Abstract
Incremental hemodialysis has been examined as a viable hemodialysis regimen for selected end-stage renal disease (ESRD) patients. Preservation of residual kidney function (RKF) has been the driving impetus for this approach given its benefits upon the survival and quality of life of dialysis patients. While clinical practice guidelines recommend an incremental start of dialysis in peritoneal dialysis patients with substantial RKF, there remains little guidance with respect to incremental hemodialysis as an initial renal replacement therapy regimen. Indeed, several large population-based studies suggest that incremental twice-weekly vs. conventional thrice-weekly hemodialysis has favorable impact upon RKF trajectory and survival among patients with adequate renal urea clearance and/or urine output. In this report, we describe a case series of 13 ambulatory incident ESRD patients enrolled in a university-based center’s Incremental Hemodialysis Program over the period of January 2015 to August 2016 and followed through December 2016. Among five patients who maintained a twice-weekly hemodialysis schedule vs. eight patients who transitioned to thrice-weekly hemodialysis, we describe and compare patients’ longitudinal case-mix, laboratory, and dialysis treatment characteristics over time. The University of California Irvine Experience is the first systemically examined twice-weekly hemodialysis practice in North America. While future studies are needed to refine the optimal approaches and the ideal patient population for implementation of incremental hemodialysis, our case-series serves as a first report of this innovative management strategy among incident ESRD patients with substantial RKF, and a template for implementation of this regimen.
Keywords: Incremental dialysis, residual kidney function, case series
In end-stage renal disease (ESRD) patients, preservation of residual kidney function (RKF) and urine output (UOP) has been associated with better solute clearance and fluid balance, as well as greater health-related quality of life and survival.1–11 Indeed, a large proportion of incident ESRD patients will have substantial RKF at the time of dialysis initiation, with 45% and 16% of patients having an estimated glomerular filtration rate (eGFR) of ≥10 ml/min/1.73m2 and ≥15ml/min/1.73m2, respectively, at the time of dialysis initiation.12 The reasons for high mortality in the first year on HD is unclear.13, 14 Inevitably, these patients will experience loss of RKF over time, which occurs more rapidly amongst hemodialysis10, 11, 13, 15–17 than peritoneal dialysis patients,18, 19 presumably due to episodic renal ischemia from intra-dialytic hypotension and hypovolemia, activation of nephrotoxic inflammatory mediators due to exposure to dialysis tubing and impurities,10, 18–21 and reduction in uremic substances that serve as the stimulus for remaining hyperfunctioning nephrons.22
Recognizing the importance of the preservation of RKF, there has been increasing interest in incremental hemodialysis as an innovative approach for initiating hemodialysis amongst incident ESRD patients.23–28 For example, in a study of 85 incident hemodialysis patients in Shanghai, those who were initiated and maintained on twice-weekly hemodialysis vs. conventional thrice-weekly hemodialysis over the entire study period were less likely to experience loss of RKF (defined as UOP <200ml/day) over the course of one year.29 In a more recent analysis of 23,645 incident US hemodialysis patients, incremental twice-weekly vs. conventional thrice-weekly hemodialysis was associated with greater preservation of RKF, defined by renal urea clearance and urine volume, after one year.30 An increasing body of data also suggests that incremental hemodialysis is associated with equivalent to better survival, particularly amongst patients with substantial RKF (renal urea clearance ≥3ml/min/1.73m2).17, 30, 31
While the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) Peritoneal Dialysis Adequacy Group recommends consideration of an incremental start of dialysis (i.e., less than a “full” dose) in peritoneal dialysis patients with substantial RKF, much less has been written about the practical implementation of incremental hemodialysis.32, 33 Although NKF-KDOQI Hemodialysis Adequacy Group guidelines endorse twice-weekly hemodialysis amongst patients with renal urea clearances (KRU) exceeding 3ml/min/1.73m2, it is not per se recommended as an initial treatment strategy amongst such patients.32 Thus, to better inform the field, we describe the largest case series of a university-based single center’s experience in implementing an Incremental Hemodialysis Program among incident ESRD patients.
METHODS
We conducted a retrospective analysis of 13 incident ESRD patients who initiated incremental twice-weekly hemodialysis in the outpatient setting over the contemporary period of January 2015 to August 2016 at the University California Irvine Dialysis Center in Orange, California. (The management of the dialysis facility has recently been transferred to Fresenius Medical Care [FMC] under the new designation “FMC University Dialysis Center of Orange.”) Among these patients, eight were transitioned to conventional thrice-weekly hemodialysis, whereas the remaining five patients have continued to receive twice-weekly hemodialysis at the time of this report (December 2016). In the overall cohort and between these two groups, we analyzed socio-demographic characteristics, laboratory parameters, dialysis treatment characteristics, and in-center hemodialysis medications at the time of dialysis initiation. We additionally compared laboratory parameters and dialysis treatment characteristics at the time of dialysis transition among those who transitioned to thrice-weekly hemodialysis vs. the most recent values (i.e., values at the end of the observation period) among those who maintained twice-weekly hemodialysis.
