Abstract
Background
Elevated stress perception and depression commonly co-occur, suggesting that they share a common neurobiology. Cortical thickness of the rostral middle frontal gyrus (RMFG), a region critical for executive function, has been associated with depression- and stress-related phenotypes. Here, we examined whether RMFG cortical thickness is associated with these phenotypes in a large family-based community sample.
Methods
RMFG cortical thickness was estimated using FreeSurfer among participants (n=879) who completed the ongoing Human Connectome Project. Depression-related phenotypes (i.e., sadness, positive affect) and perceived stress were assessed via self-report.
Results
After accounting for sex, age, ethnicity, average whole-brain cortical thickness, twin status, and familial structure, RMFG thickness was positively associated with perceived stress and sadness and negatively associated with positive affect at small effect sizes (accounting for 0.2–2.4% of variance; p-fdr: 0.0051–0.1900). Perceived stress was uniquely associated with RMFG thickness after accounting for depression-related phenotypes. Further, among siblings discordant for perceived stress, those reporting higher perceived stress had increased RMFG thickness (p=4×10−7). Lastly, RMFG thickness, perceived stress, depressive symptoms, and positive affect were all significantly heritable with evidence of shared genetic and environmental contributions between self-report measures.
Conclusions
Stress perception and depression share common genetic, environmental, and neural correlates. Variability in RMFG cortical thickness may play a role in stress-related depression, though effects may be small in magnitude. Prospective studies are required to examine whether variability in RMFG thickness may function as a risk factor for stress exposure and/or perception, and/or arises as a consequence of these phenotypes.
Introduction
Convergent evidence suggests that stress plays a prominent etiologic role in depression. Both prospective and retrospective studies have shown that stressful life events often precede depression (Kendler et al. 1999; Hammen 2005), and non-human animal models have demonstrated that stress induces depressive-like behavior (Lee et al. 2013; Zhu et al. 2014). Importantly, however, there is vast variability in how individuals respond to stressors. For instance, perceived stress, or the extent that one perceives situations in their life to be stressful, unpredictable, uncontrollable, and unmanageable, is associated with the development of depressive symptoms, including elevations in negative affect and reductions in positive affect following stress exposure (Morris et al. 2014; Oni et al. 2012; Dunkley et al. 2017). Further, consistent with converging evidence that stress may induce anhedonia (e.g., Pizzagalli 2014; Bogdan & Pizzagalli 2006), perceived stress is also coupled with reduced behavioral reward learning and positive affect, as well as elevated anhedonia (Pizzagalli et al. 2007; Bogdan, Pringle, Goetz & Pizzagalli 2012; Dunkley et al. 2017).
Twin studies showing that the association between stress perception and depression is primarily attributable to shared genetic and individual-specific environmental factors suggest that perceived stress and depression may share a common neurobiological basis (Bogdan & Pizzagalli 2009; Rietschel et al. 2014). In addition to well-documented associations between amygdala and hippocampal structure among both individuals exposed to stressful life events (Morey et al. 2012; Tottenham & Sheridan 2009; Corbo et al. 2014) and those with depressive symptoms (Whalen et al. 2002; Treadway et al. 2014; Rosso et al. 2005; Campbell & MacQueen 2004), recent work has linked rostral middle frontal gyrus (RMFG) cortical thickness to both depression and stress. Specifically, when compared to healthy controls, depressed adolescents and adults with remitted depression have increased cortical thickness within the rostral middle frontal gyrus (RMFG; Phillips et al. 2015; Reynolds et al. 2014). However, both thicker (Qiu et al. 2014) and thinner (Peng et al. 2015) RMFG1 have been observed among adults experiencing their first depressive episode. Consistent with these mixed findings, stress-related phenotypes, including posttraumatic stress disorder and circulating cortisol, have also been linked to both relatively thicker (Qiu et al. 2014; Reynolds et al. 2014; Lyoo et al. 2011) and thinner (Van Eijndhoven et al. 2013) RMFG. Because the RMFG is critical for higher-order executive functions related to stress perception and appraisal, including attention, working memory, planning, executive cognition, and emotion regulation (Koenigs & Grafman 2009; Miller & Cohen 2001; Phillips et al. 2003), it may confer vulnerability to depression and negative stress-related outcomes, in part through associations with perceived stress.
The current study examined whether RMFG cortical thickness is associated with depression-related phenotypes (i.e., sadness, positive affect) and perceived stress within a non-clinical sample of individuals who completed the ongoing family-based Human Connectome Project (n=879). We examined cortical thickness, as opposed to surface area and gray-matter volume, due to evidence that these phenotypes have separable genetic influence (Winkler et al. 2010), as well as emergent literature linking indices of RMFG cortical thickness to depression and stress-related phenotypes. Because both depression and stress-related phenotypes have been associated with increased (Qiu et al. 2014; Reynolds et al. 2014; Lyoo et al. 2011) and decreased (Peterson et al. 2009; Mackin et al. 2013) RMFG thickness, and associations with perceived stress have been unexplored, we made no directional hypotheses. We further examined whether associations between RMFG cortical thickness and stress perception remain after accounting for depression-related phenotypes and whether differences in RMFG cortical thickness are present among siblings discordant for perceived stress. These analyses can be used to evaluate support for potential sibling-shared predisposition (i.e., discordant siblings who do not differ) or causal (i.e., discordant siblings differing) effects underlying associations. Additionally, to probe regional specificity, we evaluated whether cortical thickness in other prefrontal regions previously linked to depression [i.e., anterior cingulate (Reynolds et al. 2014; van Eijndhoven 2013)] are associated with depression-related phenotypes and perceived stress. Lastly, we estimated the heritability of RMFG cortical thickness, depression-related phenotypes, and perceived stress, as well as shared genetic and environmental covariation across these phenotypes when phenotypic correlations permitted. Understanding associations between depression and stress perception with RMFG cortical thickness may inform why these behavioral constructs co-occur and contribute to our etiologic understanding of depression to ultimately inform nosology and treatment.
