Abstract
This study summarizes and compares estimates of radiation absorbed dose to the thyroid gland for typical patients who underwent diagnostic radiology examinations in the years from 1930 to 2010. We estimated the thyroid dose for common examinations, including radiography, mammography, dental radiography, fluoroscopy, nuclear medicine, and computed tomography (CT). For the most part, we observed a clear downward trend in thyroid dose over time for each procedure. Historically, the highest thyroid doses came from the nuclear medicine thyroid scans in the 1960s (630 mGy), full-mouth series dental radiography (390 mGy) in the early years of the use of x-rays in dentistry (1930s), and the barium swallow (esophagram) fluoroscopic exam also in the 1930s (140 mGy). Thyroid uptake nuclear medicine examinations and pancreatic scans also gave relatively high doses to the thyroid (64 mGy and 21 mGy, respectively, in the 1960s). In the 21st century, the highest thyroid doses still result from nuclear medicine thyroid scans (130 mGy), but high thyroid doses are also associated with chest/abdomen/pelvis CT scans (18 and 19 mGy for male and females, respectively). Thyroid doses from CT scans did not exhibit the same downward trend as observed for other examinations. The largest thyroid doses from conventional radiography came from cervical spine and skull examinations. Thyroid doses from mammography (which began in the 1960s) were generally a fraction of a mGy. The highest average doses to the thyroid from mammography were about 0.42 mGy, with modestly larger doses associated with imaging of breasts with large compressed thicknesses. Thyroid doses from dental radiographic procedures have decreased markedly throughout the decades, from an average of 390 mGy for a full-mouth series in the 1930s to an average of 0.31 mGy today. Upper GI series fluoroscopy examinations resulted in up to two orders of magnitude lower thyroid doses than the barium swallow. There are considerable uncertainties associated with the presented doses, particularly for characterizing exposures of individual identified patients. Nonetheless, the tabulations provide the only comprehensive report on the estimation of typical radiation doses to the thyroid gland from medical diagnostic procedures over eight decades (1930–2010). These data can serve as a resource for epidemiologic studies that evaluate the late health effects of radiation exposure associated with diagnostic radiologic examinations.
INTRODUCTION
Radiation has been known to be share a causal relationship with thyroid cancer for over half a century (NCRP 2009). In the context of radiation health risk studies and radiation protection, the thyroid is a particularly critical organ due to its susceptibility to develop cancer following exposure in younger individuals. According to the American Thyroid Association, the thyroid is “among the most susceptible sites to radiation-induced cancer” (American Thyroid Association 2013). Recognition that some thyroid cancers are associated with ionizing radiation exposure is based on many studies, including those of atomic bomb survivors (Prentice et al. 1982, Ron et al. 1994) and of persons exposed to moderate-to high dose radiation therapy during childhood (Ron et al. 1980, 1987). The association of low-dose radiation exposure, e.g., from diagnostic medical imaging procedures that involve radiation and other low-dose exposures, and thyroid cancer risk, has not been as well-studied.
Since the discovery of x-rays in the early 1900s, numerous radiographic and fluoroscopic diagnostic imaging examinations, utilizing either external irradiation from x-rays, internal emitters in the form of radionuclides, or a combination, have been developed. Use of administered internal emitters for nuclear medicine diagnostic imaging and mammographic screening examinations began to be used extensively in the 1960s. In the late 1970s, advances in computer processing capability allowed the development of computed tomography (CT). During the early decades of the twentieth century, when the practice of radiology grew rapidly, radiation risks were not well characterized or understood and, in some cases, both patients and medical personnel were exposed to harmful and sometimes fatal amounts of radiation. As the potential for radiation-induced carcinogenesis was appreciated, various means were utilized to reduce exposures to patients and medical radiation workers while maintaining or improving diagnostic information. These changes in technology included an increase in x-ray film speed and improvements in screens for screen-film radiography. At the same time, however, more complex procedures were developed that potentially increased individual exposure. These included CT and complex fluoroscopically-guided interventions (Miller et al. 2003, Linet et al. 2012). With the increased use of these new procedures in recent years, the average annual effective dose per person from medical procedures has increased from 0.5 mSv to 3 mSv between 1980 and 2006 (NCRP 1987, 2009).
Increasing rates of thyroid cancer have gained national and international attention in recent years. According to the National Cancer Institute thyroid cancer is the ninth most common cancer in the United States (NCI 2014); its incidence is the most rapidly increasing of all cancers (Ryerson et al. 2016). Since the early 1970s there has been a 2.4 to 2.9-fold increase in thyroid cancer incidence in the USA (Davies et al. 2014). The rate in women saw a greater increase in incidence, over four times that of the rate in men (NCI 2014). Incidence of thyroid cancer globally has also risen since 1960 (La Vecchia et al. 2015). Recent reports attribute the change mainly to increased surveillance and detection capability (Franceschi and Vaccarella 2015, Vaccarella et al. 2016, NCRP 2009), though temporal changes in known and suspected risk factors (e.g., radiation from medical sources, exposures to radioactive fallout, increasing body mass index, and lifestyle factors) may also contribute to the increasing incidence (Kitahara and Sosa, 2016; Sinnott et al. 2010). To clarify the role of diagnostic radiological procedures, dose estimates of radiation doses to the thyroid gland are needed that can be applied in epidemiologic studies to evaluate the impact of medical radiation exposure on thyroid cancer risk and estimate the proportion of thyroid cancers attributable to medical radiation exposure versus other risk factors.
MATERIALS AND METHODS
We used several approaches to estimate thyroid doses for patients who underwent diagnostic medical procedures involving radiation during 1930 to 2010 in order to account for differences in available data. Methods applied to the data for each of the imaging modalities (conventional radiography, mammography, dental x-ray, fluoroscopy, CT, and nuclear medicine) are discussed in the following sections.