RESULTS
Study Population
The baseline characteristics of the 13 enrolled are presented in Table 1. Their mean age (± SD) was 52.7 ± 18.2 years; 39% were female; and 23%, 54%, 15%, and 8% were non-Hispanic White, Hispanic, Asian, and Pacific Islander, respectively. The most common causes of ESRD were diabetic nephropathy (31%) and hypertension (15%), and other etiologies included reduced nephron mass due to partial nephrectomy, obstructive nephropathy, glomerulonephritis (e.g., membranoproliferative glomerulonephritis, IgA nephropathy), chronic interstitial nephritis, cystic disease (e.g., tuberous sclerosis), and congenital renal dysplasia (e.g., solitary kidney) (Figure 1). A large proportion of patients were diagnosed with hypertension (85%), diabetes (54%), and cardiovascular disease (31%). At the time of dialysis initiation, 46% of patients were using an arteriovenous fistula, and 54% patients were using a tunneled dialysis catheter as their primary vascular access. The average initial hemodialysis session length was 164 ± 34 minutes, and 31%, 8%, 46%, and 15% of patients were receiving 2–<2.5 hours, 2.5–<3.0 hours, 3.0–<3.5 hours, and 3.5–4.0 hours of treatment per session, respectively.
Table 1.
Baseline characteristics of 13 incident end-stage renal disease (ESRD) patients initiated on twice-weekly hemodialysis schedules.
| Patient | Age | Sex | Race/ Ethnicity |
Cause of ESRD | Diabetes (Y/N) |
HTN (Y/N) |
CVD (Y/N) |
Duration on twice- weekly HD (months) |
Transition to thrice- weekly HD (Y/N) |
Reason for transition from twice- weekly to thrice-weekly HD |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 71 | F | Hispanic | Diabetic nephropathy | Y | Y | Y | 15.5 | Y | IDWG and fluid overload |
| Patient 2 | 43 | F | Non-Hispanic White | Diabetic nephropathy | Y | Y | N | 11 | Y | IDWG and new onset HFrEF |
| Patient 3 | 59 | M | Non-Hispanic White | Diabetic nephropathy | Y | Y | N | 6 | Y | IDWG and fluid overload |
| Patient 4 | 70 | M | Hispanic | Obstructive nephropathy | Y | Y | Y | 9 | Y | IDWG |
| Patient 5 | 20 | M | Hispanic | Membranoproliferative glomerulonephritis | N | N | N | 5 | Y | Adequacy (hyperkalemia) |
| Patient 6 | 50 | F | Hispanic | Hypertension, NSAID use | N | Y | N | 5 | Y | Adequacy (acidosis, hyperkalemia, hyperphosphatemia) |
| Patient 7 | 68 | F | Asian | Diabetic nephropathy | Y | Y | Y | 4 | Y | Adequacy (hyperkalemia, acidosis, low clearance) |
| Patient 8 | 50 | M | Asian | Chronic interstitial nephritis | N | Y | N | 6 | Y | Adequacy |
| Patient 9 | 73 | M | Asian | Hypertension/Ischemic nephropathy | Y | Y | Y | 12 | N | N/A |
| Patient 10 | 66 | F | Hispanic | Reduced nephron mass (partial nephrectomy), hypertension, diabetic nephropathy | Y | Y | N | 20 | N | N/A |
| Patient 11 | 31 | M | Hispanic | Tuberous sclerosis | N | N | N | 8 | N | N/A |
| Patient 12 | 59 | M | Hispanic | IgA nephropathy & Focal segmental glomerulosclerosis | N | Y | N | 7 | N | N/A |
| Patient 13 | 25 | M | Non-Hispanic White | Congenital renal dysplasia/solitary kidney | N | Y | N | 3.5 | N | N/A |
Abbreviations: M: male; F: female; Y: yes; N: no; HTN: hypertension; CVD: cardiovascular disease; HD: hemodialysis; IDWG: interdialytic weight gain; HFrEF: heart failure with reduced ejection fraction; URR: urea reduction ratio