Methods
Participants
Participants were drawn from the December 2015 public release of the Human Connectome Project (HCP; total n=970). The HCP is an ongoing, family-based study (2–6 siblings per family, with most families including a twin pair; projected final N=1,200) designed to explore individual differences in brain circuits and their relation to behavior and genetic background (Pagliaccio et al. 2014; Van Essen et al. 2013; Barch et al. 2013). All participants were 22–37 years of age and free of the following exclusionary criteria: preterm birth, neurodevelopmental, neuropsychiatric, or neurologic disorders; a full list of exclusions is available in a prior publication (Van Essen et al. 2012). Participants were also excluded from analyses in the present study for missing or poor-quality structural MRI data (n=73), missing questionnaire data (n=1), half-sibling status (n=11), or missing parent identity (n=6). This resulted in a final sample of 879 participants [mean age: 28.82 ± 3.68 years; 393 (43.9%) female; 597 (67.4%) European-American, 143 (16.2%) African-American, 44 (5.0%) Asian-American, and 73 (8.2%) Hispanic]. Of these participants, there were 107 monozygotic twin pairs, 116 dizygotic twin pairs, 276 non-twin siblings [87 families with 2 siblings, 20 families with 3 siblings, 8 families with 4 siblings, and 2 families with 5 siblings; none of these are twins, but there may be twin pairs in their family structure], and 157 individuals who were the only member of their family to provide usable data prior to this data release. There was an average of 2.00±0.94 with a maximum of 6 siblings per family. Mean age difference between siblings within families (twin and non-twin siblings) was 2.81±2.56 years for families with 2 siblings; 2.91±2.59 years for families with 3 siblings; 3.00±2.59 years for families with 4 siblings; 3.01±2.58 years for families with 5 siblings; and 3.02±2.57 years for families with 6 siblings. Each participant provided informed written consent prior to participation in accord with the guidelines of the Washington University in St Louis Institutional Review Board and received $400 remuneration, as well as additional winnings ($5) and travel expenses.
Self-Report Scales
Perceived stress was assessed using the 10-item Perceived Stress Scale (PSS) from the NIH toolbox (NIH TB; www.nihtoolbox.org; Gershon et al. 2013). The PSS (Cohen et al. 1988) is a commonly-used measure of stress perception that is heritable (Federenko et al. 2006; Bogdan & Pizzagalli 2009) and has been associated with stress hormones, illness, and physiological responses (Cohen et al. 1993; Ebrecht et al. 2004; Cohen & Janicki-Deverts 2012). Sadness was assessed using the NIH TB Sadness Survey, which is comprised of 27 items from a depression item bank within the Patient Reported Outcome Measurement Information System (PROMIS) that shows strong convergent validity with other measures of depression (Pilkonis et al. 2014). Positive affect was assessed via 34 items from the Positive and Negative Affect Schedule – Expanded Form [PANAS-X; (Crawford & Henry 2004)] measuring higher-order positive affect, or the extent to which an individual feels pleasurable engagement with the environment (Watson & Clark 1984). While positive affect is not a direct depressive symptom measure, low positive affect is similar to the concept of anhedonia (Crawford & Henry 2004) and has been reported to be specifically associated with depression (i.e., not anxiety; Jolly et al. 1994); furthermore, positive affect as measured by the PANAS is typically negatively correlated with measures of anheodnia (e.g., Tuohy & McVey 2008).
Magnetic Resonance Imaging: Acquisition and Processing
High resolution (0.7mm isotropic voxels) 3D anatomical images, both T1-weighted (MPRAGE) and T2-weighted (T2-SPACE), were acquired using a customized Siemens 3T scanner with a 32-channel head coil. HCP acquisition and preprocessing details have been previously described in detail (Glasser et al. 2013; Van Essen et al. 2012). Briefly, relevant steps for this study from the HCP processing pipeline within Freesurfer v5.3.0 included: 1) spline-based down-sampling of the 0.7mm T1 image to 1 mm; 2) intensity normalization and Talairach transformation; 3) skull registration; 4) skull stripping; 5) subcortical segmentation; 6) creation of white and pial surfaces and their refinement using the full (0.7 mm) resolution data; 7) refinement of the pial surface using the T2-SPACE scan to help exclude CSF and dura; and 8) extraction of cortical thickness estimates for the RMFG from cortical parcellation that delineates subregions with high accuracy based on the Desikan atlas (see Supplemental Figure 1; Desikan et al. 2006).