Conventional Radiography
We evaluated 16 commonly performed radiographic examinations: skull, paranasal/sinuses, neck, cervical spine, clavicle, shoulder, chest, ribs, thoracic-cervical spine, thoracic spine, thoracic-lumbar spine, abdomen, sacrum, pelvis, lumbar spine, and lumbosacral spine. Doses to the thyroid for all radiographic exams, except the clavicle and shoulder exams during 1930 – 2010, were taken from a comprehensive dose reconstruction study of diagnostic medical radiography recently published (Melo et al. 2016). Following a comprehensive literature search, Melo et al. estimated thyroid doses from the most common radiographic examinations conducted between 1930 and 2010 by abstracting machine parameters from data provided in training textbooks for radiologic technologists and using those data to calculate typical doses. Use of training textbooks published in different periods enabled us to account for temporal trends in x-ray tube potential (kVp), tube current (mA), and exposure time. Those parameters are directly associated with organ doses (Parry et al. 1999). The number and type of projections, tube potential (kVp), current-time product (mAs), and field size were adopted from the same radiological positioning textbook series (Bontrager 1993, 1997, 2001, Clark 1939, 1949, 1956, 1967, 1973, 1986). Temporal trends in beam filtration were derived from Simon et al (2014). Melo et al. computed thyroid doses using these abstracted data and PCXMC 2.0 software (Radiation and Nuclear Safety Authority (STUK), Helsinki, Finland) which allows simulation of organ doses based on known or assumed values for tube potential (kVp), current-time product (mAs), field size, patient locations and beam filtration. The dose calculations were conducted for a reference adult phantom with a height and weight of 178.6 cm (70.31 in) and 73.2 kg (161 pounds).
Clavicle and shoulder examinations were not included in the 2016 publication of Melo et al. In this work, we replicated that paper’s methodology to compute doses for these two additional examination types.
Mammography
Although research on the use of radiography for diagnosing diseases of the breast began in the 1930s, the technique was not commonly used in medicine until the 1960s (Gold et al. 1990). We calculated thyroid doses from mammography from 1960 to 2010 and presented those results by decade. Incident air kerma values to the breast during mammography must increase with increasing compressed breast thickness in order to achieve adequate image quality and diagnostic information. Incident air kerma is one of the important technical parameters for organ dosimetry in mammography. Air kerma values were adapted from a previous study of fibroglandular tissue dose from mammography (Thierry-Chef et al. 2012). The thyroid gland is not directly exposed during mammographic examinations, though it can receive scatter dose. Chetlen et al. (2016) reported that the average skin doses over the right and left thyroid lobes from scattered radiation were 3.8% and 3.7%, respectively, of the average entrance skin doses to the breast during a routine screening mammographic examination. Whelan et al. (1999) conservatively estimated that the dose to the thyroid gland might be 10% of the skin dose that overlays the thyroid. Hence, we assumed that the dose to the thyroid gland from mammography to be about 0.375% of the entrance skin dose to the breast as shown in eq. (1).
| (1) |
where D(thyroid) is the radiation absorbed dose to the thyroid gland, K is incident air kerma over the breast during mammography, d is the conversion coefficient from skin dose to thyroid dose of 0.1, s is the conversion coefficient (0.0375) from K to skin dose, and n is number of projections, commonly two (mediolateral and craniocaudal) per breast (Thierry-Chef et al. 2012). We estimated dose to the thyroid gland from mammography for compressed breast thicknesses of 3, 5 and 8 cm.
Dental x-ray
Using PubMed, we conducted a comprehensive literature search on dose to the thyroid received from full mouth series (FMX) and dental bitewing examinations in the years 1930 – 2010. Because panoramic x-rays were not performed commonly until the early 1960s (Hallikainen 1996), our literature search was focused on identifying publications that reported thyroid dose for that exam type beginning in 1960. Some literature on doses from dental radiography did not specify the type of dental examination; in those cases, we assumed the reported thyroid doses were from FMX examinations due to the data’s similarity to other existing FMX data. Median and mean thyroid doses were obtained by decade from the data obtained in the literature review.
Some publications in the 1950s, 1960s and 1970s on exposures from dental examination reported thyroid dose in roentgen (R), specifying it as an organ dose despite the definition of R as a measure of ionization in air. Estimates of thyroid dose from dental examinations reported in R were converted to mGy for consistency using one of two conversion methods used previously (Baily 1957, Weissmann and Longhurst 1972, Richards and Webber 1964, Kuba and Beck 1968, Alcox and Jameson 1974, Hudson and Kumpula 1955, Richards and Colquitt 1981). Studies that placed dosimeters within a phantom or cadaver implicitly included consideration of the attenuation of overlying tissue. In that case, the exposure in R was multiplied by the conversion factor of 0.87 rad/R to derive absorbed dose in rad from exposure in R (Webster 2014) and by 1.07, the ratio of energy attenuation coefficient for tissue to that of air, , to account for the fact that the dosimeters were calibrated for exposure in free air. The ratio of attenuation coefficients was determined from the National Institute of Standards and Technology X-ray Mass Attenuation Coefficient Database (NIST 2004) under the assumptions that the tissue was similar to the ICRU Report 44 soft tissue description (ICRU 1989) and the average x-ray energy was 30 keV (Shulman 2006). The dose in rad was then converted to mGy by the conversion factor 10 mGy/1 rad.
| (2) |
where D is thyroid dose (mGy), R is thyroid dose (R), c is the conversion coefficient from R to rads, (0.87 rad/R), and d is the ratio of mass energy attenuation coefficient of tissue to air, 1.07.