Figure 1.
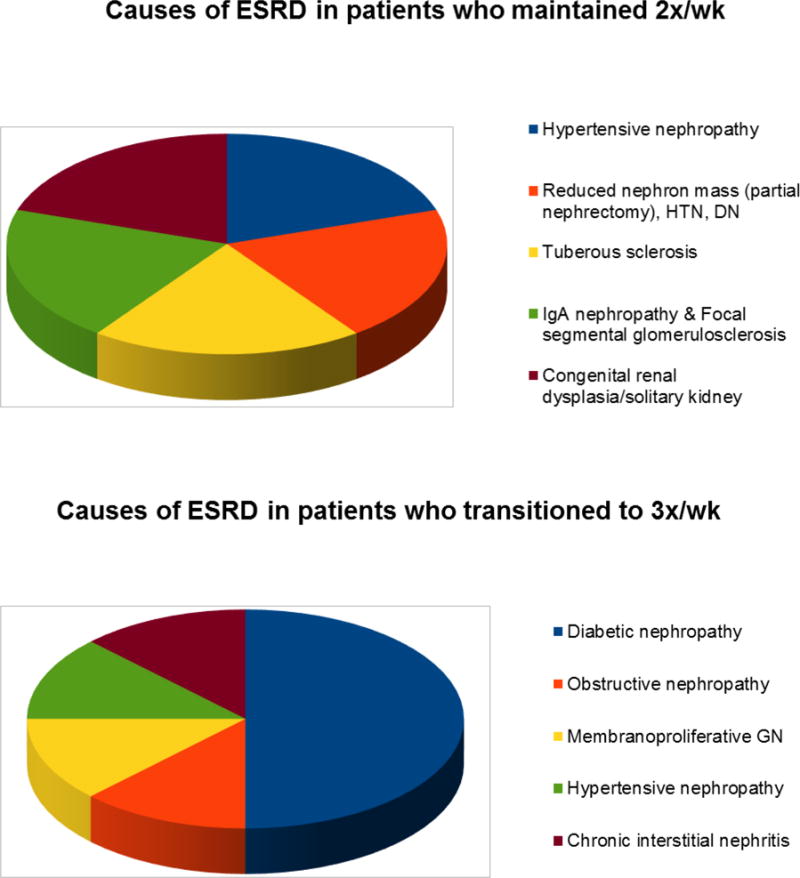
Causes of end-stage renal disease (ESRD) among patients who remained on twice-weekly hemodialysis (N=5) and those who escalated to thrice-weekly hemodialysis (N=8)
Among patients who eventually transitioned to thrice-weekly hemodialysis (N=8), the mean ± SD, median (IQR), and minimum-maximum duration of their twice-weekly hemodialysis regimens were 8.0 ± 3.9 months, 4.5 (5.0, 9.5) months, and 4.0–15.5 months, respectively. The two primary indications for transitioning to thrice-weekly hemodialysis were problems with (1) excessive interdialytic weight gain (N=4) and (2) inability to achieve adequate total clearance (N=4) (Figure 2). Among patients who experienced excessive interdialytic weight gain, one patient developed new onset systolic heart failure and three patients developed frank volume overload. Among patients with inadequate total clearance, the following laboratory aberrancies were observed: hyperkalemia (serum potassium >5.3mmol/L; N=3), metabolic acidosis (serum bicarbonate <20mmol/L; N=2), inadequate dialytic clearance per session (N=4), and hyperphosphatemia (serum phosphorus >6mg/dl; N=2). Among patients who maintained a twice-weekly hemodialysis regimen (N=5), the mean ± SD, median (IQR), and minimum-maximum duration of their twice-weekly hemodialysis regimens were 12.0 ± 6.3 months, 5.0 (9.0, 14.0) months, and 5.5–22.0 months, respectively.
Figure 2.
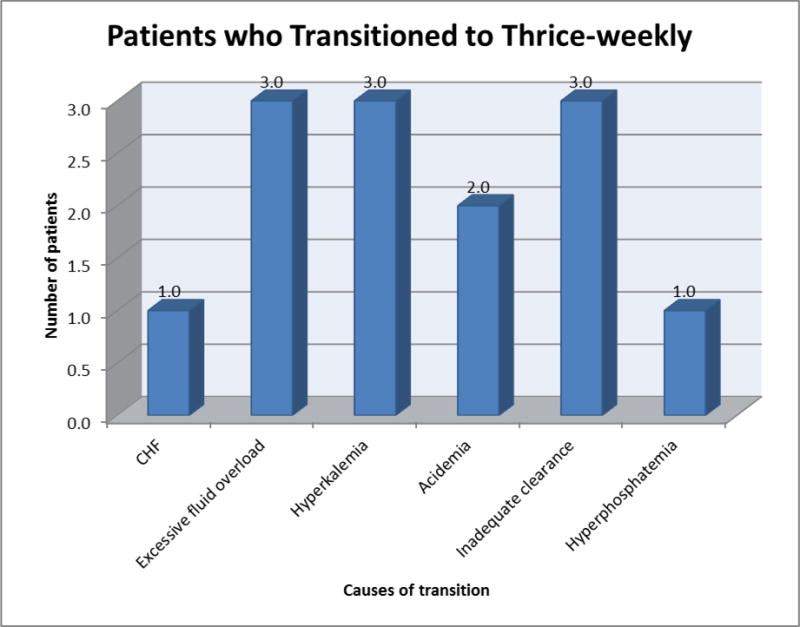
Indications for escalating to thrice-weekly hemodialysis among eight patients. *Note: patients may have had multiple concomitant indications for transition to thrice-weekly hemodialysis.
Comparison of Case-Mix Characteristics of Patients Who Maintained Twice-Weekly vs. Transitioned to Thrice-Weekly Hemodialysis Regimens
At the time of dialysis initiation, compared to patients who maintained twice-weekly hemodialysis (N=5), those who transitioned to thrice-weekly hemodialysis over the observation period (N=8) tended to be older (mean ± SD ages 54 ± 17 vs. 51 ± 21 years, respectively) and had a higher proportion of females (50% vs. 20%, respectively). Between the two groups, there were similar proportions of patients who were non-Hispanic White (25% vs. 20%, respectively), Hispanic (50% vs. 60%, respectively), and Asian/Pacific Islander (25% vs. 20%, respectively). Compared to patients who maintained twice-weekly schedules, those who transitioned to thrice-weekly hemodialysis had a higher prevalence of diabetes (63% vs. 40%, respectively), hypertension (88% vs. 80%, respectively), and cardiovascular disease (38% vs. 20%, respectively). Fifty percent of the patients who transitioned to thrice-weekly hemodialysis had diabetes as their etiology of ESRD, whereas those who were maintained on twice-weekly dialysis had non-diabetic etiologies of ESRD only (Figure 1). In comparison to twice-weekly patients, thrice-weekly patients had a higher prevalence of tunneled dialysis catheters as their primary vascular access at the time of dialysis initiation (63% vs. 40%, respectively), an observation that persisted at the time of review of the most recent characteristics (37% vs. 20% of thrice-weekly vs. twice-weekly patients, respectively).