Statistical Analyses
Data were winsorized to ± 3 SD from the mean of each variable to minimize the influence of extreme outliers. Sequential Oligogenetic Linkage Analysis Routines (SOLAR) software (http://www.sfbr.org/sfbr/public/software/solar) was used to conduct phenotypic association, heritability, and bivariate quantitative genetic analyses, while accounting for familial structure (Blangero & Almasy 1996). More specifically, to account for the non-independence of measures in related individuals, an individual’s bivariate phenotypic association (e.g., between perceived stress and RMFG cortical thickness) was modeled as a linear function of the individual’s measures and the kinship matrix coefficients for relationships among all pairs of individuals in their pedigree. In the Results section, Benjamini-Hochberg false discovery rate (FDR; Blakesley et al. 2009) corrected p-values are reported for each analysis to account for multiple testing of initial hypotheses (i.e., associations between both right and left RMFG cortical thickness with sadness, positive affect and perceived stress). We further entered depression-related phenotypes and perceived stress in a simultaneous regression to examine whether any of these constructs had unique associations with RMFG cortical thickness.
Next, we examined whether same-sex twin and non-twin sibling pairs discordant for perceived stress differed from each other on RMFG cortical thickness. These analyses examined whether PSS was associated with RMFG cortical thickness after accounting for same-sex sibling-shared genetic background and experience. If siblings discordant for perceived stress do not differ from one another with respect to RMFG cortical thickness, this would provide support for potential sibling shared predisposition effects that contribute to the relationship between perceived stress and RMFG cortical thickness. If, however, perceived stress-discordant siblings do differ from one another on RMFG cortical thickness, this would provide evidence in support of a person-specific, and potentially causal, relationship (i.e., factors unique to each sibling, that are present after accounting for sibling shared genetic and environment background) between perceived stress and RMFG cortical thickness. Siblings were considered discordant if one sibling was at least 0.5 standard deviations above the sample mean for perceived stress (“high discordant”; PSS: 55.95±5.62) while another was at least 0.5 standard deviations below (“low discordant”; PSS: 39.61±5.15; mean ΔSDdiscordant pairs±SD=1.22±0.98). This resulted in 127 non-independent discordant pairings from 55 families with one sibling pair meeting criteria, as well as 33 families with two, three, or four sibling pairs meeting criteria. Discordancy analyses were conducted using linear mixed models using the Psych (Revelle 2015) and lme4 (Bates et al. 2015) packages in R to account for the multiple-sibling structure within families.
Additionally, we examined whether cortical thickness in other prefrontal regions previously linked to depression [rostral and caudal anterior cingulate (Reynolds et al. 2014; van Eijndhoven et al. 2013)] were associated with depression-related phenotypes or perceived stress.
Lastly, univariate heritability (h2) analyses were performed on bilateral RMFG thickness estimates, perceived stress, positive affect, and sadness. We examined contributions of overlapping genetic (ρg) or individual-specific environmental (ρe) factors within bivariate phenotypic associations that were stronger than β > |.20|, to ensure that effects were large enough to warrant variance decomposition within our relatively modest sample size.
All analyses accounted for effects of sex, age, ethnicity (i.e., dummy coded for White, Black, Asian, and Hispanic), and zygosity (i.e., MZ/not MZ; DZ/not DZ) and were run with and without extreme outliers (i.e., prior to and after winsorizing), which did not affect our results. Analyses of cortical thickness also accounted for whole-brain cortical thickness. Because left-handed participants were included in our dataset, we also ran analyses with handedness as an additional covariate; results, including what was and was not significant with FDR correction, were unchanged by the inclusion of handedness in our models.
Results
Sample Characteristics
There were no significant zero-order associations between covariates (i.e., age, sex, zygosity, ethnicity) and bilateral RMFG thickness, depression-related phenotypes (i.e., positive affect, sadness), or perceived stress (all ps>0.3460), with the exception of whole brain cortical thickness, which was positively correlated with left (r=0.1998, p=2×10-8) and right (r=0.2590, p=3×10−13) RMFG cortical thickness. No variables showed evidence of significant skew (perceived stress: m=48.30±9.11, skew=0.12; positive affect: m=50.04±7.87, skew=0.10; sadness: m=46.42±8.02, skew=0.45; left RMFG thickness: m=2.57±0.12, skew=−0.33; right RMFG thickness: m=2.59±0.11, skew=0.05).
RMFG, Perceived Stress, and Depression-Related Phenotypes
Bilateral RMFG cortical thickness was positively associated with perceived stress (PSS: left: β=0.1120, r2=0.0125, p=0.0017, p-fdr=0.0051; right: β=0.1141, r2=0.0130, p=0.0013, p-fdr=0.0051; Figure 1A) as well as sadness (left: β=0.0985, r2=0.0097, p=0.0196, p-fdr=0.0240; right: β=0.0832, r2=0.0069, p=0.0186, p-fdr=0.0240; Figure 1B). Positive affect was negatively coupled with left RMFG thickness (β=−0.0824, r2=0.0070, p=0.0053, p-fdr=0.0106) but was not significantly related to right RMFG cortical thickness (β= −0.0463, r2=0.0021, p=0.1900, p-fdr=0.1900; Figure 1C; see Supplemental Table 1). A simultaneous regression examined whether our variables of interest (i.e., perceived stress, sadness, positive affect) are uniquely associated with variability in RMFG cortical thickness. In this model, perceived stress was uniquely associated with right RMFG cortical thickness (right: β=0.0828, Δr2=0.0069, p=0.0190; left: β=0.0604, Δr2=0.0036, p=0.0868), while associations with sadness and positive affect were not significant (all ps>0.3796; Supplemental Table 2). Notably, other regions in which cortical thickness has been previously associated with depression (i.e., rostral and caudal anterior cingulate cortex) were not significantly associated with depression-related phenotypes or perceived stress (all ps>0.1180; Supplemental Table 3).