For those studies reporting thyroid dose from dental examinations in R, but that placed dosimeters externally to the body, we converted the exposure to absorbed dose by three steps: (i) conversion of exposure in air (R) to rad with the conversion factor (Webster 2014) of 0.87 rad/R, (ii) accounting for backscatter and (iii) accounting for attenuation of the overlying tissue. The second step, i.e., accounting for backscatter, used a numerical factor of 1.3, typical of energies used in diagnostic medicine (Shimizu et al. 2001). The mass attenuation factor, 0.283, was derived from equation (3) using the National Institute of Standards and Technology X-ray Mass Attenuation Coefficient Database (NIST 2004) under the assumption that the tissue was the soft tissue defined in ICRU Report 44.
| (3) |
where i is mass attenuation factor, Io is the initial radiation intensity, μen/ρtissue is mass energy absorption coefficient for soft tissue, ρtissue is mass density of soft tissue, and l is the depth of the thyroid gland from the skin surface, 3.9 cm (+/− 1.6 cm) (Shultz and Rollo 1970).
The steps are summarized in Eq. 4.
| (4) |
where D is thyroid dose (mGy), R is thyroid dose (R), c is the conversion coefficient from R to rad (0.87 rad/R), b is the backscatter factor = 1.3, and i is the attenuation factor = 0.283.
Literature on x-ray usage in dentistry for the years 1930–1950 was particularly limited. Thus, the thyroid doses from full mouth series during 1930–1950 were extrapolated from the 1960s incorporating historical trends of film speed (Farman and Farman 2000) and filtration (Simon et al. 2014).
Fluoroscopy
Fluoroscopy examinations have the potential to expose the thyroid substantially if the gland is in or near the radiation imaging field. As with other imaging modalities, we used different estimation strategies for different decades depending on the type of information available in the literature.
There was very limited literature on thyroid doses from fluoroscopy procedures conducted before the 1980s. For the time period 1930 – 1950, we assumed that temporal trends of dose to the thyroid from fluoroscopy examinations would be similar to the trends for other radiographic exams. Specifically, we derived temporal trends from a study of organ doses from diagnostic medical radiographic examinations (Melo et al. 2016) performed in the same anatomical locations as the upper GI series and barium swallow examinations. All ratios were normalized to the 2000–2009 decade and were applied to the fluoroscopic examinations.
For the decades of the 1960s and 1970s, we calculated the average difference in gonadal doses between the decades as reported by the U.S. Department of Health (Public Health Service, 1969) and Bengtsson et al. (1978) and found a 6.25% difference between the 1960s and the 1970s. Therefore, we assumed that the thyroid doses between these two time periods would be similar.
For the 1980s, the two most commonly conducted fluoroscopy procedures that would have likely exposed the thyroid gland were the upper GI series and barium swallow (esophagram) (Suleiman et al. 1991). We adopted thyroid dose data for the upper GI series in the 1970s from a Swedish study of thyroid, lung and gonadal doses resulting from diagnostic radiographic examinations including fluoroscopy procedures (Bengtsson et al. 1978). That study used tube potential (kVp), current-time product (mAs), field size, and beam filtration of radiographic machines in 13 Swedish hospitals from 1973 to 1975 and measured radiation doses at various points on 1,000 patients using lithium fluoride (thermoluminescent) dosimeters (Harshaw Chemical Co., Cleveland, OH). The thyroid dose resulting from upper GI fluoroscopy in the 1980s and 1990s were adopted from a study by Bankvall et al. (1982) and Suleiman et al. (1991) which reported thyroid tissue doses from upper GI series. For the thyroid doses in the 2000s from an upper GI series, we employed average GI series time, number of images, tube potential (kVp), current-time product (mAs), and field size from the Nationwide Evaluation of X-Ray Trends (NEXT) survey reports (CRCPD and FDA 1996, 2003).
For thyroid doses from barium swallow, we adapted thyroid doses reported in the 1990s and 2000s from existing literature (Crawley et al. 2004, Ramakrishnan and Padmanabhan 2001). For the time period 1930–1989, we applied the temporal trends found in diagnostic medical radiographic examinations to our calculations for fluoroscopy examinations using the same method as for the upper GI series.
Computed Tomography
We estimated thyroid dose from CT for commonly conducted examination types including facial bone, head/brain, cervical spine (neck), chest, heart, abdomen, liver, spleen, kidney, pelvis, gall bladder, pancreas, extremities, abdomen/pelvis, and chest/abdomen pelvis (CAP). We used the National Cancer Institute dosimetry system for Computed Tomography (NCICT) (Lee et al. 2015, Bahadori et al. 2015) for thyroid dose estimation. NCICT uses organ dose coefficients (mGy/mGy), organ absorbed dose (mGy) per volumetric Computed Tomography Dose Index (CTDIvol)(mGy), which were calculated from Monte Carlo radiation simulation of a reference CT scanner coupled with a series of computational human phantoms (Lee et al. 2009). The thyroid dose coefficients for reference adult male and female individuals were derived for the examination types listed above from scan ranges based on anatomical reference points as defined in the scan protocols used in the National Institutes of Health Clinical Center. Thyroid dose (mGy) was then computed by multiplying the thyroid dose coefficients with CTDIvol values collected from the literature for each time period, as described below.
