Abstract
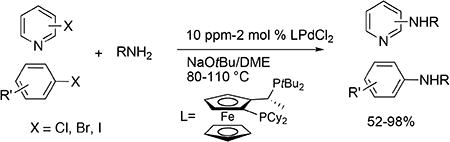
An air- and moisture-stable palladium complex, [(CyPF-tBu)PdCl2] (1), for coupling of heteroaryl chlorides, bromides, and iodides with a variety of primary amines is described. Most of these reactions occurred in high yield with 0.001-0.05 mol % catalyst loading. The reactions tolerated a wide range of functional groups.
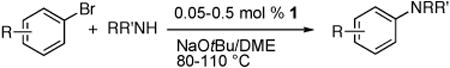
The palladium-catalyzed amination of aryl halides has emerged as a powerful tool for the synthesis of substituted anilines.1 However, catalyst loadings for this transformation have been high enough (> 0.1 mol %) to limit the use of the coupling of amines to produce less expensive intermediates and to produce commodity building blocks.2 In 2005, we reported that the combination of Pd(OAc)2 and the Josiphos ligand CyPF-tBu catalyzed, for the first time, the coupling of primary amines with heteroaryl halides with extremely low catalyst loadings (5.0-100 ppm).3 This discovery increased the potential for the use of palladium-catalyzed, carbon-nitrogen coupling processes in an industrial setting.
These reactions would be made even more practical if a stable, single-component catalyst precursor could be used. When low loadings of palladium and ligand are used separately, the rate of complexation is slow. Thus, mixing of the metal and ligand in solution must be conducted at higher concentrations prior to addition of solvent and reagents. Moreover, the complex formed from the Josiphos ligand and Pd(OAc)2 has a limited stability in solution and as a solid.
These observations led us to study C-N coupling reactions initiated with precatalysts bearing a single CyPF-tBu ligand per palladium that are more stable than the adduct of Pd(OAc)2. Herein, we report that the air- and moisture-stable palladium complex [(CyPF-tBu)PdCl2] (1) in the absence of any additional ligand is a convenient and highly active catalyst precursor for the amination of heteroaryl and aryl halides that alleviates the need to generate the metal–ligand complex in situ prior to the amination process.
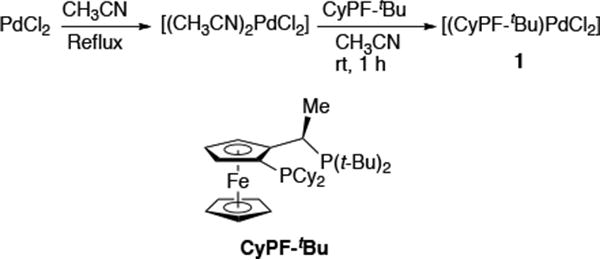
[(CyPF-tBu)PdCl2] 1 was synthesized as shown in Scheme 1 by mixing CyPF-tBu with PdCl2(CH3CN)2 as a slurry in CH3CN at room temperature under nitrogen.4 Crystalline product was obtained by layering a THF solution of the crude product with hexanes. Dichloride 1 is soluble in THF, CH2Cl2 and toluene and slightly soluble in DME. It is stable to both air and moisture and did not deteriorate after storage on the bench for three months, as determined by 1H NMR and 31P NMR spectroscopy.
Scheme 1.
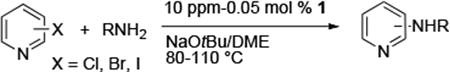
To evaluate the potential of 1 as a catalyst for the animation of heteroaryl halides, we studied the prototype reaction of 3-chloropyridine5 with octylamine in the presence of sodium tert-butoxide as base and 0.005 mol % of 1 in DME at 110 °C. This reaction was complete after 24 h and formed N-octyl-3-aminopyridine in 88% isolated yield. The reaction occurred at a similar rate and in a similar yield when conducted with 0.005 mol % Pd(OAc)2/CyPF-tBu. Reactions conducted with NaO-tBu as base were fast and occurred in high yield, while reactions conducted with the weaker base K3PO4 required higher loading (1.0 mol %) and longer time (36 h) for full conversion. Reactions in toluene with NaO-tBu as base were slightly slower than those in DME, while reactions in THF and 1,4-dioxane were much slower.