Figure 1. Bilateral RMFG thickness is associated with perceived stress, positive affect, and sadness.
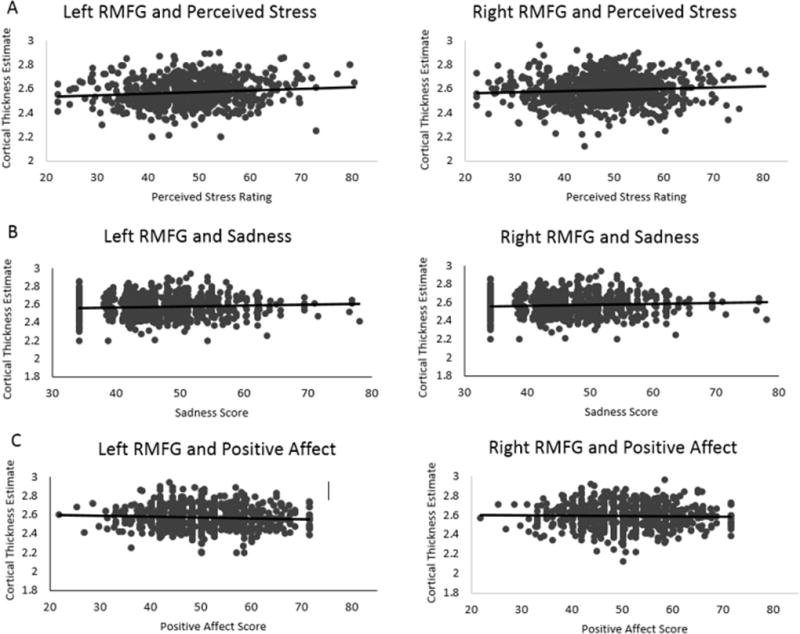
Graphs depict winsorized data points but do not represent covariate adjustment.
Perceived Stress Discordancy
Because perceived stress remained a unique predictor of right RMFG cortical thickness, even after accounting for depression-related phenotypes, we examined whether same-sex siblings (including MZ and DZ twin pairs as well as non-twin sibling pairs) discordant for perceived stress (see Methods) differed from one another on RMFG cortical thickness. These analyses revealed that siblings who reported high perceived stress (i.e., PSS: 55.95±5.62; see Methods) had increased right RMFG cortical thickness (2.6214±0.11) relative to their discordant (i.e., PSS: 39.61±5.15; see Methods) sibling who reported low perceived stress [RMFG: 2.5702±0.10; 95% bootstrapped confidence interval (CI): 0.015 – 0.034; p=4×10−7; Figure 2]. Significant results are also obtained when examining left RMFG cortical thickness (95% CI: 0.021 – 0.042; p=7.9×10−9).
Figure 2. RMFG Cortical Thickness Among Siblings Discordant for Perceived Stress.
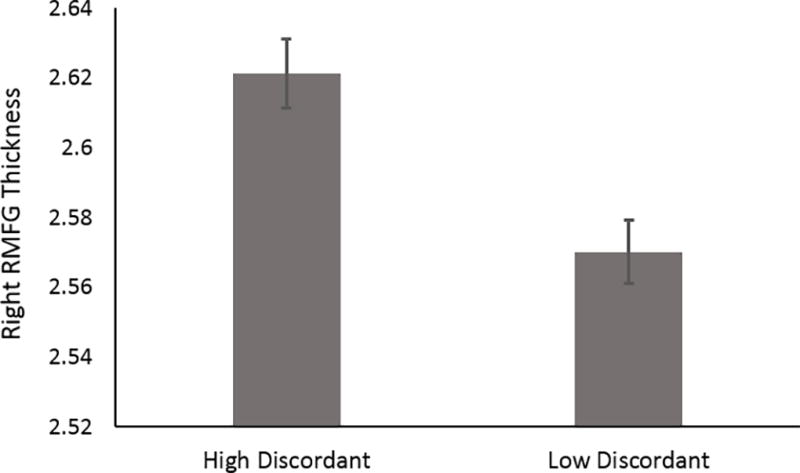
Among sibling pairs discordant for perceived stress, those who reported relatively high levels (≥ 0.5 standard deviation units above the mean) had thicker right RMFG relative to those reporting relatively low levels (≤ 0.5 standard deviation units below the mean; p=4×10−7). Error bars depict standard error of the mean.
Heritability and Sources of Variance and Covariance
Heritability estimates ranged from 22.44% (for sadness) to 71.34% (for right RMFG thickness; Figure 3). Briefly, bilateral RMFG cortical thickness, perceived stress, sadness, and positive affect were all significantly heritable. Bivariate genetic analyses revealed significant genetic and environmental correlations among self-report variables and between right and left RMFG cortical thickness (Table 1). In short, all bivariate relationships among self-report variables had significant shared genetic and environmental contributions, with shared genetic effects being largest. Because the strength of association between RMFG cortical thickness and self-report measures was small (i.e., β < |.20|), decomposition analyses were not conducted among these variables.