We collected the CTDIvol data needed to estimate the thyroid dose for the time periods of 1970–1979, 1980–1989, 1990–1999, and 2000–2009, where CTDIvol is the output of CT scanners for either a head or body cylindrical CTDI phantom (16 cm and 32 cm, respectively), as follows. For the period 1970–1979, McCullough (McCullough and Payne 1976, McCullough et al. 1978) reported maximum surface dose (rad) using thermoluminescent dosimeters (TLD) placed in an 8.5 inch diameter and 3 inch thick Plexiglas cylindrical phantom for head scans and a 8 × 13 inch and 3 inch thick elliptical phantom for body scans. The location of these “surface dose” measurements was equivalent to that of “peripheral dose” measurements as currently defined. In accordance with a study (McKnitt-Gray, 2002) that showed that the central dose in the head phantom is equivalent to the surface dose and the central dose is about the half of the peripheral dose in the body phantom, we derived the weighted CTDI (CTDIw) by adding two-thirds of peripheral dose to one-third of the central dose. From that, the CTDIvol was calculated by using a pitch of 1 (McKnitt-Gray, 2002). For the period 1980–1989, we adopted the data from Shope et al. (1982) and McCrohan et al. (1987). Shope et al. reported minimum surface dose, central dose, and maximum surface dose using two cylindrical phantoms with diameters of 16 cm and 32 cm. We derived CTDIw from the maximum surface and central doses again by adding two-thirds of peripheral dose to one-third of central dose (McKnitt-Gray 2002). McCrohan et al. (1987) reported the “multiple scan average dose” (MSAD), which is equivalent to the CTDI when pitch is unity (Payne et al. 2005), which was the case for most single-detector CT scanners. For the time period 1990–1999, we employed the data reported by Conway et al. (1992) in the 1990 NEXT survey. That survey reported MSAD measurements from 252 CT scanners using the 16-cm diameter, 15-cm-long cylindrical phantom coupled with 10 cm-long pencil ion chamber. We also used the data from the 2000 NEXT survey of CT (Stern 2007), which reported CTDIvol for head and body phantoms.
Nuclear Medicine
Nuclear medicine (NM) procedures that involve administration of radioiodine have the greatest potential to deliver high absorbed doses to the thyroid gland since these procedures result in concentration of radioactivity in the gland. The usual way of estimating absorbed dose to the thyroid of a patient from a nuclear medicine procedure is to multiply the administered activity and the relevant dose coefficient. However, many different radiopharmaceuticals have been used over the decades for NM procedures, each giving rise to different thyroid doses. For that reason, for the purpose of this work, we provide weighted average organ doses from nuclear medicine which is simply the weighted average thyroid dose to all patients who underwent nuclear medicine thyroid examinations. We defined the weighting factors to be the relative usage proportions of each radiopharmaceutical (Drozdovitch et al. 2015) from individual radiopharmaceuticals as described by Villoing et al. (2017).
Weighted average estimates of absorbed doses per examination to the thyroid (Villoing et al. 2017) among all patients receiving nuclear medicine procedures were calculated as the product of three factors: the percentage of use of a given radiopharmaceutical during a given time period and for a given procedure (Drozdovitch et al. 2015), the administered activity for this radiopharmaceutical per time period per procedure combination (Drozdovitch et al. 2015), and the dose coefficients (mGy/MBq) derived from ICRP publications 53, 80 and 106 (ICRP 1987, 1998, 2008). In the special case of the weighted dose to thyroid, absorbed dose can be derived as follows.
| (5) |
where DT,k,l is the absorbed dose (mGy) to the thyroid, weighted by the procedure type k, within a year l, due to radiopharmaceutical m. pk,l,m is the proportion of the k procedures conducted in year l with radiopharmaceutical m. Ak,l,m is the activity (MBq) for the specified, k, l, and m, and dm is the dose coefficient (mGy MBq−1) expressed in absorbed dose per unit administered activity for the specific T and m.