Table 1 and 2 summarize the coupling of heteroaryl and aryl chlorides, bromides and iodides with primary alkylamines, catalyzed by dichloride 1. In general, the rates for animation of aryl bromides were slightly faster than those for animation of aryl chlorides. The rates for animation of aryl iodides were markedly slower than those for animation of either aryl chlorides or bromides. Consequently, the catalyst loadings for reactions of aryl iodides were 5-10 times higher than those for reactions of aryl chlorides and bromides, but reactions of aryl iodides still occurred with much greater efficiency than with catalysts reported previously containing other ligands.6
Table 1. Coupling of Heteroaryl Halides with Primaryl Alkylamines Using (CyPF-tBu)PdCl21a.
| |||||
|---|---|---|---|---|---|
| entry | halide | product | catalyst loading (%) | conditions | yield (%b) |
| 1 |
|
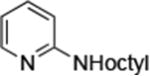
|
0.001 | 110°C,24 h | 92 |
| 2 |
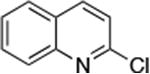
|
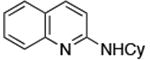
|
0.01 | 110 °C, 24 h | 85 |
| 3 |
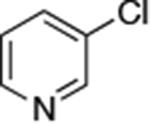
|
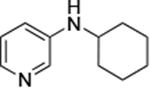
|
0.05 | 100 °C, 24 h | 88 |
| 4 |
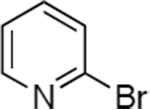
|
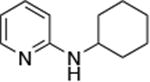
|
0.005 | 80 °C, 36 h | 86 |
| 5 |
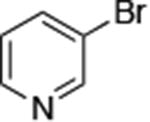
|
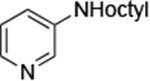
|
0.005 | 100 °C, 36 h | 74 |
| 6c | 0.005 | 110 °C, 12 h | 87 | ||
| 7 |
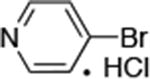
|
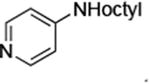
|
0.05 | 100 °C, 30 h | 88 |
| 8 |
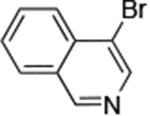
|
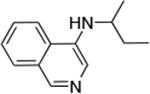
|
0.05 | 100 °C, 24 h | 96 |
| 9 |
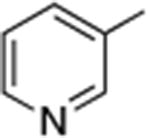
|
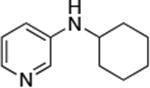
|
0.05 | 110 °C, 24 h | 70 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 1.4 equiv NaO-tBu in 1 mL DME.
Isolated yield.
Reaction with 7.90 g of 3-bromopyridine (50.0 mmol).
Table 2. Coupling of Aryl Halides with Primary Alkylamines Using (CyPF-tBu)PdCl2 1a.
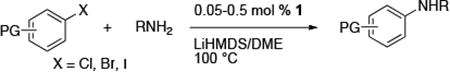
| |||||
|---|---|---|---|---|---|
| entry | halide | product | catalyst loading (%) | conditions | yield (%)b |
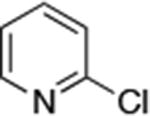
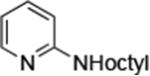
| 1 |
|
|
0.001 | 110 °C, 24 h | 92 |
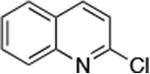
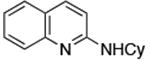
| 2 |
|
|
0.01 | 110°C,24 h | 85 |
| 3 |
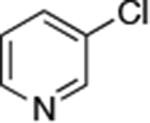
|
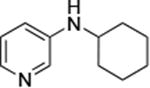
|
0.05 | 100°C,24 h | 88 |
| 4 |
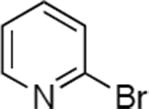
|
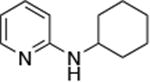
|
0.005 | 80 °C, 36 h | 86 |
| 5 |
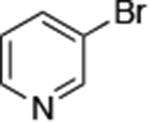
|
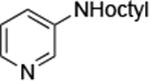
|
0.005 | 100°C,36 h | 74 |
| 6c | 0.005 | 110 °C, 12 h | 87 | ||
| 7 |
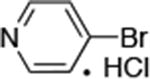
|
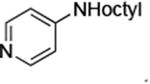
|
0.05 | 100°C,30 h | 88 |
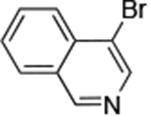
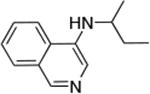
| 8 |
|
|
0.05 | 100°C,24 h | 96 |
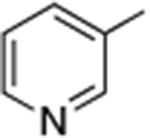
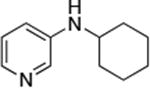
| 9 |
|
|
0.05 | 110 °C, 24 h | 70 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 1.4 equiv NaO-tBu in 1 mL DME.