Figure 3. Heritability of Phenotypes.
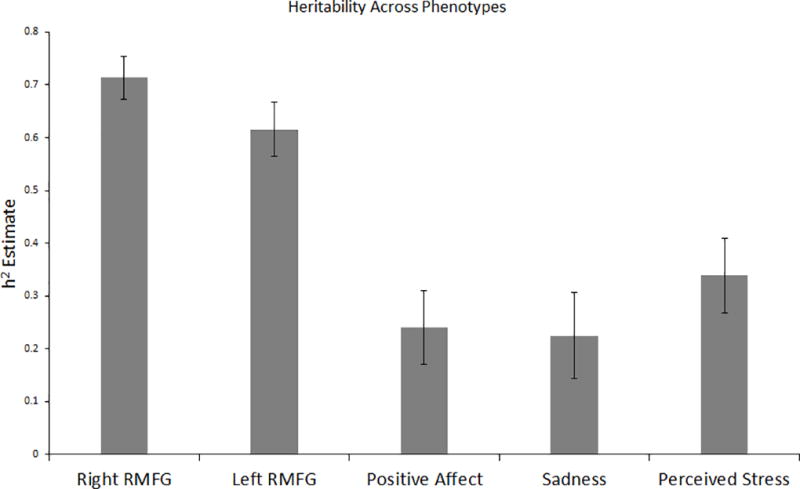
Heritability estimates: right RMFG thickness = 71.34%, left RMFG thickness = 61.56%, positive affect = 23.96%, sadness = 22.44%, perceived stress = 33.85%. * = significant at p<0.05. Household effects (i.e., living with the same biological mother) were used to test for shared/rearing environment and found to be nonsignificant (p >.05 across all phenotypes). Thus, any remaining variance can be attributed to individual specific environmental factors or error.
Table 1.
Bivariate Variance Decomposition
| Left RMFG Thickness | Sadness | Perceived Stress | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | ρe (S.E.) | ρg (S.E.) | β | ρe (S.E.) | ρg (S.E.) | β | ρe (S.E.) | ρg (S.E.) | |
| Right RMFG Thickness | 0.832 | 0.4390 (0.0626) | 0.9971 (0.0164) | 0.083 | - | - | 0.114 | - | - |
| Positive Affect | 0.082 | - | - | − 0.468 | −0.3804 (0.0583) | −0.7526 (0.1486) | −0.484 | −0.4655 (0.0561) | −0.5398 (0.1411) |
| Sadness | 0.098 | - | - | - | - | - | 0.565 | 0.4564 (0.0566) | 0.8639 (0.1130) |
| Perceived Stress | 0.112 | - | - | 0.565 | - | - | - | - | - |
Variance decomposition analyses were conducted for bivariate pairs that were phenotypically correlated at β > |.20|. Significant ρg and ρe estimates (all ps < 0.0065) are listed above with standard error values in parentheses.
Discussion
We examined associations among depression-related phentoypes (i.e., sadness, positive affect), perceived stress, and RMFG cortical thickness. We found that bilateral RMFG cortical thickness was positively associated with sadness and perceived stress and that left RMFG thickness was negatively associated with positive affect, though at relatively small effect sizes. Further, among siblings discordant for perceived stress, those with relatively high levels had thicker RMFG cortex. This suggests that the association between RMFG thickness and perceived stress remains after accounting for sibling-shared genetic background and experience, providing support for potential causation. Consistent with prior literature, depression-related phenotypes (Sullivan, Neale & Kendler 2000) and stress perception (Bogdan & Pizzagalli 2009; Federenko et al. 2006) were significantly heritable, and much like heritability estimates of average cortical thickness across the entire brain (Panizzon et al. 2009), RMFG cortical thickness was highly heritable. Further, we found that the correlation between self-report measures of depression-related phenotypes (i.e., sadness and low positive affect) and perceived stress is due to shared genetic and environmental factors, while the correlation between cortical thickness estimates across hemispheres can be attributed primarily to shared genetic influence. Collectively, these data suggest that perceived stress and depression-related phenotypes share common genetic, environmental, and neural correlates, and that relatively increased RMFG cortical thickness may contribute to stress-related depressive symptomology.
Our results linking increased RMFG cortical thickness with depression-related phenotypes and perceived stress in a non-clinical sample are consistent with observations among depressed youth (Reynolds et al. 2014), adults experiencing their first depressive episode (Qiu et al. 2014; but see also: Peng et al. 2015), and trauma-exposed individuals (Lyoo et al. 2011). As the RMFG is involved in a host of executive functions – including mood and behavior regulation (Koenigs & Grafman 2009; Miller & Cohen 2001) – that are impaired in depression (Murrough et al. 2011), our findings bolster the putative role of RMFG structure in depression-related phenotypes. However, these results contrast with reports that unaffected individuals at familial risk for depression (Peterson et al. 2009) and those experiencing depression in later life (Mackin et al. 2013) have relatively thinner RMFG. Importantly, however, controlling for illness duration seems to abolish some significant structural differences between early- and late-onset depressed patients (Truong et al. 2013). One possibility that may explain these discrepant results is that increased cortical thickness is associated with first or early episodes of depression as well as non-clinical levels of depression and stress perception, which may transition to decreases in cortical thickness over time alongside the expression of recurrent depressive symptoms and/or stress generation (Liu & Alloy, 2010; Kendler & Gardner 2016). Notably, cortical thickness in other prefrontal regions previously associated with depression and/or stress exposure (i.e., rostral and caudal anterior cingulate cortex) showed no nominally-significant associations with depression-related phenotypes or perceived stress in our study (all ps>0.118). While the lack of significance here may reflect specific relationships between RMFG cortical thickness, depression, and perceived stress, it is also possible that our general population sample was underpowered to detect differences in these other regions.