Weighted average absorbed doses per examination to the thyroid from NM procedures are presented as time-averaged values within five-year time periods between 1960 and 2010 and for seventeen types of diagnostic NM procedures: blood volume, bone marrow scan, brain scan and brain blood flow, cardiac procedures, GI bleeding and Meckel’s scan, hepatobiliary scan, iron metabolism, liver scan, lung ventilation, lung perfusion, pancreas scan, kidney scan, bone scan, thyroid scan, thyroid uptake and tumor localization.
RESULTS
Our results are presented in tables of estimates of organ absorbed dose (mGy) to the thyroid for each type of imaging modality.
Conventional Radiography
Table 1 presents our best estimates of the thyroid doses from 17 different radiography examinations during the period 1930–2010 based on technical parameters derived from radiologic technologists training materials. All doses to the thyroid in recent years are 2.3 mGy or less.
Table 1.
Average thyroid doses (mGy) to typical patients from 16 conventional radiographic procedures from 1930–2009.
| 1930– 1939 |
1940– 1949 |
1950– 1959 |
1960– 1969 |
1970– 1979 |
1980– 1989 |
1990– 1999 |
2000– 2009 |
|
|---|---|---|---|---|---|---|---|---|
| Skulla | 23 | 16 | 9.2–10 | 3.6 | 3.6 | 3.6 | 0.42 | 0.42 |
| Paranasal sinusesa | 0.07 | 0.07 | 0.06 | 0.02 | 0.02 | 0.04 | 0.04 | 0.04 |
| Neck (soft tissue)a | 6.5 | 6.5 | 4.8 | 1.5 | 1.5 | 1.5 | 0.84 | 0.84 |
| Cervical spinea | 49 | 49 | 49 | 7.8 | 7.8 | 7.8 | 2.3 | 2.3 |
| Clavicle | 0.26 | 0.26 | 0.15 | 0.09 | 0.09 | 0.07 | 0.05 | 0.05 |
| Shoulder | 0.07 | 0.07 | 0.04 | 0.25 | 0.31 | 0.08 | 0.03 | 0.03 |
| Chesta | 0.19 | 0.23 | 0.23 | 0.16 | 0.16 | 0.31 | 0.19 | 0.15 |
| Ribsa | 0.53 | 0.53 | 1.0 | 1.7 | 1.8 | 1.5 | 1.04 | 1.04 |
| Thoracic-cervical spinea | 9.6 | 8.5 | 14 | 2.6 | 2.8 | 4.5 | 0.99 | 0.99 |
| Thoracic spinea | 3.1 | 2.0 | 4.4 | 0.96 | 1.2 | 1.4 | 0.23 | 0.23 |
| Thoracic-lumbar spinea | 3.1 | 2.0 | 4.4 | 0.96 | 1.2 | 1.4 | 0.23 | 0.23 |
| Abdomena | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sacruma | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pelvisa | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lumbar spine | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lumbosacral spine | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Adapted from Melo et al. 2016
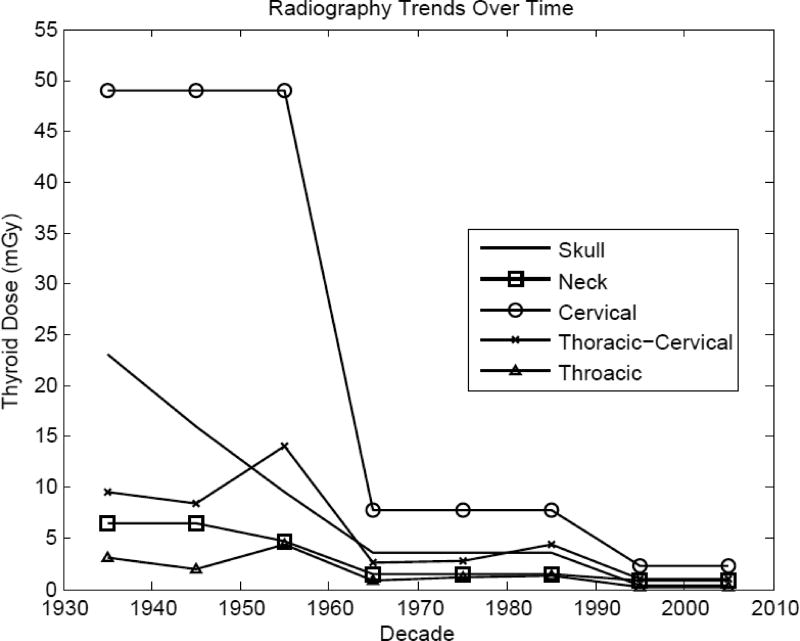
Examinations with the thyroid directly in the field to be imaged or near the x-ray field boundary, e.g., cervical spine, clearly resulted in the largest doses, about 2.3 mGy from 2000 to 2009. Doses to the thyroid from these exams have decreased over 20-fold since the 1930s. Examinations where the thyroid may have been partially in the field or near to the field, e.g., neck, ribs, and thoracic-cervical spine, resulted in the next highest doses in recent years, about 1 mGy from 2000 to 2009. Doses from these exams have decreased much less, presumably because the dose is more of a function of scatter than direct irradiation.
The temporal trends of decade-averaged typical thyroid doses from five examination types are shown in Figure 1. This figure demonstrates the large temporal decrease in thyroid dose from cervical spine radiography where the gland is directly in the field, compared with more modest decreases from exams where the exposure to the gland is more due to scattered radiation.
Figure 1.
Temporal trends of decade-average typical thyroid doses for radiographic procedures yielding the highest doses to the thyroid.
Mammography
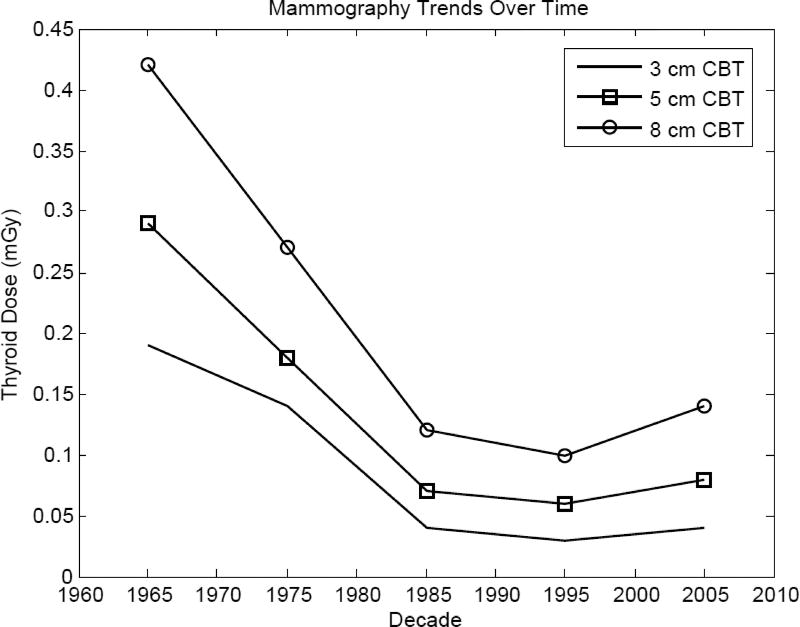
Doses to the thyroid gland from mammography are a result of scattered radiation since the field does not normally include the gland. Present day doses from mammography were estimated to be about 0.1 mGy for 3 cm compressed breast thickness (CBT) to about 0.4 mGy for 8 cm CBT.
Average estimated doses to the thyroid from mammography (Figure 2) have been relatively constant since the 1980s though were about 10-fold greater in the 1960s and 1970s (Tables 2a–2c). Because dose to the thyroid is largely scatter dose, the same technological advances that led to decreases in breast dose from mammography (see Thierry-Chef et al. 2012) have resulted in decreases to thyroid dose.
Figure 2.
Temporal trends of decade-average typical thyroid doses from mammography based on the most common range of compressed breast thickness.
