Abstract
Background
Non-adherence to antiretroviral (ART) treatment remains a prevalent problem even among the segment of the U.S. HIV population that is ‘linked’ to medical care.
Methods
Controlled pilot feasibility study with ART experienced adult patients (n=20) linked to HIV medical care without suppressed viral load. Patients were randomized to a peer-led HIV medication adherence intervention named ‘Ready’ or a time equivalent ‘healthy eating’ control arm. Lay individuals living with HIV were trained to facilitate ‘Ready’.
Results
Patients had been prescribed a mean of three prior ART regimens. The group randomized to ‘Ready’ had significantly improved adherence. MEMS and pharmacy refill data correlated with viral load log drop. Higher readiness for healthful behavior change correlated with viral load drop and approached significance.
Conclusion
A peer-led medication adherence intervention had a positive impact among adults who had experienced repeated non-adherence to HIV treatment. A larger study is needed to examine intervention dissemination and efficacy.
The national goal of establishing an AIDS-free generation1 will be thwarted if HIV-infected individuals do not adhere to their HIV treatment regimens. Rates of adherence to HIV medications vary (33–88%) among the HIV-infected U.S. population and it has been reported that only a quarter of those with HIV are in care and have a suppressed viral load.2, 3 Non-adherence to antiretroviral (ART) medications is costly at many levels. Not only the non-adherent person pays a price by risking his or her individual health: non-adherence causes drug-resistance, and drug-resistant strains can be transmitted to others. HIV-infected individuals with drug-resistance require more complex and expensive treatment to suppress viral load.4,5
Although ‘test and treat’ and ‘linkage to care’ initiatives are addressing the need to identify previously undiagnosed HIV cases and establish medical care for these individuals, the high level of non-adherence remains a vexing issue even among the “linked” segment of the HIV population.2,6,7 Effective, practical interventions are needed that can help the subgroup of individuals who are linked to HIV care but are struggling with adherence to treatment and do not have a suppressed HIV viral load. Further, as healthcare resources become more limited, adherence interventions must be cost-effective, require little or no additional time from health care providers, and be acceptable to patients.8
This pilot feasibility study translated and tailored a successful nurse-led HIV treatment adherence intervention9 to a peer-led format. In this study, the term peer refers to a lay individual living with HIV disease. The study was undertaken to test the hypothesis that tailoring our intervention to the participant’s unique barriers to adherence and delivering it via a peer would increase its cultural relevance and thereby enhance acceptability and impact.
Methods
The target population for this pilot feasibility study was a group of HIV-infected adults who repeatedly had problems with adherence to HIV treatment despite being linked to HIV medical care and having access to ART medications. Researchers collaborated with one urban Midwestern U.S. clinic that serves a large number of vulnerable HIV-infected adults. The clinic, located within a safety-net hospital, predominantly serves low-income adults of color living with HIV disease. The research team consisted of: (1) lay individuals living with HIV who had worked in HIV medical care settings as paid, and/or volunteer, HIV peer educators, (2) a nurse who was a member of the clinic staff, (3) a pharmacist who was a member of the hospital staff and had expertise in medication adherence research and (4) researchers from an urban university. The members of the research team worked together to translate and tailor the intervention to a peer-led format, implement the feasibility study, and evaluate the results.
Participants and Recruitment
The pilot feasibility study was conducted in 2012 and 2013. Inclusion criteria were: (1) 18 years of age or older, (2) ability to speak English or Spanish, (3) medical record documentation of non-adherence to ART medications, (4) HIV-1 RNA by PCR not suppressed (i.e. >200 copies), (5) community-dwelling individual, and (6) access to ART medications. Recruitment was done with flyers that were placed in the waiting room of the clinic, the exam rooms, and the offices of case managers who provided social services to the target population. Health care providers, social workers and case managers also distributed the flyer to inpatients with a diagnosis of HIV who had dropped out of HIV care or who had non-suppressed HIV viral loads. The flyer briefly described the study and directed patients to call the study nurse for more details. Those interested in participating called the study nurse and were then given an appointment for an enrollment visit at which time the study was explained and informed consent was obtained. The participants also consented to collection of HIV viral load and CD4 cell count results from their medical record and medication refill information from their pharmacy.