Due to its cross-sectional nature, the current study cannot inform whether individual differences in stress perception and depressive symptoms may precede and/or follow the associated differences in RMFG anatomical variability. However, based on prior literature, we can make some speculations. It is possible that stress exposure leads to elevations in perceived stress and depression-related phenotypes, as well as increased RMFG cortical thickness. Consistent with this proposition, increased RMFG thickness has been observed among disaster survivors following trauma exposure (i.e., after 1.42 years); additionally, greater thickness here predicted better recovery from PTSD, and thickness normalized (i.e., decreased) to the extent that symptoms remitted by five-year follow-up (Lyoo et al. 2011). These results suggest trauma-dependent increases in RMFG cortical thickness, which resolve alongside psychological recovery. It is plausible that, within our sample, increased cortical thickness may reflect stress exposure and unresolved recovery, resulting in greater perceptions of stress as well as expression of depression-related phenotypes.
Alternatively, RMFG cortical thickness may serve as a preexisting vulnerability factor that influences stress perception and/or confers vulnerability to depression. In support of this explanation, increased DLPFC (including a region within the Desikan-atlas-defined RMFG ROI) gray-matter volume, a different structural metric than cortical thickness which was evaluated in our study, has been correlated with rumination, or the tendency to dwell repetitively on negative emotional experiences (Wang et al. 2015). Rumination is a key risk factor for depression that also mediates the relationship between chronic perceived stress and psychological health risk indicators (e.g., depressive symptoms and sleep quality; Zawadzki, 2015). The relatively high heritability estimates of RMFG cortical thickness that we observe (Right RMFG: 71.34%; left RMFG: 61.56%) are consistent with this notion. However, other findings contradict this interpretation. First, we observed relatively increased cortical thickness among those reporting elevated perceived stress relative to their discordant sibling. Thus, these data suggest that these differences arise from individual specific genetic and/or environmental effects, and that sibling shared genetic and environmental factors are not predisposing in this manner. Other evidence also contradicts this interpretation, as relatively decreased cortical thickness in the DLPFC (including within the RMFG) in adolescent females has been associated with decreased cognitive reappraisal, a form of emotion regulation (Vijayakumar et al. 2014), which has been correlated with reduced stress perception across stages of adulthood (Prakash et al. 2015).
Consistent with a large and established prior literature (e.g., Polderman et al. 2015), heritability analyses suggest that phenotypic variation in perceived stress, depression-related phenotypes, and bilateral RMFG cortical thickness is, in part, attributable to genetic factors. The relatively-high heritability estimates of bilateral RMFG cortical thickness (i.e., 61.56% – 71.34%) are at the upper end of heritability estimates in psychiatric phenotypes (Polderman et al. 2015) and consistent with estimates of average whole-brain cortical thickness (Panizzon et al. 2009). These findings suggest that RMFG cortical thickness may contribute to familial transmission of stress perception and depression risk. Furthermore, decomposition analyses suggest that the association between perceived stress and depression-related phenotypes is attributable to common sources of environmental (e.g., potentially stress) and genetic variation (e.g., potentially brain structure; Bogdan & Pizzagalli 2009).
It is important to consider study limitations when interpreting the present results. First, the study is cross-sectional, leaving uncertain both the underlying temporal nature of associations and their etiologic plausibility. Longitudinal work is needed to elucidate temporal effects, which would bolster confidence in the potential mechanisms underlying these associations (e.g., that perceived stress causes structural differences and/or that structural differences alter the perception of stress). Second, it is important to consider the limitations of large-scale studies assessing multiple phenotypes such as the HCP. To facilitate broad phenotypic coverage and large samples, such generalist studies are often unable to provide comprehensive within-phenotype assessment. For example, in our study, we were limited by the lack of an explicit anhedonia measure, and relied on a correlated measure of positive affect. Similarly, because the HCP did not measure trauma or stressful life event exposure, we are unable to ascertain whether associations between RMFG cortical thickness and perceived stress are attributable to heightened subjective perceptions of stress and/or heightened stress exposure that may lead to increased perception. Third, because this is a relatively healthy sample, it is unclear whether the results might generalize to clinical levels of depression and perceived stress. Indeed, such differences in sample makeup may underlie conflicting directional associations within the literature between RMFG cortical thickness and depression- and stress-related phenotypes (Reynolds et al. 2014; Peterson et al. 2009; Peng et al. 2015).
