relatively normal stromal tissue, along with adipocytes and hair follicles, but no epidermal layer was present (0/12). Cells in these grafts were overwhelmingly GFP+, although some GFP− cells were seen that could have been either of host or rSLC origin (data not shown). As expected, control tissue recombinants containing GFP−-FD + GFP+-FSE formed large grafts (Figure 3E) consisting of keratinized epidermis, hair follicles, sebaceous glands and dermis containing adipocytes (Figure 3B), and was similar to host skin (Figure 2C), while negative control GFP−-FD grafts lacked epithelium but did contain hair follicles (not shown). Thus, rSLCs did not form epidermis, an ectodermal derivative, when grown in association with dermal mesenchyme.
Figure 3. Rat stem Leydig cells (rSLCs) do not transdifferentiate into epidermis.
Tissue recombinants of GFP+-fetal dermis (FD) + rSLCs (A, D) produced small grafts that did not contain histologically identifiable skin and lacked an epidermis. In contrast, control GFP−-FD + GFP+-fetal skin epidermis (FSE) tissue recombinants (B, E) produced large grafts of histologically normal skin containing keratinized epidermis (epi), dermis (de), sebaceous glands (sg) and hair follicles (hf) similar to host skin (C). Magnification bars are 50 µm for panels A–C and 5 mm for panels D and E.
Discussion
The rodent testis contains SLCs that can form new Leydig cells and restore serum testosterone concentrations when differentiated Leydig cells are removed (Chen et al., 2016; Li et al., 2016). Our current results show that adult rSLCs are also able to differentiate into other non-Leydig cell types in vivo, in this case, prostatic and uterine epithelium, when exposed to appropriate inductive cues from fetal/neonatal mouse mesenchyme from other tissues. Thus, adult rSLCs are multipotent and plastic and capable of responding to inductive signals from mouse mesenchyme to transdifferentiate into other tissues.
The GFP+-UGM + rSLCs grafts histologically resembled prostate and consisted of ducts lined by cuboidal GFP− epithelial cells, reflecting a rSLC origin. Prostatic epithelium in tissue recombinants is derived from the GFP− rSLCs used in these grafts, as UGM grafts alone form only fibromuscular tissue as previously reported (Cunha et al., 1992). Critically, prostatic epithelium in GFP+-UGM + SLC grafts, control GFP−-UGM + GFP+-UGE grafts and host prostate all robustly expressed NKX3.1, an important marker of prostatic epithelium not expressed in Leydig cells or other testicular cell types (Simon et al., 2009; Cooke et al., 2015). Mouse NKX3.1 is a homeobox transcription factor that induces prostatic epithelial differentiation and is essential for normal prostate ductal branching and secretory protein production (Tanaka et al., 2000). In addition, androgen receptor expression, a hallmark of prostatic epithelium, is comparable in GFP+-UGM + SLC grafts, control GFP−-UGM + GFP+-UGE grafts and host prostate. Prostatic epithelium in GFP+-UGM + SLC grafts, control GFP−-UGM + GFP+-UGE grafts and host prostate all robustly express dorsolateral prostate secretory proteins, indicating that the SLCs not only form a tissue that is histologically similar to prostatic epithelium, but is also produces characteristic secretions. Thus, adult rSLCs form functional prostatic epithelium expressing normal histological and molecular characteristics when combined with fetal mouse UGM. These findings are similar to previous results showing that UGM recombined in heterotypic tissue recombinations with urinary bladder or vaginal epithelium (Cunha, 1975; Cunha et al., 1980; Cunha et al., 1983a) or spermatogonial stem cells (Simon et al., 2009; Cooke et al., 2015) could induce prostatic epithelial differentiation in these cells.
Grafts of GFP+-UtM + rSLCs contained epithelium that was histologically similar to uterine epithelium and GFP−, in contrast to the GFP+-UtM used in the tissue recombinations. In addition, DAPI staining in epithelium of GFP+-UtM + rSLCs tissue recombinants showed a more homogenous nuclear staining pattern, indicative of a rat origin, whereas mouse stroma in these grafts as well as mouse UtE in control GFP−-UtM + GFP+-UtE grafts showed the more punctuate nuclear staining pattern reflective of mice (Lipschutz et al., 1996; Li et al., 2000). Thus, epithelial cells in these grafts are derived from GFP− rSLCs, since grafts of UtM alone formed only fibromuscular tissue as previously reported (Cunha et al., 1989; Cunha et al., 1992).