Study Design
A randomized controlled design was used in this pilot study to examine the feasibility and preliminary impact of a peer-led HIV medication adherence intervention. Participants (n=20) were randomized to the peer-led adherence intervention (n=10) or a time and contact matched comparison intervention (n=10). Prior to randomization, both interventions were presented to potential participants as active interventions that were the same in length and format. Participants were informed that both interventions were being evaluated for possible benefit in the HIV-infected population. In addition, potential participants were told up front that all participants would receive the same compensation for participation in the study regardless of randomization group.
‘Ready’ peer-led medication adherence intervention
The peer-led intervention, named ‘Ready’, addressed the multi-faceted problem of non-adherence among individuals who were linked to HIV medical care, were prescribed antiretroviral medications, but were unable to take medications as prescribed and did not have a suppressed HIV viral load. The intervention was delivered at the individual level, based on the readiness stage of Wellness Motivation Theory10 and framed in understanding the process of initiating and maintaining healthful behavior change. Readiness often has been associated with the cessation of an unhealthy behavior such as smoking. In this intervention, readiness was conceptualized as a process that precedes the behavior of HIV medication adherence and leads to the ability to incorporate and sustain behavior. The theory purports that readiness consists of goal setting and identifying barriers and facilitators to a desired behavior. Further, social support networks enhance readiness for healthful behavior change. Hence in our intervention resources were provided for social support networks within the HIV community.
Five peers (i.e. lay individuals living with HIV) partnered with the investigators to translate the nurse-led intervention to a peer-led format. The peer-led intervention was tailored to enhance its cultural relevance for a target population who was living day to day in a culture of HIV. The peers who worked with researchers in this project were selected based on their willingness to participate in the project, their past experience working in the HIV medical care setting as lay HIV educators, and their diversity. In an effort to enhance cultural sensitivity the peers selected were diverse with regard to gender, ethnicity and language (Spanish/English).
In summary, our team developed the original adherence intervention, named Ready, which was driven by Wellness Motivation Theory and facilitated by a nurse.9 The intervention was then translated to a peer-led format under Social Cognitive Theory (SCT) (see Table 1). In SCT, a person’s behavior and environment interact at the levels of individual, group, and community. SCT is well suited for an HIV treatment adherence intervention because it accounts for both the personal and socio-cultural determinants of health.11 According to SCT, one’s attitudes about treatment adherence and self-efficacy strongly influence behavior. That is, people must believe they can produce the desired adherence behavior before undertaking a new treatment or lifestyle change. This theory focuses on behavior change at cognitive and social levels and purports that change occurs through the modeling of others. Six key concepts lead to the desired behavior change: reciprocal determinism, behavioral capability, expectations, self-efficacy, observational learning and reinforcement.12,13
Table 1.
Peer-led Ready adherence intervention under Social Cognitive Theory
| Theoretical Concept | Definition | Intervention activities |
|---|---|---|
| Reciprocal determinism | Environment can act either as a facilitator of or a barrier to a health behavior; individuals can change environments to have a positive influence on health. | Create strategies together with peer to change environment. Example: seek housing assistance to move to a new location and avoid contact with drug-using friends if drug use is a barrier to adherence. |
| Behavioral capability | Knowledge and skills to implement a desired action | Peer teaches medication adherence techniques. Example: Peers teach and then patients correctly demonstrate back how to set up a pill-box with weekly medications, and set timer as a reminder to take medications. |
| Expectations | Probability of a desired outcome from performance of behavior | Peer discusses his/her own past struggles with medication adherence. Provides an understanding that others (i.e. peers) have had problems with medication adherence and have been able to become adherent. |
| Self-efficacy | Belief in one’s ability to perform a particular action | Role-play telling friends/relatives about HIV status and medications. |
| Observational learning | Interactive knowledge transmission | Peers, who are influential others, are adherent to HIV treatment and share tips on how to become/stay adherent. |
| Reinforcement | Use or practice of desired behavior by influential others | Peers discuss how they stopped drug or alcohol use and became adherent to HIV treatment. |
Two of the peers who participated in the translation process then facilitated the peer-led Ready intervention reported in this article. The interventionists were one male and one female, who had worked as peer educators in the HIV medical care setting that served as the recruitment site for the study. The peer interventionists received their own HIV care at a different site and were not patients of the HIV medical care setting where the study was conducted.