Importantly, the association between RMFG cortical thickness and self-reported phenotypes – including depression-related phenotypes and perceived stress – were small in magnitude (0.2%–2.4% of variance explained), which prohibited our ability to evaluate shared genetic and environmental covariation across these phenotypes. One reason for these small effects may be our use of a relatively healthy community sample. Given prior reports linking both increased and decreased RMFG cortical thickness to depression and stress-related phenotypes, it is possible that heterogeneous presentations or correlates of depression and stress perception may have oppositional associations with RMFG cortical thickness that reduced our observed effect size. The large effects observed in our discordant sibling analyses that account for unmeasured sibling shared factors support this speculation. Further, prior reports of larger associations (e.g., Reynolds et al. 2014) between RMFG cortical thickness and depression as well as stress-related phenotypes have been observed in smaller patient or trauma-exposed samples (e.g., Reynolds et al. 2014; Peng et al. 2015; Qiu et al. 2014; depressed patient n ranging from 16–46). Such small samples, when combined with publication bias, may result in imprecise and enlarged effect size estimates. For example, a recent meta-analysis including over 17,000 individuals with major depressive disorder found that the effect size of the association between hippocampal volume and depression is small (i.e., 0.5% of variance explained) and less than half of what was found in a prior meta-analysis drawn from fewer participants (n=351 patients; Schmaal et al. 2015; Videbech and Ravnkilde 2004). While such observations suggest that small studies of patients may have led to overestimated effects between brain structure and illness, it is also important to consider that large meta-analyses may also result in more heterogeneous patient groupings that diminish effect sizes.
These limitations notwithstanding, our study suggests that relatively increased RMFG cortical thickness is a common neural substrate of stress perception and depression-related phenotypes that may promote depression/stress vulnerability and/or result from such experience. Though our results suggest that RMFG cortical thickness is positively coupled with both depression-related phenotypes and stress perception, the effect of this association is small and presently would not be informative on an individual level in isolation regarding treatment or risk assessment. Notably, however, our discordancy analyses of perceived stress suggest that this association cannot be attributed to sibling shared genetic factors and familial environment (and indeed becomes much larger when accounting for these factors), providing support for a potential causal relationship between RMFG cortical thickness and perceived stress, though the directionality of causation cannot be determined.
Supplementary Material
Supplemental Figure 1. Desikan Atlas ROIs. The rostral middle frontal gyrus ROI, as defined by the Desikan Atlas (image reproduced with modification from Desikan et al. 2006).
Acknowledgments
We extend thanks to the Human Connectome Project (HCP) study and affiliated staff.
Financial Support
Data for this study were provided by the Human Connectome Project, WU-Minn Consortium (principal investigators: David Van Essen, PhD, and Kamil Ugurbil, PhD; grant 1U54MH091657) funded by the 16 National Institutes of Health institutes and centers that support the National Institutes of Health Blueprint for Neuroscience Research, as well as by the McDonnell Center for Systems Neuroscience at Washington University. LJM (T32-GM081739), CHD (T32-DA007313, T32-GM081739), DAAB (T32-GM008151), DMB (1U54MH091657), GCB (1U54MH091657), MPH (1U54MH091657), and RB (AG045231, HD083614, AG052564) were supported by NIH. DAAB was also supported by NSF (DGE-1143954; non-overlapping with NIH support).
Footnotes
For prior studies that report results in the DLPFC, but not in the RMFG specifically, we probed coordinates associated with the reported DLPFC ROI to certain whether they were within the Desikan-atlas-defined RMFG.
Conflict of Interest
None
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews. Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. Available at: http://dx.doi.org/10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, et al. Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, et al. Package lme4. Journal Of Statistical Software. 2015;67(1):1–91. [Google Scholar]
- Blangero J, Almasy L. Solar: Sequential Oligogenic Linkage Analysis Routines: Population Genetics Laboratory Technical Report No. 6. Southwest Foundation for Biomedical Research 1996 [Google Scholar]
- Bogdan R, Pizzagalli DA. The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychological medicine. 2009;39(2):211–8. doi: 10.1017/S0033291708003619. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2628414&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of Psychiatry and Neuroscience. 2004;29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. Journal of Applied Social Psychology. 2012;42(6):1320–1334. [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Negative Life Events, Perceived Stress, Negative Affect, and Susceptibility to the Common Cold. Journal of Personality and Social Psychology. 1993;64(1):131–140. doi: 10.1037//0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. The Social Psychology of Health. 1988:31–67. Available at: http://doi.apa.org/psycinfo/1988-98838-002.