Table 2.
| a. Estimates of average thyroid doses (mGy) to typical female patients from mammography for 3 cm CBTa). | ||||
|---|---|---|---|---|
| Mean | Min | Max | Protocols | |
| 1960 – 1969 | 0.19 | 0.03 | 0.74 | Egan, Gershon-Cohen |
| 1970 – 1979 | 0.14 | 0.00 | 1.7 | Egan, Typical (non-screen), Xeroradiography, Screen-Film (low-dose) |
| 1980 – 1989 | 0.04 | 0.00 | 0.13 | Xeroradiography, Screen-Film (low-dose) |
| 1990 – 1999 | 0.03 | 0.00 | 0.11 | Xeroradiography, Screen-Film (low-dose) |
| 2000 – 2009 | 0.04 | 0.01 | 0.11 | Screen-Film (low-dose) |
| b. Estimates of average thyroid doses (mGy) to typical female patients from mammography for 5 cm CBT. | ||||
|---|---|---|---|---|
| Mean | Min | Max | Protocols | |
| 1960 – 1969 | 0.29 | 0.03 | 1.2 | Egan, Gershon-Cohen |
| 1970 – 1979 | 0.18 | 0.00 | 1.8 | Egan, Typical (non-screen), Xeroradiography, Screen-Film (low-dose) |
| 1980 – 1989 | 0.07 | 0.01 | 0.23 | Xeroradiography, Screen-Film (low-dose) |
| 1990 – 1999 | 0.06 | 0.00 | 0.20 | Xeroradiography, Screen-Film (low-dose) |
| 2000 – 2009 | 0.08 | 0.02 | 0.19 | Screen-Film (low-dose) |
| c. Estimates of average thyroid doses (mGy) to typical female patients from mammography for 8 cm CBT. | ||||
|---|---|---|---|---|
| Mean | Min | Max | Protocols | |
| 1960 – 1969 | 0.42 | 0.02 | 1.3 | Egan, Gershon-Cohen |
|
| ||||
| 1970 – 1979 | 0.27 | 0.00 | 3.7 | Egan, Typical (non-screen), Xeroradiography, Screen-Film (low-dose) |
|
| ||||
| 1980 – 1989 | 0.12 | 0.01 | 0.52 | Xeroradiography, Screen-Film (low-dose) |
|
| ||||
| 1990 – 1999 | 0.10 | 0.01 | 0.43 | Xeroradiography, Screen-Film (low-dose) |
|
| ||||
| 2000 – 2009 | 0.14 | 0.03 | 0.44 | Screen-Film (low-dose) |
CBT is compressed breast thickness (cm)
Dental radiography
Present-day dental radiography (without the use of a thyroid shield) results in doses to thyroid of a few hundredths of a mGy to a few tenths of a mGy, depending on the exam type. Similar to mammography, our calculations show that dose to the thyroid from dental radiography has been relatively constant since the 1980s.
Good data on which to base organ dose estimates in the 1930s through the 1950s are extremely sparse and the few data available result in highly skewed distributions due to occasional large dose estimates reported in the literature.
FMX dental radiography in the 1930s resulted in the highest thyroid dose from any radiographic procedure in any decade (Table 3). However, by the 1960s, thyroid dose associated with this procedure was markedly reduced. Overall, bitewing examinations resulted in the smallest doses overall and, by 1970, there was little to no dose to the thyroid from those procedures.
Table 3.
Estimates of average thyroid doses (mGy) from three types of diagnostic dental procedures
| 1930– 1939 |
1940– 1949 |
1950– 1959 |
1960– 1969 |
1970– 1979 |
1980– 1989 |
1990– 1999 |
2000– 2009 |
|
|---|---|---|---|---|---|---|---|---|
| Full-Mouth Series (FMX) | 390 (84a) b | 250 (53a) b | −96 (21a) | −0.32 | −0.28 | −0.12 | −0.56 | −0.31 |
| Bitewing | 3.1b | 2.0 b | 0.49 b | 0.39 | 0.01 | 0.01 | 0.01 | 0.00 |
| Panoramic | - | - | 19c | 0.34 | 0.56 | 0.07 | 0.02 | 0.04 |
Median value if mean is skewed to due combination of sparse and widely varying data
Data Extrapolated
Note: Panoramic examinations did not start commercially until the 1960s
Sources:
FMX:
1950s: Hudson and Kumpula 1955, Bailey 1957
1960s: Richards and Webber 1964, Winkler 1968
1970s: Greer 1972, Jerman et al. 1973, Alcox and Jameson 1974
1980s: Richards and Colquitt 1981, Kircos et al. 1987, Underhill et al. 1988, Brand et al. 1989, Bristow et al. 1989
1990s: Avendanio et al. 1996, Cederberg et al. 1997
2000s: Lambrecht et al. 2004, Ludlow et al. 2008
Bitewing:
1960s: Richards 1964
1970s: Alcox and Jameson 1974, Lee 1974
1980s: Kircos et al. 1987, Brand et al. 1989
1990s: Velders et al. 1991 (2), Avendanio et al. 1996
2000s: Ludlow et al. 2008, Dauer et al. 2014
Panoramic:
1950s: Hudson and Kumpula 1955
1960s: Kuba and Beck 1968
1970s: Block et al. 1977
1980s: Nilsson et al. 1985, Underhill et al. 1988, Bristow et al. 1989
1990s: Dula et al. 19982000s: Danforth and Clark 2000, Lecomber et al. 2001, Gijbels et al. 2001, Cohnen et al. 2002, Lambrecht et al. 2004, Ekestubbe et al. 2004, Gavala et al. 2009
Fluoroscopy
Fluoroscopic procedures result in a very wide range of thyroid doses due to the diversity of the procedures and the wide range of important dose-determining variables, e.g., exposure-time. Dose from Upper GI series since the 1980s has ranged from a few tenths of a mGy to about 0. 6 mGy while barium swallow (esophagram) gives doses of 10–22 mGy. Table 4 summarizes the thyroid doses from fluoroscopy examinations.
Table 4.
Estimates of average thyroid doses (mGy) to typical patients for three common types of fluoroscopic procedures from 1930 – 2009.
| 1930–1939 | 1940–1949 | 1950–1959 | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | 2000–2009 | |
|---|---|---|---|---|---|---|---|---|
| Upper GI seriesa | 1.8 – 7.4 (up to 49)b | 1.8 – 7.4 (up to 49)b | 1.4 – 5.9 (up to 39)b | 0.29 (0.15–1.2)b,c | 0.29 (0.15–1.2)d,e | 0.15 – 0.55 (up to 3.5)f | 0.15 – 0.55 (up to 3.5)f | 0.13 – 0.53 (up to 3.5) |
| Barium Swallow (Esophagram) | 140b | 140b | 110b | 25b | 25b | 22b | 10g | 10g |
Ranges are included for the upper GI series due to the variation of data from the literature searches. Outliers are also included in parentheses.