The peer interventionists received didactic training by the researchers in preparation to facilitate the intervention and then role-played facilitation of the intervention with one of the investigators portraying a participant. The peer-led Ready intervention consisted of six individual sessions, lasting approximately one hour, which occurred once weekly for six consecutive weeks. A booster intervention session was delivered one month after the initial six weekly intervention sessions. Participants were led through a process to identify and create a list of their past barriers to HIV medication adherence. A list of common barriers and facilitators to HIV treatment adherence, identified through a review of the literature14 was used to aid participants in this process.
The intervention focused on the peer interventionist leading the participant through a process of identifying, addressing, and strategizing how to overcome the individual participant’s barriers to adherence. In keeping with Wellness Motivation Theory10, participants were asked to rank their identified barriers from ‘easiest’ to ‘most difficult’ and then the barriers were addressed in that order, beginning with the ‘easiest’ barriers. In addition the participant set health related goals which included becoming adherent to HIV medications. A nurse served as the resource person to the two peer interventionists. The nurse also assisted with addressing any medical issues that were identified as adherence barriers.
Healthy eating control comparison intervention
The comparison intervention was time and contact matched and provided identical compensation. This intervention was chosen because it was felt to be valuable to the target population given the prevalence of metabolic syndrome among people with HIV and the importance of addressing cardiac risk factors.15 In addition, the comparison intervention had been successfully implemented with another population.16 A graduate health psychology student facilitated the comparison intervention, which provided education about the benefits of healthy eating and exercise and strategies to improve diet and increase physical activity. The graduate health psychology student was trained on the comparison intervention by a nurse researcher with expertise in implementing physical activity interventions aimed to reduce cardiac risk factors in vulnerable populations.17 In addition the control participants received two workbooks endorsed by the American Heart Association about healthy eating and exercise.
Measures
Using web-based survey software (Survey Monkey®), the participants completed questionnaires at baseline about their demographic and health characteristics including age, gender, ethnicity, education level, year of HIV diagnosis and number of previous HIV medication regimens. Questionnaires on readiness for healthful behavior change, social support, and depression symptoms were completed at baseline and at the 12 and 24-week follow-up time points. Laboratory results (HIV viral load, CD4 cell count) and pharmacy refill dates were collected by study personnel from each participant’s electronic health record and pharmacy respectively.
Adherence
Medication adherence was measured using three approaches. HIV viral load suppression (HIV-1 RNA by PCR) was the primary clinical measure of interest to this study. Medical record extraction was used to collect HIV1 RNA by PCR (viral load) and CD4 cell count information. Viral load and CD4 cell count were drawn within 1 month prior to the enrollment date and at the 24-week post-enrollment follow-up time point. A viral load of <200 copies was used as the cut-off point for suppression based on the U.S. Department of Health and Human Resources (HRSA) HIV/AIDS Bureau Performance Measures for HIV viral load suppression.18 High levels of adherence are directly correlated with the suppression of HIV viral load.19
MEMS (Medication Event Monitoring System) and pharmacy refills were used as additional measures of adherence. MEMS (Aradex Group, Switzerland) are electronic caps that measure the time and date a medication bottle is opened. Electronic monitoring has been shown to be an accurate measure of adherence to ART therapy.20 The study pharmacist placed a MEMS cap on the participant’s ART medication bottle. If the participant had multiple pill bottles, the primary protease inhibitor (i.e. not ritonavir) was used for the MEMS cap placement. MEMS data were downloaded at the 2-week study time point to ensure that the MEMS cap was working correctly. The MEMS cap data were again downloaded at the 12-week booster session study visit and at the 24-week post-intervention time point. The mean 30-day adherence percentage preceding each time point was calculated for each participant.
Various pharmacy refill measures of adherence have been shown to be important predictors of HIV virologic and CD4 outcome measures.21,22 In addition, on-time pharmacy refills have been shown to be an accurate and reliable measure of adherence to ART therapy in previous studies.23 Pharmacy refill dates were used to determine the amount of ART medication the participant possessed during the study period. The study pharmacist contacted pharmacies by phone to collect the number and timeliness of ART medication pharmacy refill dates over the 24-week course of the study for each participant.