Comparison of Laboratory Characteristics of Patients Who Maintained Twice-Weekly vs. Transitioned to Thrice-Weekly Hemodialysis Regimens
A comparison of the initial laboratory characteristics among patients who remained on a twice-weekly hemodialysis regimen vs. those who eventually transitioned to conventional thrice-weekly hemodialysis showed notable differences (Table 2). Compared to patients who remained on twice-weekly hemodialysis, those who transitioned to thrice-weekly hemodialysis tended to have higher serum potassium (4.7 ± 1.0 vs. 3.7 ± 0.6mmol/L, respectively) and lower serum sodium levels (134 ± 4 vs. 139 ± 2mmol/L, respectively). In terms of mineral bone disease parameters, compared to twice-weekly hemodialysis patients, thrice-weekly patients had higher parathyroid hormone (766 ± 1154 vs. 525 ± 422pg/ml, respectively), lower serum phosphorus (5.5 ± 1.5 vs. 6.3 ± 1.6mg/dl, respectively), and higher serum calcium levels (8.6 ± 0.7 vs. 8.0 ± 1.1mg/dl, respectively). With respect to anemia parameters, they were also observed to have lower hemoglobin (8.6 ± 0.7 vs. 9.9 ± 1.1g/dl, respectively), lower iron saturation (23 ± 10 vs. 26 ± 18%, respectively), and higher serum ferritin levels (421 ± 288 vs. 233 ± 226ng/ml, respectively). In terms of nutritional markers, they had lower serum albumin (3.3 ± 0.4 vs. 3.5 ± 0.7g/dl, respectively) and serum creatinine levels (6.6 ± 2.4 vs. 7.2 ± 1.1mg/dl, respectively).
Table 2. Dialysis treatment and laboratory characteristics at the initiation of twice-weekly hemodialysis.
| Patient | IDWG* (kg) |
Serum K (mmol/L) |
Phos (mg/dl) |
PTH (pg/ml) |
Hb (mg/dl) |
ESA* (units) |
Serum bicarbonate (mmol/L) |
BMI | PreHD SBP (mmHg) |
HD treatment time (min) |
Dialysate K (meq) |
Vascular access type |
Calcitriol dose (mcg)** |
Serum albumin (mg/dl) |
Serum creatinine (mg/dl) |
Ca (mg/dl) |
Tsat (%) |
Ferritin (ng/ml) |
Na (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 1.3 | 3.7 | 3.7 | 7 | 8.4 | 2200 | 23 | 33.1 | 151 | 180 | 3 | TC | 0.25 qd | 3.1 | 3.4 | 9.9 | 16 | 430 | 124 |
| Patient 2 | 1.4 | 4.4 | 7.9 | 92 | 8.8 | 2200 | 17 | 37.0 | 190 | 120 | 3 | AVF | none | 2.8 | 8.5 | 8.3 | 18 | 65 | 140 |
| Patient 3 | 2.0 | 4.7 | 5.7 | 272 | 9.7 | 0 | 18 | 27.1 | 167 | 210 | 2 | TC | none | 3.0 | 4.1 | 8.4 | 38 | 552 | 137 |
| Patient 4 | 1.0 | 4.6 | 3.9 | 244 | 8.8 | 2200 | 27 | 27.6 | 166 | 180 | 3 | TC | none | 2.9 | 6.4 | 9.2 | 30 | 204 | 134 |
| Patient 5 | 0.4 | 7.1 | 4.5 | 895 | 7.3 | 4400 | 19 | 19.9 | 147 | 180 | 1 | AVF | 0.25/HD | 3.8 | 5.4 | 8.8 | 13 | 916 | 138 |
| Patient 6 | 0.2 | 4.4 | 6.9 | 3475 | 8.1 | 2000 | 22 | 22.6 | 135 | 150 | 3 | TC | 0.25/HD | 3.7 | 7.8 | 8.3 | 17 | 322 | 137 |
| Patient 7 | 1.5 | 4.3 | 5.1 | 156 | 8.7 | 3000 | 16 | 23.5 | 155 | 180 | 3 | AVF | 0.25/HD | 3.8 | 10.7 | 8.4 | 14 | 697 | 136 |
| Patient 8 | 1.7 | 4.4 | 6.5 | 990 | 8.8 | 2000 | 16 | 23.7 | 140 | 180 | 2 | TC | 0.25/HD | 3.6 | 6.5 | 7.5 | 35 | 181 | 136 |
| Patient 9 | 1.6 | 4.4 | 7.5 | 311 | 9.3 | 4400 | 25 | 33.2 | 179 | 120 | 3 | AVF | 0.25 qd | 3.9 | 8.0 | 8.2 | 22 | 306 | 138 |
| Patient 10 | 0.3 | 3.3 | 7.3 | 71 | 10.2 | 0 | 23 | 24.8 | 138 | 120 | 3 | AVF | none | 3.4 | 6.3 | 8.7 | 12 | 46 | 140 |
| Patient 11 | 1.2 | 3.0 | 3.8 | 779 | 9.9 | 0 | 18 | 18.4 | 113 | 180 | 3 | TC | 0.25/HD | 2.5 | 7.4 | 6.1 | 57 | 72 | 139 |
| Patient 12 | 1.5 | 4.2 | 7.4 | 339 | 11.6 | 0 | 10 | 35.0 | 149 | 120 | 3 | AVF | 0.5 qd | 4.3 | 8.3 | 8.3 | 20 | 145 | 138 |
| Patient 13 | 0.1 | 3.5 | 5.7 | 1125 | 8.6 | 5000 | 24 | 29.9 | 174 | 210 | 3 | TC | none | 3.5 | 5.8 | 8.8 | 21 | 594 | 135 |
Abbreviations: K: serum potassium; Phos: serum phosphorus; HTN: hypertension; PTH; parathyroid hormone; Hg: hemoglobin; ESA: erythropoietin stimulating agent; BMI: body mass index; HD: hemodialysis; IDWG: interdialytic weight gain; Ca: serum calcium; Tsat: transferrin saturation; Na: serum sodium; TC: tunneled catheter; qd: daily.