Data extrapolated
Derived from Public Health Service, 1964
Derived from Bentgsston et al 1978
Derived from Bankvall et al. 1982
In decades past, before efficient image intensifiers and other technologies were available, doses for upper GI series were 2- to 10-fold greater and doses from barium swallows were 10-fold or more greater.
Computed Tomography (CT)
CTDIvol data collected from the literature is presented in Table 5a for head and body CTDI phantoms over four decades: the 1970s, 1980s, 1990s, and 2000s. In the most recent decade, CDTIvol values for head and body were about 62 and 17 mGy, respectively. In contrast to other imaging modalities, we observed that CTDIvol increased by 200% and 189% for head and body phantoms, respectively, from the 1970s to the 2000s. The increase is consistent with other literature where radiation dose from CT is reported (Smith-Bindman et al. 2012, NCRP 2009). The demand for higher resolution images to provide increased diagnostic information has been a factor in the increase of doses.
Table 5.
| a. CTDIvol measurements for the head and body CTDI phantoms from 1970s to 2000s collected from the literature. | ||||
|---|---|---|---|---|
| CTDI Phantom | 1970 – 1979 | 1980 – 1989 | 1990 – 1999 | 2000–2009 |
| Head | 31 | 35 | 46 | 62 |
| Body | 9 | 12 | 13 | 17 |
| b. Estimates of average thyroid dose (mGy) for typical adult male from 15 different CT examination types. | ||||
|---|---|---|---|---|
| 1970 – 1979 | 1980 – 1989 | 1990 – 1999 | 2000 – 2009 | |
| Facial Bone | 0.31 | 0.35 | 0.46 | 0.62 |
| Head/Brain | 0.37 | 0.42 | 0.55 | 0.74 |
| Cervical Spine (Neck) | 17 | 24 | 25 | 34 |
| Chest | 14 | 19 | 21 | 28 |
| Heart | 1.1 | 1.6 | 1.7 | 2.3 |
| Abdomen | 0.26 | 0.35 | 0.38 | 0.51 |
| Liver or Spleen | 0.30 | 0.41 | 0.44 | 0.60 |
| Kidney | 0.21 | 0.29 | 0.32 | 0.43 |
| Gall Bladder or Pancreas | 0.30 | 0.41 | 0.44 | 0.60 |
| Pelvis | 0.01 | 0.01 | 0.01 | 0.02 |
| Extremities | 0.00 | 0.00 | 0.00 | 0.00 |
| Abdomen/Pelvis | 0.27 | 0.36 | 0.39 | 0.53 |
| Chest/Abdomen/Pelvis (CAP) | 16 | 22 | 23 | 32 |
| c. Estimates of average thyroid dose (mGy) for by decade for typical adult females from 15 different CT examinations. | ||||
|---|---|---|---|---|
| 1970 – 1979 | 1980 – 1989 | 1990 – 1999 | 2000 – 2009 | |
| Facial Bone | 0.37 | 0.42 | 0.55 | 0.74 |
| Head/Brain | 0.50 | 0.56 | 0.74 | 0.99 |
| Cervical Spine (Neck) | 17 | 24 | 26 | 34 |
| Chest | 16 | 22 | 23 | 31 |
| Heart | 1.2 | 1.6 | 1.7 | 2.4 |
| Abdomen | 0.30 | 0.41 | 0.44 | 0.60 |
| Liver or Spleen | 0.34 | 0.47 | 0.50 | 0.68 |
| Kidney | 0.21 | 0.29 | 0.32 | 0.43 |
| Gall Bladder or Pancreas | 0.34 | 0.47 | 0.50 | 0.68 |
| Pelvis | 0.01 | 0.01 | 0.01 | 0.02 |
| Extremities | 0.00 | 0.00 | 0.00 | 0.00 |
| Abdomen/Pelvis | 0.31 | 0.42 | 0.45 | 0.62 |
| Chest/Abdomen/Pelvis (CAP) | 17 | 23 | 25 | 34 |
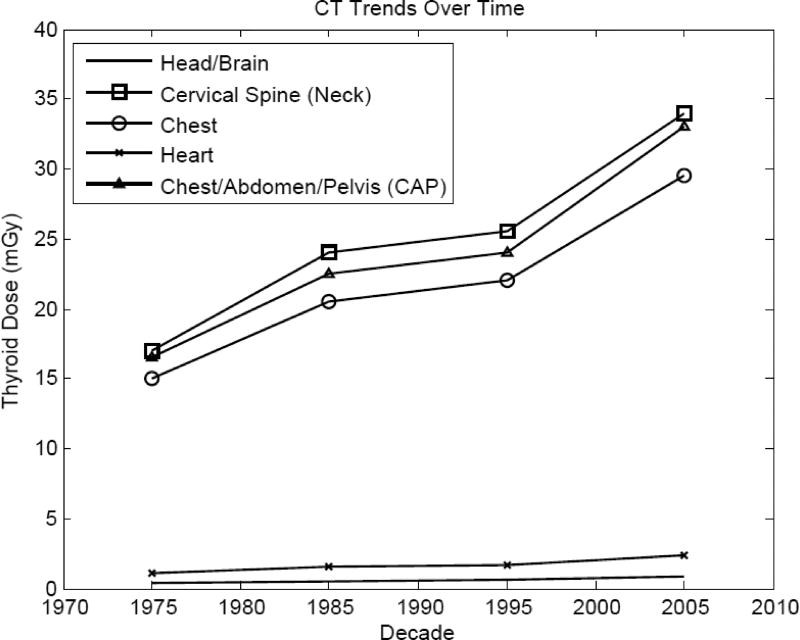
Tables 5b and 5c present the thyroid dose received by a typical adult male and female for 15 different types of CT examinations in the period 1970–2010. CT of the liver and spleen and CT of the gall bladder and pancreas cover the same field and, therefore, their computed thyroid doses were the same. As with other imaging modalities, the presence of the thyroid gland in the imaging field is the primary determinant of dose to the gland. For example, in the most recent decades, CT imaging of the cervical spine (which includes the gland in the radiation field) results in doses of about 34 mGy both for males and females. CT imaging of parts of the body near the thyroid, e.g., the head/brain, result in thyroid doses in recent years of about 0.74 and 0.99 mGy for male and females, respectively. Overall, as is the case for CDTIvol, doses to thyroid have increased in recent years by about 2-fold since the 1970s.