Isolated yield.
Reactions of primary alkylamines with a variety of heteroaryl chlorides, bromides and iodides occurred in high yield with catalyst loadings between 0.001-0.05 mol% (Table 1). These loadings are one or two orders of magnitude lower than those reported previously for the reactions of aryl chlorides with primary amines in the presence of catalysts based on other ligands.7,8 These loadings are just as low as those with the combination of Pd(OAc)2 and the Josiphos ligand. For example, reaction of 3-bromopyridine with octylamine occurred in 74% yield with only 0.005 mol % catalyst. Of course, these reactions can be conducted without a drybox. The same process conducted on an 8.0 g scale using Schlenk methods formed the product in a comparable 87% yield.
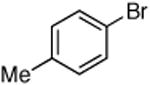
Reactions of aryl chlorides, bromides, and iodides with primary alkylamines occurred in high yield with low catalyst loading without changing the reaction conditions from that of entry 3 in Table 1. These results are summarized in Table 2. Electron-poor, electron-neutral and electron-rich aryl halides reacted with a variety of primary alkylamines with low catalyst loading (0.005-0.05 mol %). For instance, in the presence of only 0.05 mol % of the catalyst, the reaction of sec-butylamine with 3-chloroanisole and 4-bromotoluene occurred in 75% and 81% yields, respectively (Table 2, entries 3 and 6). However, the coupling of an electron-rich aryl iodide with an α-branched primary alkylamine required a slightly higher loading (0.3 mol %) to proceed to completion (Table 2, entry 9). Typically, reactions of more hindered α-branched primary alkylamines were slower and required higher loadings than those of linear primary amines. However, reactions of these amines with aryl chlroides and bromides still occurred with just 0.05 mol % catalyst and with aryl iodides with 0.3 mol % catalyst.
This catalyst is not only highly reactive for coupling of primary amines, it is highly selective for monoarylation. No products from diarylation of amines were observed by GC/MS for any of the reactions in Tables 1 or 2.
The use of the strong base NaO-tBu in catalytic animation reactions can be incompatable with certain functional groups. However, the use of two equivalents of lithium bis(trimethylsilyl)amide (LiN(TMS)2) as base9,10 greatly improves the functional group compatibility of these reactions. Reactions of aryl halides containing protic functional groups,10,11 including a free alcohol, phenol, carboxylic acid, amide, and protected amine, are summarized in Table 3. These reactions occurred in high yield in the presence of 0.05-0.5 mol % complex 1 and lithium bis(trimethylsilyl)amide (LiN(TMS)2)10 as the base (Table 3, entries 2-7). Reactions of aryl chlorides, bromides, and iodides bearing carboalkoxy and nitro groups, as well as acyl groups containing enolizable hydrogens, occurred in higher yields when using K3PO4 as base, instead of LiN(TMS)2 (Table 3, entries 8-11).10,11 In these reactions, higher loadings of the catalyst and higher temperatures were required for full conversion, due to the decreased basicity and/or solubility of K3PO4.
Table 3. Amination of Functionalized Aryl halides with Primary Alkylamine Using (CyPF-tBu)PdCl2 1a.