Epithelium in GFP+-UtM + rSLCs grafts expressed functional uterine epithelial markers, in addition to characteristic uterine epithelial morphology. Cytokeratin expression in GFP+-UtM + rSLCs grafts was similar to host uterus and control GFP−-UtM + GFP+-UtE grafts, and cytokeratin is not expressed in rSLCs. In addition, expression of the uterine epithelial secretory protein C3 was similar in GFP+-UtM + rSLCs and GFP−-UtM + GFP+-UtE grafts and host uterus. Thus, rSLCs showed both morphological and functional differentiation into UtE, as well as the production of a characteristic uterine epithelial secretory protein. Although we did not directly test whether UtE of rSLC origin responds mitogenically to estrogen, the epithelial proliferative pattern in GFP+-UtM + rSLCs grafts, as assessed by MKI67 staining, was similar to UtE of host uterus, suggesting that UtE in the grafts and host uteri respond similarly to proliferative effects of host estrogen.
The ability of rSLCs to differentiate into UtE under the inductive influence of neonatal UtM is similar to heterotypic tissue recombinations that have been reported previously for neonatal UtM recombined with neonatal vaginal epithelium (Cunha, 1976; Kurita et al., 2001), spermatogonial stem cells (Simon et al., 2009; Cooke et al., 2015) or aorta-derived mesangioblasts (Simon et al., 2013), all of which form morphologically and functionally normal uterine epithelium. Thus, various differentiated cell types can transdifferentiate into UtE expressing key markers for this tissue under the inductive influence of neonatal UtM.
In this study, adult rSLCs + fetal mouse dermis tissue recombinants did not form epidermis, an ectodermal derivative. This suggests that adult rSLCs have restricted plasticity compared to other stem cells such as spermatogonial stem cells (Simon et al., 2009; Cooke et al., 2015), which are capable of differentiating into epithelium from all germ layers. Similar to rSLCs, aorta-derived mesangioblasts also did not transdifferentiate into epidermis (Simon et al., 2013). This is consistent with previous studies showing that mesenchyme is typically capable of instructively inducing epithelial or other cells (e.g., stem cells) of the same germ layer origin but may not be able to instructively induce tissues of other germ layers (Boutin et al., 1991; Taylor et al., 2009). Leydig cells have historically been considered to be mesodermally derived (Hardy et al., 1989) although recent studies questioned this (Davidoff et al., 2009; DeFalco et al., 2011). Assuming a mesodermal origin for rSLCs, their ability to form prostatic epithelium indicates that they can produce tissues derived from different primordial germ layers. However, lineage-specific adult stem cells such as SLCs may not be able to give rise to tissues derived from all germ layers, and appear to be developmentally restricted despite their plasticity.
Our results are the first to indicate that adult rSLCs can form various other tissues. The ability of some tissues to undergo instructive induction is age-related. For example, neonatal vaginal epithelium can be instructively induced to become UtE only during the neonatal period (Cunha, 1975; 1976). Conversely, instructive induction of bladder epithelium into prostatic epithelium by UGM can be obtained using bladder epithelium from rodents ranging from neonatal to adult (Cunha et al., 1983a; Hayashi et al., 1993). The ability of adult rSLCs to respond to inductive UGM or UtM signals show that these cells maintain plasticity into adulthood and responsiveness to these signals from heterologous mesenchyme.
The adult rSLCs are capable of forming prostatic or uterine epithelium that is morphologically normal and expresses various functional markers. Responsivity of rSLCs to inductive signals from mouse tissue suggests conservation in mouse UtM and UGM signals that instructively induce full differentiation in rat epithelium. Instructive induction by mouse UGM of epithelial tissue from another species to undergo prostatic differentiation has been shown previously with both human and rat bladder epithelium (Cunha et al., 1983b; Hayashi et al., 1993; Aboseif et al., 1999; Li et al., 2000).