Readiness
The 30-item Index of Readiness (IR) was used to assess readiness for the healthful behavior change of adherence.24 The IR is grounded in Wellness Motivation Theory and is based on its readiness stage. The subscales represent the three critical components of the readiness stage: identification of barriers/creation of strategies, reevaluation of lifestyle, and goal commitment. A mean total readiness score is calculated, as is a mean for each subscale. Higher scores indicate more readiness for healthful behavior change. Past studies using the IR with the HIV population showed internal consistency reliability estimates of 0.89.9 In this study, readiness was hypothesized to be the intervention’s mediator for the healthful behavior change of adherence to HIV medications.
Social Support
The Medical Outcomes Study (MOS) Social Support Survey (SSS) was used to assess social support. This 19-item, 5-point Likert scale instrument was developed for use with patients with chronic conditions and is in the public domain. The MOS SSS assesses four types of support, emotional/informational, tangible, affectionate and positive social interaction, and has high internal consistency. Higher scores indicate the availability of greater social support.25 Having social support has been reported in the HIV literature to be a facilitator of adherence14 hence in this study it was treated as a moderator.
Depression
The CES-D (Center for Epidemiologic Studies Depression Scale) was used to assess depressive symptoms.26 The 20-item CES-D has been widely used in research with the general population and the HIV population. Scores are summed and a total score of over 16 indicates symptoms of depression. As depression has been linked to non-adherence in the HIV literature14 depressive symptoms were treated as a moderator in this study.
Data Analysis
Descriptive statistics and independent samples t-tests were used to examine differences between the intervention and comparison groups with regard to demographic and health data, readiness, social support, and depression symptoms at the pre-and post-intervention time points. Missing data were imputed using the last observation carried forward (LOCF) method.27 Log 10 transformations were performed for viral load measurements at baseline, 6, 12 and 24 weeks post intervention. The log drops of viral load at 6, 12 and 24 weeks post intervention were calculated and used to compare the treatment effect. Independent sample t tests were conducted to estimate and test the differences on the viral load log drop. The Pearson correlation coefficient was used to examine correlations between readiness, social support, depressive symptoms and viral load log drop from baseline to 6,12 and 24 weeks post-intervention.
Results
Demographic and Health Characteristics of Participants
The baseline characteristics of the 20 adults (15 males and 5 females) who participated in this study were similar. Participants in this study had an average of three prior virologic failures to ART regimens before entering the study. Most (75%) were individuals of color and half were over the age of 45. Participants had no significant differences with regard to demographic and health characteristics of age, gender, ethnicity, education level, years living with HIV, and medication dosing schedule. The majority of participants (85%) who enrolled in this study were on once-daily dosing ART regimens. All participants reported having depressive symptoms and limited social support at baseline. There were differences between the intervention group and comparison group on baseline HIV viral load and CD4 cell count. The intervention group had a mean viral load at baseline of 48,012 whereas that of the control group was 20,954 (p=.002). The intervention group had a mean CD4 cell count of 405 at baseline, compared to 301 for the control group (p=.03).
Attendance at Intervention Sessions
Participants in both groups received identical compensation for study participation. All ten participants in the peer-led Ready group completed the intervention and attended 100% of the intervention sessions. In the comparison group, eight participants completed a healthy eating intervention with a 60% session attendance rate overall. Of the two participants in the control group who did not complete the comparison intervention, one participant attended no sessions and the other participant attended only one intervention session.
Medication Adherence
There were significant differences between groups on medication adherence from baseline to post-intervention (see Figure 1 and Table 2). Those randomized to the ‘Ready’ peer-led intervention group had significantly improved adherence at 12 and 24 weeks with log drops of 2.62 and 3.34 respectively. Complete viral load data were available for 16 of the 20 participants, MEMS data for 18 of 20 and pharmacy refill data for 19 of 20. There were four participants in the control comparison group who did not have viral load results at the 24-week follow-up time point. For these participants the LOCF was used for the analysis and also adherence was examined using MEMS and pharmacy refill information which indicated non-adherence to their HIV medications (i.e. MEMS cap opened periodically and missed/late medication refills). There was one participant in the peer-led Ready group who did not have the MEMS cap at the 24-week follow-up time point as the MEMS cap was lost by the participant: for that patient, viral load results and pharmacy refill information were used to examine adherence. For one additional participant in the peer-led intervention group whose pharmacy could not retrieve refill data, viral load results and MEMS cap data were used to examine adherence. As has been shown in previous HIV medication adherence research, in our study the suppression of viral load was negatively correlated with consistent and on-time pharmacy refills and consistent MEMS cap events.28 In other words suppression of viral load was correlated with higher MEMS events and higher numbers of on-time pharmacy refills (Pearson correlation coefficient 0.446).