- Corbo V, et al. Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Research - Neuroimaging. 2014;223(2):53–60. doi: 10.1016/j.pscychresns.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. The British journal of clinical psychology / the British Psychological Society. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ebrecht M, et al. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. 2004 doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- Eijndhoven P Van, et al. Paralimbic cortical thickness in first-episode depression: Evidence for trait-related differences in mood regulation. American Journal of Psychiatry. 2013;170(12):1477–1486. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, et al. The Human Connectome Project: A data acquisition perspective. NeuroImage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, et al. The WU-Minn Human Connectome Project: An overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federenko IS, et al. The heritability of perceived stress. Psychological medicine. 2006;36(3):375–385. doi: 10.1017/S0033291705006616. [DOI] [PubMed] [Google Scholar]
- Gershon RC, et al. NIH Toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11):S2–S6. doi: 10.1212/WNL.0b013e3182872e5f. Available at: http://www.neurology.org/cgi/doi/10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of abnormal psychology. 1991;100(4):555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and Depression. Annu. Rev. Clin. Psychol. 2005;1(1):293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. Available at: http://ezp-prod1.hul.harvard.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2005-07171-011&site=ehost-live&scope=site%5CnHammen@psych.ucla.edu. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Depressive vulnerability, stressful life events and episode onset of major depression: a longitudinal model. Psychological Medicine. 2016:1–10. doi: 10.1017/S0033291716000349. Available at: http://www.journals.cambridge.org/abstract_S0033291716000349. [DOI] [PMC free article] [PubMed]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. Available at: c:%5Cpdfd%5Cxc11583.pdf. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research. 2009;201(2):239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, et al. Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. The Korean journal of physiology & pharmacology?: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2013;17(5):393–403. doi: 10.4196/kjpp.2013.17.5.393. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3823951&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, et al. The Neurobiological Role of the Dorsolateral Prefrontal Cortex in Recovery From Trauma Longitudinal Brain Imaging Study Among Survivors of the South Korean Subway Disaster. Arch Gen Psychiatry. 2011;68(7):701–713. doi: 10.1001/archgenpsychiatry.2011.70. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews in Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morey RA, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of general psychiatry. 2012;69(11):1169–78. doi: 10.1001/archgenpsychiatry.2012.50. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3647246&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, et al. Interactive models of depression vulnerability: The role of childhood trauma, dysfunctional attitudes, and coping. British Journal of Clinical Psychology. 2014;53(2):245–263. doi: 10.1111/bjc.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, et al. Brain-behavior relationships in the experience and regulation of negative emotion in healthy children: Implications for risk for childhood depression. Development and Psychopathology. 2014;26:1289–1303. doi: 10.1017/S0954579414001035. Available at: http://www.scopus.com/inward/record.url?eid=2-s2.0-84913603141&partnerID=40&md5=0a2970100aaf2182db327c1c0c68a1e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, et al. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, et al. Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, et al. A Prospective, Longitudinal Study of the Effect of Remission on Cortical Thickness and Hippocampal Volume in Patients with Treatment-Resistant Depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2015;18(8):pyv037. doi: 10.1093/ijnp/pyv037. Available at: http://ijnp.oxfordjournals.org/content/early/2015/04/22/ijnp.pyv037.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkonis PA, et al. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS??) in a three-month observational study. Journal of Psychiatric Research. 2014;56(1):112–119. doi: 10.1016/j.jpsychires.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Hussain MA, Schirda B. The role of emotion regulation and cognitive control in the association between mindfulness disposition and stress. Psychology And Aging. 2015;30(1):160–171. doi: 10.1037/a0038544. Available at: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=25545683&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Qiu L, et al. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Translational psychiatry. 2014 Feb;4:e378. doi: 10.1038/tp.2014.18. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4012282&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W. Package “psych” - Procedures for Psychological, Psychometric and Personality Research. R Package. 2015:1–358. Available at: http://personality-project.org/r/psych-manual.pdf.
- Reynolds S, et al. Cortical thickness in youth with major depressive disorder. BMC psychiatry. 2014;14:83. doi: 10.1186/1471-244X-14-83. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3994552&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel L, et al. Perceived Stress has Genetic Influences Distinct from Neuroticism and Depression. Behavior Genetics. 2014;44(6):639–645. doi: 10.1007/s10519-013-9636-4. [DOI] [PubMed] [Google Scholar]
- Rosso IM, et al. Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry. 2005;57(1):21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Schmaal L, et al. Suvcortical brain alterations in major depressive disorder: findingins from the ENIGMA Major Depressive Disorder working group. Molecular Psychiatry. 2015;21(6):806–12. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan Ma. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in human neuroscience. 2009 Jan;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, et al. Illness Progression, Recent Stress, and Morphometry of Hippocampal Subfields and Medial Prefrontal Cortex in Major Depression. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong W, et al. Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Research. 2013;214:204–11. doi: 10.1016/j.pscychresns.2013.09.003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24099630. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, et al. Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Social Cognitive and Affective Neuroscience. 2014;9(11):1845–1854. doi: 10.1093/scan/nst183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, et al. Individual differences in rumination in healthy and depressive samples: association with brain structure, functional connectivity and depression. Psychological medicine. 2015:1–10. doi: 10.1017/S0033291715000938. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26219340. [DOI] [PubMed]
- Watson D, Clark LA. The PANAS-X: Manual for the positive and negative affect schedule-expanded form. The British journal of clinical psychology the British Psychological Society. 1994;65:836–851. Available at: http://ir.uiowa.edu/cgi/viewcontent.cgi?article=1011&context=psychology_pubs. [Google Scholar]
- Whalen PJ, et al. Functional neuroimaging studies of the amygdala in depression. Seminars in clinical neuropsychiatry. 2002;7(4):234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Zawadzki MJ. Rumination is independently associated with poor psychological health: Comparing emotion regulation strategies. Psychology & Health, (March) 2015:1–18. doi: 10.1080/08870446.2015.1026904. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25748334. [DOI] [PubMed]
- Zhu S, et al. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport. 2014:1151–1155. doi: 10.1097/WNR.0000000000000243. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25089805. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Desikan Atlas ROIs. The rostral middle frontal gyrus ROI, as defined by the Desikan Atlas (image reproduced with modification from Desikan et al. 2006).