In the present experiment, adult rSLCs could form cells of endodermal or mesodermal origin only. In contrast, a previous study has shown that nestin-positive SLCs from neonatal mice can be programmed in vitro to form tissues of endodermal, mesodermal and ectodermal origin (Jiang et al., 2014). This may be due to age, experimental conditions (in vitro vs in vivo) and/or species of the SLCs used, since Jiang et al used neonatal mouse SLCs, in contrast to our adult rat SLCs (Stanley et al., 2012; Chen et al., 2016; Li et al., 2016). Previous studies have shown that the ability of differentiated epithelium to transdifferentiate decreases with age (Cunha, 1975; 1976). A similar process may explain why adult SLCs are developmentally restricted and show more limited plasticity than neonatal SLCs.
In summary, adult rSLCs can transdifferentiate into prostatic and uterine epithelium when combined with inductive fetal/neonatal mesenchyme that reprograms the rSLCs. However, rSLCs were unable transdifferentiate into epidermis when combined with fetal dermis. The adult SLC may have obvious applicability as a treatment for testosterone deficiency in males. Our results indicate that adult SLCs are multipotent but are not pluripotent, and although they could have some applications in regenerative medicine, this may be constrained by their limited developmental repertoire.
Footnotes
This work was supported by a New Florida Scholar Boost Award from the State of Florida (PSC), NIH grant R37 AG21092 (BRZ), and National Natural Science Foundation of China grants NSFC31271252 (HC) and NSFC81471411 (HC).
Preliminary results were presented at the 41st Annual Conference of American Society of Andrology, April, 2016, New Orleans, Louisiana, USA.
References
- Aboseif S, El-Sakka A, Young P, Cunha G. Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation. 1999;65:113–118. doi: 10.1046/j.1432-0436.1999.6520113.x. [DOI] [PubMed] [Google Scholar]
- Benton L, Shan LX, Hardy MP. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: prostatic inductions. Differentiation. 1991;48:99–105. doi: 10.1111/j.1432-0436.1991.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Jin S, Huang S, Folmer J, Liu J, Ge R, Zirkin BR. Transplantation of alginate-encapsulated seminiferous tubules and interstitial tissue into adult rats: Leydig stem cell differentiation in vivo? Mol Cell Endocrinol. 2016;436:250–258. doi: 10.1016/j.mce.2016.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Simon L, Nanjappa MK, Medrano TI, Berry SE. Plasticity of spermatogonial stem cells. Asian J Androl. 2015;17:355–359. doi: 10.4103/1008-682X.148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Uchima FD, Fujii DK, Bern HA, Cunha GR. Restoration of normal morphology and estrogen responsiveness in cultured vaginal and uterine epithelia transplanted with stroma. Proc Natl Acad Sci U S A. 1986;83:2109–2113. doi: 10.1073/pnas.83.7.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Age-dependent loss of sensitivity of female urogenital sinus to androgenic conditions as a function of the epithelia-stromal interaction in mice. Endocrinology. 1975;97:665–673. doi: 10.1210/endo-97-3-665. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Baskin L. Mesenchymal-epithelial interaction techniques. Differentiation. 2016a;91:20–27. doi: 10.1016/j.diff.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Baskin L. Use of sub-renal capsule transplantation in developmental biology. Differentiation. 2016b;91:4–9. doi: 10.1016/j.diff.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, Kinbara H. Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol. 1992;1:76–83. [PubMed] [Google Scholar]
- Cunha GR, Donjacour A. Mesenchymal-epithelial interactions: technical considerations. Prog Clin Biol Res. 1987;239:273–282. [PubMed] [Google Scholar]
- Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer L, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983a;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Lung B, Reese B. Glandular epithelial induction by embryonic mesenchyme in adult bladder epithelium of BALB/c mice. Invest Urol. 1980;17:302–304. [PubMed] [Google Scholar]