Figure 1.
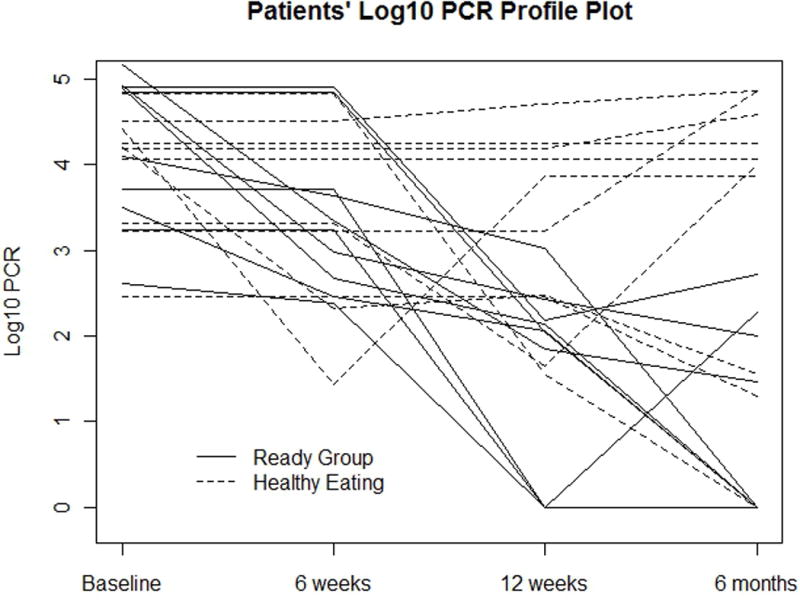
Table 2.
HIV RNA PCR log drop: baseline to 12 & 24 weeks post-intervention
| ‘Ready’ peer-led adherence intervention M (SD) (n=10) | ‘Healthy Eating’ control intervention M (SD) (n=10) | Difference M (SD) | Test statistics | P | |
|---|---|---|---|---|---|
| 12 weeks post viral load log drop | 2.6180(0.8113) | 0.7036(1.1492) | 1.9144(0.9947) | −4.30 | 0.0004 |
| 24 weeks post viral load log drop | 3.3439(1.1101) | 0.6143(1.8760) | 2.7295(1.5414) | −3.96 | 0.0009 |
Readiness, Social support and depression symptoms
Higher readiness for healthful behavior change was correlated with log 10 viral load log drop and approached significance at the 24-week post-intervention time point (See Table 3). Relationships between social support, depression symptoms and log 10 viral load log drop were not significant.
Table 3.
Pearson correlations between Log10 viral load log drop and readiness measures at 24 weeks post intervention (n=20)
| Readiness (subscales and total score) | Viral Load Log Drop Correlation (P value) |
|---|---|
| Identification of Barriers/Creation of Strategies | −0.0501 (.097) |
| Re-evaluation of Life Style | −0.077 (.812) |
| Goal Commitment | −0.509 (.091) |
| Total readiness score | −0.408 (.188) |
Discussion
This paper describes the successful translation of an HIV medication adherence intervention from a nurse-led to a peer-led format and reports the results of subsequent testing of the peer-led intervention. Our findings demonstrate positive feasibility and significantly improved adherence among patients randomized to receive the peer-led adherence intervention.
Successful peer-led (i.e. facilitated by a person living with the disease) interventions have been developed and implemented with a variety of non-infectious chronic diseases.29–32 However, the majority of peer-led interventions in HIV have focused on prevention and increasing HIV testing uptake. Peers have exhibited the ability to act successfully as change agents in HIV prevention endeavors.33 Some peer-led HIV medication adherence interventions have shown promising results and have indicated that peers are capable and effective in delivering complex behavioral HIV medication interventions.34 Peers have effectively engaged populations in treatment adherence which traditionally have been labeled as difficult-to-reach, such as injection drug users.35 Peers also serve as educators in some HIV medical care settings nationwide and have promise as behavior change agents in HIV disease.36 Now, this pilot study has shown the positive feasibility of using a peer-led intervention in a community setting to improve adherence among HIV infected individuals who are engaged in care but do not have suppressed viral loads.