| |||||
|---|---|---|---|---|---|
| entry | halide | product | catalyst loading (%) | conditions | yield (%)b |
| 1 |
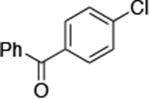
|
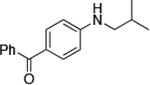
|
0.05 | 100 °C 48 h | 84 |
| 2 |
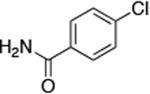
|
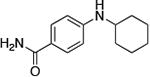
|
0.5 | 100 °C 36 h | 52 |
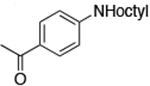
| 3c |
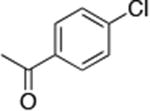
|
|
0.5 | 110 °C 24 h | 67 |
| 4 |
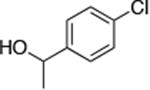
|
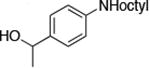
|
0.5 | 100 °C 24 h | 77 |
| 5 |
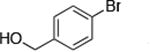
|
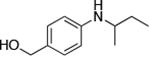
|
0.5 | 100 °C 24 h | 69 |
| 6 |
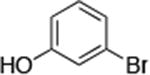
|
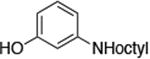
|
0.05 | 100 °C 24 h | 84 |
| 7 |
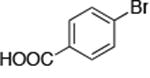
|
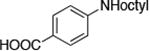
|
0.05 | 100 °C 24h | 78 |
| 8c |
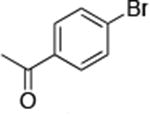
|
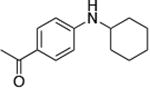
|
1.0 | 110 °C 24 h | 75 |
| 9c |
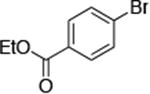
|
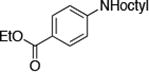
|
0.5 | 110 °C 24 h | 88 |
| 10c |
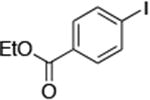
|
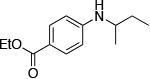
|
2.0 | 110 °C 48h | 71 |
| 11c |
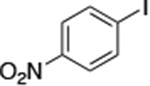
|
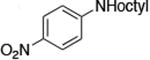
|
1.0 | 110 °C 24 h | 85 |
| 12c |
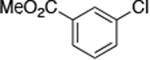
|
|
1.0 | 110 °C 24 h | 89 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 2.4 equiv LiN(SiMe3)2 in 1 mL DME.
Isolated yield.
Using K3PO4 as the base.
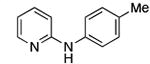
Reactions of primary arylamines are summarized in Table 4. These reactions were slower than the reactions of primary alkylamines. Nevertheless, high yields of coupled product from reactions of heteroaryl and aryl chlorides and bromides with electron rich arylamines were obtained using only 0.05-0.1 mol % catalyst (Table 4, entries 1-3, 5-6). Reactions of electron-deficient primary heteroarylamines were slower and required higher catalyst loadings than the reactions of primary arylamines. For example, the reaction of 2-aminopyrazine with the unactivated 3-chloropyridine ccurred in high yield, but required 1 mol % catalyst at 110 °C (Table 4, entry 4).
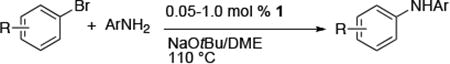
Table 4. Coupling of Heteroaryl and Aryl Halides with Primary Arylamines Using (CyPF-tBu)PdCl2 1a.
| |||||
|---|---|---|---|---|---|
| entry | halides | product | catalyst loading (%) | conditions | yield (%)b |
| 1 |
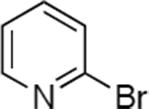
|
|
0.05 | 110 °C, 24 h | 92 |
| 2 |
|
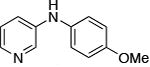
|
0.1 | 110°C, 36 h | 89 |
| 3 | 0.05 | 110 °C, 24 h | 83 | ||
| 4 |
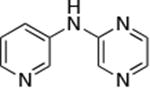
|
1.0 | 110 °C, 24 h | 86 | |
| 5 |
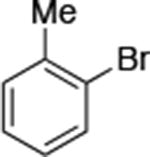
|
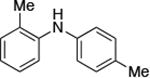
|
0.05 | 110 °C, 48 h | 95 |
| 6 |
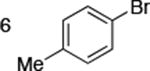
|
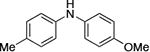
|
0.05 | 110 °C, 48 h | 98 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 1.4 equiv NaO-tBu in 1 mL DME.
Isolated yield.
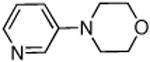
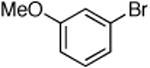
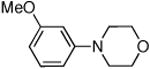
Dichloride complex 1 was less effective as a catalyst for reactions of secondary amines8,12 with heteroaryl and aryl halides. Reactions were slower and substantial amounts of hydrodehalogenation product were observed. Only a limited number of combinations of secondary amines and aryl halides reacted in high yield at low catalyst loading. These data are summarized in Table 5. For instance, the cyclic secondary amine morpholine reacted with 3-bromoanisole in the presence of 0.05 mol % catalyst to give the desired products in 89% yield (Table 5, entry 3).