- Cunha GR, Sekkingstad M, Meloy BA. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit and human tissues. Differentiation. 1983b;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Brody JR. Role of uterine epithelium in the development of myometrial smooth muscle cells. Biol Reprod. 1989;40:861–871. doi: 10.1095/biolreprod40.4.861. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Muller D, Holstein AF. The neuroendocrine Leydig cells and their stem cell progenitors, the pericytes. Adv Anat Embryol Cell Biol. 2009;205:1–107. [PubMed] [Google Scholar]
- DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donjacour AA, Rosales A, Higgins SJ, Cunha GR. Characterization of antibodies to androgen-dependent secretory proteins of the mouse dorsolateral prostate. Endocrinology. 1990;126:1343–1354. doi: 10.1210/endo-126-3-1343. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci U S A. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Cunha GR, Parker M. Permissive and instructive induction of adult rodent prostatic epithelium by heterotypic urogenital sinus mesenchyme. Epithelial Cell Biol. 1993;2:66–78. [PubMed] [Google Scholar]
- Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update. 2001;7:363–369. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MH, Cai B, Tuo Y, Wang J, Zang ZJ, Tu X, Gao Y, Su Z, Li W, Li G, Zhang M, Jiao J, Wan Z, Deng C, Lahn BT, Xiang AP. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24:1466–1485. doi: 10.1038/cr.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L. Testosterone therapy and prostate cancer--safety concerns are well founded. Nat Rev Urol. 2015;12:48–54. doi: 10.1038/nrurol.2014.338. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Z, Jiang Z, Guo J, Zhang Y, Li C, Chung J, Folmer J, Liu J, Lian Q, Ge R, Zirkin BR, Chen H. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci U S A. 2016;113:2666–2671. doi: 10.1073/pnas.1519395113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, Hayward SW, Cunha GR, Baskin LS. Plasticity of the urothelial phenotype: effects of gastro-intestinal mesenchyme/stroma and implications for urinary tract reconstruction. Differentiation. 2000;66:126–135. doi: 10.1046/j.1432-0436.2000.660207.x. [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Young P, Taguchi O, Cunha GR. Urothelial transformation into functional glandular tissue in situ by instructive mesenchymal induction. Kidney Int. 1996;49:59–66. doi: 10.1038/ki.1996.8. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, Song J, Zhang Y. Strategies to Optimize Adult Stem Cell Therapy for Tissue Regeneration. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17060982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes ES, Foster BA, Donjacour AA, Cunha GR. Initiation of secretory activity of rat prostatic epithelium in organ culture. Endocrinology. 1996;137:4225–4234. doi: 10.1210/endo.137.10.8828481. [DOI] [PubMed] [Google Scholar]
- Nanjappa MK, Medrano TI, March AG, Cooke PS. Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biol Reprod. 2015;92:78. doi: 10.1095/biolreprod.114.125724. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Armstrong JM, Lipshultz LI. Preserving fertility in the hypogonadal patient: an update. Asian J Androl. 2015;17:197–200. doi: 10.4103/1008-682X.142772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Cooke PS, Berry SE. Aorta-derived mesoangioblasts can be differentiated into functional uterine epithelium, but not prostatic epithelium or epidermis, by instructive mesenchymes. Cells Tissues Organs. 2013;198:169–178. doi: 10.1159/000354900. [DOI] [PubMed] [Google Scholar]
- Simon L, Ekman GC, Kostereva N, Zhang Z, Hess RA, Hofmann MC, Cooke PS. Direct transdifferentiation of stem/progenitor spermatogonia into reproductive and nonreproductive tissues of all germ layers. Stem Cells. 2009;27:1666–1675. doi: 10.1002/stem.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Chen H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153:5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219:248–260. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Wang H, Wilkinson SE, Richards MG, Britt KL, Vaillant F, Lindeman GJ, Visvader JE, Cunha GR, St John J, Risbridger GP. Lineage enforcement by inductive mesenchyme on adult epithelial stem cells across developmental germ layers. Stem Cells. 2009;27:3032–3042. doi: 10.1002/stem.244. [DOI] [PubMed] [Google Scholar]
- Walters KA, Simanainen U, Handelsman DJ. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update. 2010;16:543–558. doi: 10.1093/humupd/dmq003. [DOI] [PubMed] [Google Scholar]
- Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202. doi: 10.1097/MED.0000000000000161. [DOI] [PubMed] [Google Scholar]