The intervention was translated in partnership with individuals living with HIV to enhance cultural relevance. Wellness motivation theory was used as the theoretical framework.10 Our randomized controlled study design examined the feasibility and preliminary impact of the peer-led intervention on medication adherence. Participants in this study were adults living with HIV (n=20) who had an average of three previous virologic failures to HIV medication regimens due to non-adherence and did not have a suppressed HIV viral load at the time of study enrollment. Medication adherence was examined using three strategies: suppression of HIV viral load, MEMS cap events and timing of pharmacy refills. In addition the intervention’s mediator (readiness) and selected moderators (social support and depression symptoms) were also examined.
Participants were randomized to receive the peer-led medication adherence intervention named “Ready” or a time and contact-matched intervention, delivered by a health psychologist, which focused on healthy eating to enhance cardiovascular health. Both arms of the study were presented to participants, prior to randomization, as active interventions as both were being examined for their impact. Because the HIV population carries a high risk for metabolic and cardiovascular health problems, yet is likely to be unaware of such risk,37 an intervention that focused on heart health was felt to be of value to the target population. Of interest was the anecdotal fact that several participants stated at the enrollment visit that he/she hoped to be randomized to the comparison intervention that focused on healthy eating.
There was a statistically significant difference in viral load suppression/medication adherence between participants who received the peer-led ‘Ready’ medication adherence intervention and those who received the control comparison healthy eating intervention. Results indicated that 90% of the participants in the peer-led medication adherence intervention group had suppressed viral loads at the 24 week follow-up time point, compared to 30% of control participants (p<.01). Moreover, participants in the peer-led medication adherence intervention group attended 100% of intervention sessions while participants in the control arm attended only 60% of their intervention sessions.
Higher readiness for healthful behavior change correlated with HIV viral load drop and approached significance. The readiness components (i.e. subscales) of identifying barriers, creating strategies and setting goals were associated with medication adherence. No significant relationships, or changes over time, were observed on the moderators of social support and depression from baseline to 24-weeks post-intervention. While it could be hypothesized that the interaction with the peer would enhance social support and decrease depression, the intervention was specifically designed to increase medication adherence and not to impact these two variables. Moreover, the sample size was small which could have limited the ability to detect such findings. Also, because Social Cognitive Theory (SCT) was used to translate the intervention to its peer-led format, perhaps key components of SCT (e.g. self-efficacy) should have been examined as mediators of behavior change.
Although the results of this study are encouraging, there were limitations that must be considered when interpreting the findings. Recruitment occurred in one clinic located in the Midwestern United States with a sample of 20 adults. All participants in this study had access to ART medications. Hence, the findings of this study are not generalizable. It should also be noted that there were statistically significant differences at baseline between the treatment and control groups with regard to mean HIV viral load and CD4 cell count. However, it is unlikely that these differences were clinically significant as both groups had mean viral loads <50,000 and mean CD4 cell counts >300 at enrollment and thus were not profoundly immune compromised.5
Despite these limitations in the current study, the peer-led Ready intervention appears to have promise as a strategy that could help individuals who are struggling with taking their HIV medications as prescribed. The peer-led intervention was acceptable to the target population, as demonstrated by 100% attendance at the peer-led intervention sessions by participants. Moreover, the peer-led approach aimed to enhance the cultural relevance of the ‘Ready’ intervention. Individuals living with HIV worked with researchers to tailor ‘Ready’ and translate it to a peer-led format. This process infused relevant elements into the intervention from a culture of living with HIV. Focusing on cultural relevance has been proposed as a strategy to enhance intervention efficacy by other researchers.38,39
The ability to prevent HIV transmission and enhance individual health outcomes with ART medications and suppression of HIV viral load is one of the greatest scientific accomplishments of this era.6 Yet, non-adherence to HIV treatment persists as a challenge in the medical care arena and in the treatment and eradication of this disease. Effective and practical medication adherence interventions are needed that can help people with HIV and other chronic diseases to achieve the maximum benefit from available treatment.