Table 5. Coupling of Heteroaryl and Aryl Bromides with Secondary Amines Using (CyPF-tBu)PdCl2 1a.
| |||||
|---|---|---|---|---|---|
| entry | halide | product | catalyst loading (%) | conditions | yield (%)b |
| 1 |
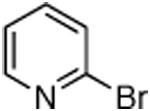
|
|
0.5 | 110 °C, 20 h | 76 |
| 2 |
|
|
0.5 | 110 °C, 48 h | 90 |
| 3 |
|
|
0.05 | 110 °C, 48 h | 89 |
| 4 |
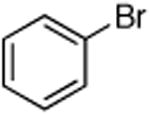
|
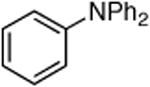
|
0.5 | 110 °C, 20 h | 81 |
| 5 |
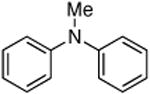
|
0.05 | 110 °C, 48 h | 69 | |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 1.4 equiv NaO-tBu in 1 mL DME.
Isolated yield.
In summary, we have shown that the palladium complex [(CyPF-tBu)PdCl2] (1) is a general, highly efficient catalyst for the coupling of heteroaryl and aryl chlorides, bromides and iodides with primary nitrogen nucleophiles. Reactions catalyzed by [(CyPF-tBu)PdCl2] occur with 0.001-0.05 mol % catalyst, and these catalyst loadings are, in most cases, two orders of magnitude lower than those of analogous couplings by catalysts containing other ligands. These data are similar to those obtained with the combinatinon CyPF-tBu and Pd(OAc)2 without the need to first bind the ligand to the metal precursor. The process exhibits a broad scope and a high tolerance for functional groups, such as cyano, keto, free carboxylate, amido, carboalkoxy, aromatic and aliphatic hydroxyl and amino functional groups.
Supplementary Material
Acknowledgments
We thank the NIH (GM-55382) for support of this work, Johnson-Matthey for a gift of PdCl2, Solvias for a gift of the Josiphos ligands.
Footnotes
Supporting Information Available All experimental procedures and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.orge.
References
- 1.a Hartwig JF, Shekhar S, Shen Q, Barrios-Landeros F. In: Chemistry of Anilines. Rappoport Z, editor. Vol. 1. John Wiley & Sons Ltd: Chichester, UK; 2007. p. 455. [Google Scholar]; b Hartwig JF. In: Modern Arene Chemistry. Austruc C, editor. Wiley-VCH: Weinheim; 2002. p. 107. [Google Scholar]; c Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negoshi E, editor. Wiley-Intescience: New York; 2002. p. 1051. [Google Scholar]; d Muci AR, Buchwald SL. Topics in Current Chemistry. 2002;219:131. [Google Scholar]; e Belfield AJ, Brown GR, Foubister AJ, Ratcliffe PD. Tetrahedron. 1999;55:13285. [Google Scholar]; f Yang BH, Buchwald SL. J Organomet Chem. 1999;576:125. [Google Scholar]
- 2.a Buchwald SL, Mauger C, Mignani G, Scholz U. Adv Synth Catal. 2006;348:23. [Google Scholar]; b King AO, Yasuda N. In: Organometallics in Process Chemistry. Larsen RD, editor. Springer; Berlin: 2004. p. 205. [Google Scholar]
- 3.Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Angew Chem Int Ed. 2005;44:1371. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]
- 4.Schlosser M. Organometallics in Synthesis: A Manual. 2nd. John Wiley & Sons, Ltd.; Chichester: 2002. [Google Scholar]