Equivalent ESA dose among patients receiving varying ESA formulations.
Equivalent calcitriol dose among patients receiving varying activated vitamin D/vitamin D analog formulations.
When the most recent laboratory values of patients who remained on twice-weekly hemodialysis were compared to those who transitioned to thrice-weekly dialysis at the time of dialysis transition, those on thrice-weekly treatment persisted in having higher serum potassium (5.3 ± 0.8 vs. 4.3 ± 0.5mmol/L, respectively), higher parathyroid hormone (661 ± 946 vs. 526 ± 414pg/ml, respectively), lower hemoglobin (9.2 ± 1.0 vs. 10.7 ± 1.1g/dl, respectively), and lower iron saturation levels (17 ± 4 vs. 37 ± 23%) (Table 3).
Table 3. Dialysis treatment and laboratory characteristics immediately prior to transition to thrice-weekly hemodialysis and most recent values among those who maintained twice-weekly hemodialysis.
| Patient | IDWG* (kg) | Serum K (mmol/L) |
Phos (mg/dl) |
PTH (pg/ml) |
Hb (mg/dl) |
ESA* (units) |
Serum bicarbonate (mmol/L) |
BMI | PreHD SBP (mmHg) |
HD treatment time (min) |
Dialysate K (meq) |
Vascular access type |
Calcitriol dose (mcg)** |
Serum albumin (mg/dl) |
Serum creatinine (mg/dl) |
Ca (mg/dl) |
Tsat | Ferritin (ng/ml) |
Na (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 2.9 | 5.3 | 4.5 | 129 | 9.8 | 3000 | 26 | 35 | 175 | 180 | 3 | AVF | 0.25 qd | 3.7 | 6.4 | 9.7 | 24 | 1210 | 128 |
| Patient 2 | 2.7 | 4 | 5.2 | 61 | 10.1 | 3000 | 25 | 34.3 | 194 | 180 | 2 | AVF | none | 3.4 | 7 | 9 | 18 | 598 | 128 |
| Patient 3 | 5.8 | 5.1 | 4.5 | 321 | 8 | 2000 | 20 | 28.5 | 160 | 240 | 2 | TC | none | 3.1 | 7.24 | 8.4 | 18 | 416 | 133 |
| Patient 4 | 3.6 | 5.4 | 5.7 | 415 | 8.3 | 3000 | 23 | 26.8 | 153 | 180 | 2 | TC | 0.25/HD | 3.5 | 8.1 | 7.2 | 16 | 348 | 137 |
| Patient 5 | 3.4 | 6.5 | 9.1 | 1125 | 7.8 | 3000 | 17 | 21.5 | 152 | 225 | 2 | AVF | 0.75 | 4 | 12.9 | 8.3 | 10 | 1164 | 134 |
| Patient 6 | 0.5 | 6.4 | 10.6 | 2846 | 9.6 | 4600 | 13 | 25.6 | 161 | 150 | 3 | AVF | 0.5/HD | 3.8 | 11 | 7.6 | 13 | 129 | 135 |
| Patient 7 | 0.3 | 5.3 | 5.6 | 296 | 10 | 3000 | 23 | 23.7 | 154 | 180 | 2 | AVF | 0.5/HD | 4 | 8.7 | 8.8 | 20 | 296 | 134 |
| Patient 8 | 1.7 | 4.5 | 5 | 93 | 10 | 6000 | 25 | 22.9 | 141 | 180 | 2 | TC | 0.75/HD | 4 | 12.1 | 9.2 | 14 | 120 | 140 |
| Patient 9 | 2.83 | 4.5 | 8.3 | 504 | 11 | 4400 | 20 | 31.6 | 177 | 210 | 3 | AVF | none | 3.6 | 7.9 | 7.7 | 29 | 288 | 141 |
| Patient 10 | 1.7 | 4.9 | 5 | 17 | 9.3 | 3000 | 27 | 23.4 | 149 | 180 | 3 | AVF | none | 4.2 | 11.9 | 9.5 | 25 | 1168 | 138 |
| Patient 11 | 1.6 | 4.1 | 3.6 | 1154 | 10 | 4000 | 23 | 21.4 | 136 | 180 | 3 | AVF | 4.5/HD | 3.9 | 8.7 | 8.1 | 77 | 262 | 139 |
| Patient 12 | 3.2 | 3.6 | 6.5 | 361 | 11.1 | 0 | 22 | 36 | 146 | 210 | 3 | AVF | none | 4.1 | 9.7 | 7.6 | 21 | 65 | 137 |
| Patient 13 | 3.6 | 4.2 | 6.2 | 595 | 12.2 | 7500 | 27 | 26.8 | 171 | 200 | 3 | TC | 0.25/HD | 3.3 | 4.7 | 9.1 | 32 | 774 | 138 |
Abbreviations: K: serum potassium; Phos: serum phosphorus; HTN: hypertension; PTH; parathyroid hormone; Hg: hemoglobin; ESA: erythropoietin stimulating agent; BMI: body mass index; HD: hemodialysis; IDWG: interdialytic weight gain; Ca: serum calcium; Tsat: transferrin saturation; Na: serum sodium; TC: tunneled catheter; qd: daily.