The Ready intervention, with its culturally relevant, tailored and peer-led format, offers a novel approach to enhance HIV medication adherence and also has the potential to be implemented in a variety of settings. This intervention format appears to enhance receptivity by the subgroup of patients who have had repeated virologic failure to HIV treatment and who may not have responded to previous strategies to enhance adherence. Further, such peer-led medication adherence strategies could be considered for use in other chronic diseases where patients may be struggling with adherence.
Acknowledgments
This study was funded by research grants from the American Nurses Foundation and from Gilead Sciences. We thank the Department of Medicine at the University of Missouri-Kansas City for providing the computers to collect survey data and for technical support. The authors are grateful to the study participants for their willingness to be a part of this endeavor, and to research assistant Mary Kay Jesson, MSN, RN for her hard work and attention to detail.
References
- 1.The White House Office of National AIDS Policy. HIV/AIDS Strategy for the United States, update of 2011–2012. http://aids.gov/federal-resources/national-hiv-aids-strategy/overview/. Accessed: June 16, 2014.
- 2.Gardner EM, McLees MP, Stenier JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Vital signs: HIV prevention through care and treatment: United States. MMWR. 2011;60(47):1618–1623. [PubMed] [Google Scholar]
- 4.Clavel F, Hance AJ. HIV drug resistance. NEJM. 2004;350:1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 5.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed January 21 2014. [Google Scholar]
- 6.Cohen J. Breakthrough of the year: HIV treatment as prevention. Science. 2011;334:1628. doi: 10.1126/science.334.6063.1628. [DOI] [PubMed] [Google Scholar]
- 7.Montaner JS. Treatment as Prevention. Topics in Antiretroviral Medicine. 2013 Jul-Aug;21(3):110–114. [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco WP, Malone JI, Morrison AD, et al. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. J Behavioral Medicine. 2009;32:349–359. doi: 10.1007/s10865-009-9209-4. [DOI] [PubMed] [Google Scholar]
- 9.Enriquez M, Cheng A, McKinsey D, Stanford J. Development and efficacy of an intervention to enhance readiness for adherence among adults who had previously failed HIV treatment. AIDS Patient Care STDs. 2009;23(3):177–184. doi: 10.1089/apc.2008.0170. [DOI] [PubMed] [Google Scholar]
- 10.Fleury J. Empowering potential: A theory of wellness motivation. Nurs Res. 1991;13:31–35. [PubMed] [Google Scholar]
- 11.Bandura A. Health promotion by social cognitive means. Health Education & Behavior. 2004;31(2):143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 12.Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13:623–649. [Google Scholar]
- 13.Bandura A. Self-efficacy: The Exercise of Control. W.H. Freeman and Company; p. 1997. [Google Scholar]
- 14.Enriquez M, McKinsey D. Strategies to improve HIV treatment adherence in developed countries: clinical management at the individual level. HIV/AIDS Research and Palliative Care. 2011;3:45–51. doi: 10.2147/HIV.S8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LiVecchi V, Maggi P, Rizzo M, Montalto G. The metabolic syndrome and HIV infection. Current Pharmaceutical Design. 2014;19:1. doi: 10.2174/1381612819666131206104209. [DOI] [PubMed] [Google Scholar]
- 16.Peterson JA, Cheng AL. Physical activity counseling intervention to promote weight loss in overweight rural women. Journal American Association of Nurse Practitioners. 2013;25(7):385–94. doi: 10.1111/j.1745-7599.2012.00794.x. (2013) [DOI] [PubMed] [Google Scholar]
- 17.Peterson J. Get moving! Physical activity counseling in primary care. Journal of the American Academy of Nurse Practitioners. 2007;19(7):349–357. doi: 10.1111/j.1745-7599.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 18.HRSA Core Measures HIV/AIDS Programs. National Quality Forum #2082. 2013 Nov; http://hab.hrsa.gov/deliverhivaidscare/coremeasures.pdf. Accessed: July 16, 2014.