- 5.a Hooper MW, Utsunomiya M, Hartwig JF. J Org Chem. 2003;68:2861. doi: 10.1021/jo0266339. [DOI] [PubMed] [Google Scholar]; b Yin J, Zhao MM, Huffman MA, McNamara JM. Org Lett. 2002;4:3481. doi: 10.1021/ol0265923. [DOI] [PubMed] [Google Scholar]; c Jonckers THM, Maes BUW, Lemiere GLF, Dommisse R. Tetrahedron. 2001;57:7027. [Google Scholar]; d Maes BUW, Loones KTJ, Lemiere GLF, Dommisse RA. Synlett. 2003:1822. [Google Scholar]; e Burton G, Cao P, Li G, Rivero R. Org Lett. 2003;5:4373. doi: 10.1021/ol035655u. [DOI] [PubMed] [Google Scholar]; f Wagaw S, Buchwald SL. J Org Chem. 1996;61:7240. doi: 10.1021/jo9612739. [DOI] [PubMed] [Google Scholar]; g Ji J, Li T, Bunnelle WH. Org Lett. 2003;5:4611. doi: 10.1021/ol0357696. [DOI] [PubMed] [Google Scholar]
- 6.a Wolfe JP, Buchwald SL. J Org Chem. 1996;61:1133. [Google Scholar]; b Hamann BC, Hartwig JF. J Am Chem Soc. 1998;120:7369. [Google Scholar]; c Ali MH, Buchwald SL. J Org Chem. 2001;66:2560. doi: 10.1021/jo0008486. [DOI] [PubMed] [Google Scholar]; d Parrot I, Ritter G, Wermuth CG, Hibert M. Synlett. 2002:1123. [Google Scholar]
- 7.a Littke AF, Fu GC. Angew Chem Int Ed. 2002;41:4176. doi: 10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; b Stambuli JP, Kuwano R, Hartwig JF. Angew Chem Int Ed. 2002;41:4746. doi: 10.1002/anie.200290036. [DOI] [PubMed] [Google Scholar]; c Wolfe JP, Tomori H, Sadighi JP, Yin J, Buchwald SL. J Org Chem. 2000;65:1158. doi: 10.1021/jo991699y. [DOI] [PubMed] [Google Scholar]; d Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am Chem Soc. 2003;125:6653. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]; e Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. J Org Chem. 2002;67:5553. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]; f Urgaonkar S, Xu JH, Verkade JG. J Org Chem. 2003;68:8416. doi: 10.1021/jo034994y. [DOI] [PubMed] [Google Scholar]; g Viciu MS, Kissling RM, Stevens ED, Nolan SP. Org Lett. 2002;4:2229. doi: 10.1021/ol0260831. [DOI] [PubMed] [Google Scholar]; h Grasa GA, Viciu MS, Huang J, Nolan SP. J Org Chem. 2001;66:7729. doi: 10.1021/jo010613+. [DOI] [PubMed] [Google Scholar]; i Li GY. Angew Chem Int Ed. 2001;40:1513. doi: 10.1002/1521-3773(20010417)40:8<1513::aid-anie1513>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]; j Rataboul F, Zapf A, Jackstell R, Harkal S, Riermeier T, Monsees A, Dingerdissen U, Beller M. Chem Eur J. 2004;10:2983. doi: 10.1002/chem.200306026. [DOI] [PubMed] [Google Scholar]
- 8.Stauffer SR, Lee S, Stambuli JP, Hauck SI, Hartwig JF. Org Lett. 2000;2:1423. doi: 10.1021/ol005751k. [DOI] [PubMed] [Google Scholar]
- 9.Louie J, Hartwig JF. Tetrahedron Lett. 1995;36:3609. [Google Scholar]
- 10.a Harris MC, Huang X, Buchwald SL. Org Lett. 2002;4:2885. doi: 10.1021/ol0262688. [DOI] [PubMed] [Google Scholar]; b Urgaonkar S, Verkade JG. Adv Synth Catal. 2004;346:611. [Google Scholar]
- 11.a Wolfe JP, Buchwald SL. Tetrahedron Lett. 1997;38:6359. [Google Scholar]; b Wolfe JP, Buchwald SL. J Org Chem. 2000;65:1144. doi: 10.1021/jo9916986. [DOI] [PubMed] [Google Scholar]; c Lee S, Jorgensen M, Hartwig JF. Org Lett. 2001;3:2729. doi: 10.1021/ol016333y. [DOI] [PubMed] [Google Scholar]
- 12.a Nishiyama M, Yamamoto T, Koie Y. Tetrahedron Lett. 1998;39:617. [Google Scholar]; b Navarro O, Marion N, Mei J, Nolan SP. Chem Eur J. 2006;12:5142. doi: 10.1002/chem.200600283. [DOI] [PubMed] [Google Scholar]; c Marion N, Navarro O, Mei J, Stevens ED, Scott NM, Nolan SP. J Am Chem Soc. 2006;128:4101. doi: 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]; d Marion N, Ecarnot EC, Navarro O, Amoroso D, Bell A, Nolan SP. J Org Chem. 2006;71:3816. doi: 10.1021/jo060190h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