Equivalent ESA dose among patients receiving varying ESA formulations.
Equivalent calcitriol dose among patients receiving varying activated vitamin D/vitamin D analog formulations.
Comparison of Dialysis Treatment Characteristics of Patients Who Maintained Twice-Weekly vs. Transitioned to Thrice-Weekly Hemodialysis Regimens
Differences in the initial dialysis treatment characteristics between the two groups were also observed (Table 2). Compared to patients who remained on twice-weekly hemodialysis, those who transitioned to thrice-weekly hemodialysis tended to have higher interdialytic weight gain (1.2 ± 0.6 vs. 0.9 ± 0.7kg, respectively) and higher pre-dialysis blood pressures (156 ± 18 vs. 151 ± 27mmHg, respectively). In terms of dialysis prescriptions, compared to twice-weekly patients, thrice-weekly patients had longer dialysis session lengths (173 ± 27 vs. 150 ± 42minutes, respectively) and greater use of dialysate with lower potassium concentrations (i.e., at initiation 75% of thrice-weekly patients were treated with a 2K dialysate bath whereas 100% of twice-weekly patients were treated with a 3K dialysate bath).
Compared to the most recent values of the twice-weekly group, the thrice-weekly group’s characteristics at the time of transition showed similar interdialytic weight gain (2.6 ± 1.8 vs. 2.6 ± 0.9kg, respectively), pre-dialysis blood pressures (161 ± 16 vs. 156 ± 17mmHg, respectively), and dialysis session lengths (~3.0 hours across both groups) (Table 3).
Changes in Characteristics over Time among Patients Who Transitioned to Thrice-Weekly Hemodialysis
Among patients who eventually transitioned to thrice-weekly hemodialysis, baseline characteristics at the time of initiation of twice-weekly dialysis vs. those at the time of transition to thrice-weekly hemodialysis are outlined in Tables 2 and 3. Over time, higher serum potassium levels were observed (5.3 ± 0.8 vs. 4.7 ± 1.0mmol/L, respectively). In terms of mineral bone disease parameters, they developed higher serum phosphorus (6.3 ± 2.3 vs. 5.5 ± 1.5mg/dl, respectively), lower parathyroid hormone (661 ± 946 vs. 766 ± 1154pg/ml, respectively), and maintained similar calcium levels (8.5 ± 0.8 vs. 8.6 ± 0.7mg/dl, respectively). In terms of anemia parameters, they had higher hemoglobin (9.2 ± 1.0 vs. 8.6 ± 0.7g/dl, respectively), lower iron saturation (17 ± 4 vs. 23 ± 10%, respectively), and higher ferritin levels over time (535 ± 431 vs. 421 ± 288ng/ml, respectively). In terms of nutritional parameters, they had higher serum albumin (3.7 ± 0.3 vs. 3.3 ± 0.4g/dl, respectively) and higher serum creatinine levels over time (9.2 ± 2.5 vs. 6.6 ± 2.4mg/dl, respectively). With respect to dialysis treatment characteristics, they had higher interdialytic weight gain (2.6 ± 1.8 vs. 1.2 ± 0.6kg, respectively), higher pre-dialysis blood pressure (161 ± 16 vs. 156 ± 18mmHg, respectively), longer dialysis session length (189 ± 29 vs. 173 ± 27minutes, respectively), and greater use of dialysate with lower potassium concentrations (i.e., 2K dialysate baths) (75% vs. 25%, respectively) over time.
Changes in Characteristics over Time among Patients Who Maintained Twice-Weekly Hemodialysis
Baseline characteristics at the time of initiation compared to the most recent values among patients maintained on a twice-weekly hemodialysis regimen are shown in Tables 2 and 3. Similar to those converted to thrice-weekly dialysis, serum potassium concentrations rose albeit to lower levels (4.3 ± 0.5 vs. 3.7 ± 0.6mmol/L, respectively). In terms of mineral bone disease parameters, they developed higher serum phosphorus (6.3 ± 2.3 vs. 5.5 ± 1.5mg/dl, respectively), lower parathyroid hormone (661 ± 946 vs. 766 ± 1154pg/ml, respectively), and maintained similar calcium levels (8.5 ± 0.8 vs. 8.6 ± 0.7mg/dl, respectively). In terms of anemia parameters, they had higher hemoglobin (9.2 ± 1.0 vs. 8.6 ± 0.7g/dl, respectively), lower iron saturation (17 ± 4 vs. 23 ± 10%, respectively), and higher ferritin levels over time (535 ± 431 vs. 421 ± 288ng/ml, respectively). In terms of nutritional parameters, they had higher serum albumin (3.7 ± 0.3 vs. 3.3 ± 0.4g/dl, respectively) and higher serum creatinine levels over time (9.2 ± 2.5 vs. 6.6 ± 2.4mg/dl, respectively). With respect to dialysis treatment characteristics, they had higher interdialytic weight gain (2.6 ± 1.8 vs. 1.2 ± 0.6kg, respectively), higher pre-dialysis blood pressure (161 ± 16 vs. 156 ± 18mmHg, respectively), longer dialysis session length (189 ± 29 vs. 173 ± 27minutes, respectively), and greater use of dialysate with lower potassium concentrations (i.e., 2K dialysate baths) (75% v. 25%, respectively) over time.