- 19.Bangsberg D. Less then 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clinical Infectious Diseases. 2006;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 20.Lylmo RA, Vanden Boogaard J, Msoka E, et al. Measuring adherence to antiretroviral therapy in northern Tanzania: feasibility and acceptability of the Medication Event Monitoring System. BMC Public Health. 2011;11:92. doi: 10.1186/1471-2458-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnsten JH, Farzadegan H, Grant RW, et al. Antiertroviral therapy adherence and viral suppression in HIV-infected dug users: comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33(8):1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNabb J, Ross J, Abriola K, et al. Adherence to highly active antiretorivral therapy predicts virologic outcome at an inner city human immunodeficiency virus clinic. Clinical Infectious Diseases. 2001;33(5):700–705. doi: 10.1086/322590. [DOI] [PubMed] [Google Scholar]
- 23.Marconi VC, Wu B, Hampton J, et al. Early warning indicators for first-line virologic failure independent of adherence measures in a South African urban clinic AIDS Patient Care STDs. 2013;27(12):657–68. doi: 10.1089/apc.2013.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleury J. The index of readiness: Development and psychometric analysis. J Nurs Measure. 1994;31:470–477. [PubMed] [Google Scholar]
- 25.Sherborune CD, Stewart AL. The MOS Social Support Survey. Social Science and Medicine. 1991;3:705–14. doi: 10.1016/0277-9536(91)90150-b. (1991) [DOI] [PubMed] [Google Scholar]
- 26.Hann D, Winter K, Jacobsen P. Measurement of depression symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale CES-D. J Psychosomat Res. 1999;46:385–401. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 28.Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Adherence to HIV antiretrovirals among persons with serious mental illness. AIDS Patient Care STDs. 2003 Apr;17(4):179–86. doi: 10.1089/108729103321619782. [DOI] [PubMed] [Google Scholar]
- 29.Coull AJ, Taylor VH, Elton R, et al. A randomised controlled trial of senior lay health mentoring in older people with ischaemic heart disease: The Braveheart Project. Age and Aging. 2004;33:348–54. doi: 10.1093/ageing/afh098. [DOI] [PubMed] [Google Scholar]
- 30.Druss BG, Zhao L, vonEsenwien SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophrenia Research. 2010;118:264–70. doi: 10.1016/j.schres.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothrock JF, Parada VA, Sims C, et al. The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache. 2006;46:726–731. doi: 10.1111/j.1526-4610.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 32.Truncali A, Dumanovsky T, Stollman H, Angell SY. Keep on Track: a volunteer-run community-based intervention to lower blood pressure in older adults. JAGS. 2010;58:1177–1183. doi: 10.1111/j.1532-5415.2010.02874.x. [DOI] [PubMed] [Google Scholar]
- 33.Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Education and Prevention. 2009;21:181–206. doi: 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deering KN, Shannon K, Sinclair H, et al. Piloting a peer-driven intervention model to increase access and adherence to ART and HIV care among street-entrenched HIV-positive women in Vancouver. AIDS Patient Care STDs. 2009;23:603–609. doi: 10.1089/apc.2009.0022. [DOI] [PubMed] [Google Scholar]
- 35.Chaisson RE, Barnes GL, Hackman J, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. 2001;110(8):610–5. doi: 10.1016/s0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- 36.Enriquez M, Farnan R, Neville S. What experienced HIV-infected lay peer educators working in Midwestern U.S. HIV medical care settings think about their role and contributions to patient care. AIDS Patient Care STDs. 2013;27(8):474–480. doi: 10.1089/apc.2013.0021. [DOI] [PubMed] [Google Scholar]
- 37.Cioe P, Crawford S, Stein M. Cardiovascular risk factor knowledge and risk perception among HIV-infected adults. JANAC. 2014;25(1):60–69. doi: 10.1016/j.jana.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller CS, Coe K, Moore N. Addressing the demand for cultural relevance in intervention design. Health Promot Practice. 2014 doi: 10.1177/1524839914526204. (in press) [DOI] [PubMed] [Google Scholar]
- 39.Conn V, Enriquez M, Ruppar T. Cultural relevance in medication adherence interventions with under-represented adults: meta-analysis of outcomes. 2014 State of the Science Congress; Washington DC. 2014, September; [DOI] [PMC free article] [PubMed] [Google Scholar]