DISCUSSION
In this report, we describe the largest case series of ambulatory incident ESRD patients enrolled in a university-based Incremental Hemodialysis Program. Over a 23-month follow up period, 13 patients initiated incremental hemodialysis, among whom eight patients eventually transitioned to thrice-weekly hemodialysis and five patients have continued on a twice-weekly hemodialysis regimen.
In this descriptive evaluation, we observed that patients who transitioned to conventional thrice-weekly hemodialysis had a higher comorbidity burden (e.g., diabetes, hypertension, cardiovascular disease) compared to their counterparts who maintained twice-weekly hemodialysis. Notably, half of the patients who transitioned to thrice-weekly hemodialysis had diabetic nephropathy as the underlying cause of their ESRD compared to none of those who were maintained on a twice weekly regimen. This is consistent with observations in pre-dialysis chronic kidney disease patients and incident hemodialysis patients in whom diabetic nephropathy is associated with faster kidney function decline vs. non-diabetic etiologies.34, 35
Those who eventually transitioned to thrice-weekly hemodialysis had mildly worse metabolic status (e.g., hyperkalemia, hyponatremia), mineral bone disease parameters (e.g., higher parathyroid hormone levels), hematologic values (e.g., lower hemoglobin, lower iron saturation), malnutrition-inflammation-complex characteristics (e.g., lower serum albumin, lower serum creatinine, higher serum ferritin), and fluid balance (e.g., higher interdialytic weight gain, higher pre-dialysis blood pressure) than those remaining on twice-weekly hemodialysis.
We did not have access to data on serial renal urea clearance and urine output measurements over time. While it is possible that, compared to the twice-weekly group, the thrice-weekly patients may have had lower baseline RKF and faster decline over time, behavioral factors such as compliance with diet, medications, and dialysis treatments may also have contributed to these initial differences and eventual transition to more frequent hemodialysis. Longitudinal follow up of these patients also showed that, compared to the twice-weekly group’s most recent values, the thrice-weekly group’s values at the time of transition continued to show worse metabolic (e.g. hyperkalemia), mineral bone disease (e.g., higher parathyroid hormone), and anemia parameters (e.g., lower hemoglobin and iron saturation). Although updated fluid status parameters showed that average interdialytic weight gain and pre-dialysis blood pressures were similar across the two groups, excessive fluid gain was cited as an indication for transitioning to more frequent hemodialysis among 50% of the thrice-weekly patients.
Beyond the NKF-KDOQI’s recommendations regarding a requisite amount of RKF (i.e., renal urea clearance of >3ml/min/1.73m2), there are limited data on the optimal subpopulations and patient characteristics for the implementation of incremental hemodialysis.25 Experts have proposed criteria that can be used to select appropriate patients for the incremental hemodialysis regimen,25 including those with (1) renal urea clearance >3ml/min/1.73m2 AND (2) urine output >500ml/day, as well as five additional criteria: (a) limited fluid retention between two consecutive hemodialysis treatments with an interdialytic weight gain <2.5kg, or <5% of the ideal dry weight without hemodialysis for three to four days; (b) limited or readily manageable cardiovascular or pulmonary symptoms without excessive fluid overload; (c) infrequent or readily manageable hyperphosphatemia; (d) ESA-responsiveness and an absence of profound anemia (hemoglobin <8g/dl); (e) suitable body size, particularly if not hypercatabolic; (f) infrequent or readily manageable hyperkalemia; (g) adequate nutritional status without hypercatabolism; (h) infrequent hospitalization and easily manageable comorbidities; and (i) adequate health-related quality of life.
At this time, there remain multiple questions with respect to the practical implementation of incremental hemodialysis among incident ESRD patients, such as (1) What is the optimal dialysis prescription for incremental hemodialysis?; (2) Beyond assessing quarterly renal urea clearance as per the NKF-KDOQI Hemodialysis Adequacy Group guidelines, are there additional accurate and efficient tools that can be used to serially monitor patients’ RKF?; (3) What adjunctive management strategies (i.e., medications, diet, physical activity) can be concurrently implemented with incremental hemodialysis to best preserve RKF?; (4) What characteristics predict patients who will be able to maintain incremental hemodialysis vs. eventually transition to thrice-weekly hemodialysis?; (5) What are the optimal transition points for escalation from twice- to thrice-weekly hemodialysis?; and (6) Does the incremental hemodialysis regimen provide a more cost-effective management strategy than conventional hemodialysis?
Our report has several limitations which bear acknowledgement. First, we lacked data on baseline and longitudinal renal urea clearance and urine output data that could be correlated with patients’ characteristics and trajectories towards escalation to thrice-weekly vs. continuation of twice-weekly hemodialysis. Second, due to data limitations, we were unable to examine and compare relevant outcomes such as health-related quality of life, hospitalization, and mortality risk amongst patients in the Incremental Hemodialysis Program; they are being examined in corollary studies. Third, given that our case-series was restricted to a single university-based center with experience in the implementation of incremental hemodialysis, our findings may not be generalizable to other patient populations. However, we aspire for our experience to serve as a template for the broader implementation of incremental hemodialysis across other medical centers.
In conclusion, we describe a novel strategy for initiating hemodialysis among incident ESRD patients as a means to preserve RKF in this population. Future studies are needed to refine the optimal approaches for implementation of the incremental twice-weekly hemodialysis regimen, as well as the ideal patient populations for this management strategy.
Acknowledgments
Funding Support:
The authors are supported by the research grants from the NIH/NIDDK including K23-DK102903 (CMR), K24-DK091419 (KKZ), R01-DK096920 (KKZ), U01-DK102163 (KKZ), and philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, and Dr. Joseph Lee.
Footnotes
Financial Disclosures:
None of the authors have any disclosures to report.
References
- 1.Bargman JM, et al. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–62. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 2.Canaud B. Residual renal function: the delicate balance between benefits and risks. Nephrol Dial Transplant. 2008;23(6):1801–5. doi: 10.1093/ndt/gfn089. [DOI] [PubMed] [Google Scholar]
- 3.Chandna SM, Farrington K. Residual renal function: considerations on its importance and preservation in dialysis patients. Semin Dial. 2004;17(3):196–201. doi: 10.1111/j.0894-0959.2004.17306.x. [DOI] [PubMed] [Google Scholar]
- 4.Morduchowicz G, et al. Effects of residual renal function in haemodialysis patients. Int Urol Nephrol. 1994;26(1):125–31. doi: 10.1007/BF02768252. [DOI] [PubMed] [Google Scholar]
- 5.Obi Y, et al. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J Am Soc Nephrol. 2016;27(12):3758–3768. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paniagua R, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–20. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 7.Shafi T, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–58. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Termorshuizen F, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–70. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 9.van der Wal WM, et al. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26(9):2978–83. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 10.Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24(5):487–94. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 11.Vilar E, et al. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24(8):2502–10. doi: 10.1093/ndt/gfp071. [DOI] [PubMed] [Google Scholar]
- 12.Bethesda M. US Renal Data System, USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases. 2011;10 [Google Scholar]
- 13.Bradbury BD, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 14.Lukowsky LR, et al. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35(6):548–58. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugirdas JT, et al. Effect of frequent hemodialysis on residual kidney function. Kidney international. 2013;83(5):949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diao Z, et al. Preservation of residual renal function with limited water removal in hemodialysis patients. Ren Fail. 2011;33(9):875–7. doi: 10.3109/0886022X.2011.605535. [DOI] [PubMed] [Google Scholar]
- 17.Lin YF, et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology (Carlton) 2009;14(1):59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 18.Jansen MA, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–53. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 19.Lysaght MJ, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans. 1991;37(4):598–604. [PubMed] [Google Scholar]
- 20.Burton JO, et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–20. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyodo T, Koutoku N. Preservation of residual renal function with HDF. Contrib Nephrol. 2011;168:204–12. doi: 10.1159/000321762. [DOI] [PubMed] [Google Scholar]
- 22.Bricker NS, Morrin PA, Kime SW., Jr The pathologic physiology of chronic Bright's disease. An exposition of the “intact nephron hypothesis. Am J Med. 1960;28:77–98. doi: 10.1016/0002-9343(60)90225-4. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A, Saran R, Nolph KD. Continuum and integration of pre-dialysis care and dialysis modalities. Perit Dial Int. 1999;19(Suppl 2):S276–80. [PubMed] [Google Scholar]
- 24.Daugirdas JT, Kjellstrand C. Chronic hemodialysis prescription: a urea kinetic approach. Handbook of dialysis. 1994;4:146–70. [Google Scholar]
- 25.Kalantar-Zadeh K, et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. American Journal of Kidney Diseases. 2014;64(2):181–186. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keshaviah PR, Emerson PF, Nolph KD. Timely initiation of dialysis: a urea kinetic approach. Am J Kidney Dis. 1999;33(2):344–8. doi: 10.1016/s0272-6386(99)70310-0. [DOI] [PubMed] [Google Scholar]
- 27.Mehrotra R, Nolph KD, Gotch F. Early initiation of chronic dialysis: role of incremental dialysis. Perit Dial Int. 1997;17(5):426–30. [PubMed] [Google Scholar]
- 28.Rosansky SJ, et al. Dialysis initiation: what’s the rush? Semin Dial. 2013;26(6):650–7. doi: 10.1111/sdi.12134. [DOI] [PubMed] [Google Scholar]
- 29.Lin X, et al. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33(1–3):66–72. doi: 10.1159/000334634. [DOI] [PubMed] [Google Scholar]
- 30.Obi Y, et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. American Journal of Kidney Diseases. 2016;68(2):256–265. doi: 10.1053/j.ajkd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson JA, et al. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19(6):625–33. doi: 10.1159/000013533. [DOI] [PubMed] [Google Scholar]
- 32.Hemodialysis Adequacy Work, G. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 33.Eknoyan G, et al. NKF-DOQI clinical practice guidelines for hemodialysis adequacy. American Journal of Kidney Diseases. 1997;30(3):S17–S63. doi: 10.1016/s0272-6386(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 34.Jones C, et al. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant. 2006;21(8):2133–43. doi: 10.1093/ndt/gfl198. [DOI] [PubMed] [Google Scholar]
- 35.Moist LM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11(3):556–64. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]