For the same examination types, thyroid doses to females, as compared to males, are estimated to be about the same for most examination types. Only for head/brain CT scans are the doses higher in females (up to 50% greater) reflecting different body sizes.
Nuclear Medicine
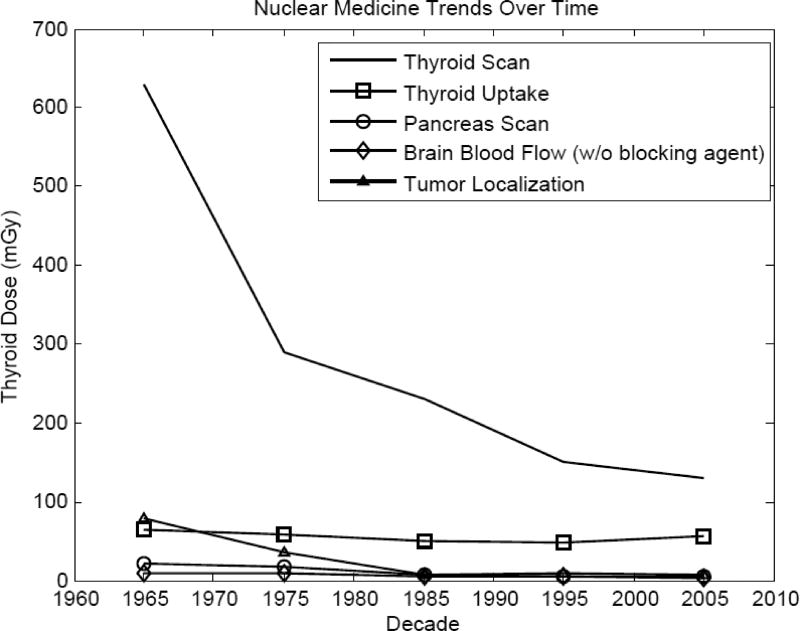
Estimates of weighted average dose per examination to the thyroid from 17 NM procedures in five successive decades are presented in Table 6. Examinations of the thyroid gland resulted in the largest radiation doses since the gland actively accumulates radioiodine.
Table 6.
Estimates of weighted average absorbed dose per examination (mGy) to thyroid, from 17 nuclear medicine procedures.
| 1960–1969 | 1970 – 1979 | 1980 – 1989 | 1990 – 1999 | 2000–2009 | |
|---|---|---|---|---|---|
| Thyroid scan | 630 | 290 | 230 | 150 | 130 |
| Thyroid uptake | 64 | 58 | 50 | 48 | 56 |
| Brain scana | 1.8 | 1.3 | 3.6 | 9.0 | 6.9 |
| Brain blood flowb | 10/7.6 | 8.5/2.0 | 4.1/1.1 | 5.0/4.8 | 2.9 |
| Lung perfusion | 0.48 | 0.35 | 0.44 | 0.46 | 0.46 |
| Lung ventilation | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 |
| Bone scan | 2.1 | 1.1 | 0.96 | 1.1 | 1.2 |
| Liver scan | 0.23 | 0.15 | 0.15 | 0.15 | 0.14 |
| Hepatobiliary scan | 0.01 | 0.01 | 0.03 | 0.03 | 0.03 |
| Bone marrow scan | 1.9 | 0.60 | 0.51 | 0.51 | 0.52 |
| Pancreas scan | 21 | 17 | 6.7 | 4.2 | 4.2 |
| Renal Scan | 0.14 | 0.17 | 0.31 | 0.17 | 0.07 |
| Cardiac procedures | 0.72 | 11 | 17 | 12 | 9.2 |
| GI bleeding and Meckel’s scan | 4.2 | 4.2 | 4.2 | 4.6 | 5.4 |
| Tumor localization | 78 | 35 | 6.9 | 8.4 | 7.6 |
| Blood Volume | 0.02 | 0.06 | 0.09 | 0.08 | 0.05 |
| Iron Metabolism | 0.31 | 0.31 | 0.31 | 0.31c | - |
With administration of blocking agent for Tc-99m pertechnetate
Without/with administration of blocking agent for Tc-99m pertechnetate
Procedure not performed in latter half of decade
As shown in Table 6 and Figure 4, absorbed doses to the thyroid from thyroid scans were very high in the early years, about 630 mGy. Thyroid scans (i.e., imaging of the gland) resulted in the largest doses in all time periods. Substantially lower doses were received from thyroid uptake examinations (assessments of physiology without imaging) (Table 6 and Figure 4). Thyroid uptake studies delivered radiation doses to the thyroid of 64 mGy in the early years, remaining nearly constant with a gradual decrease to 48 mGy between 1960 and 1999. While improvements in technology have allowed thyroid doses from thyroid scans to be reduced 5-fold over time, imaging of the thyroid still results in exposure greater than all other imaging modalities.
Figure 4.
Temporal trends of decade-average typical thyroid doses for five different nuclear medicine examination types.
Cardiac NM examinations give the next highest thyroid doses (about 9 mGy in recent years) followed by brain scans (about 7 mGy in recent years). Thyroid doses from both procedures have varied considerably over the decades with highest doses received most recently. Changes in doses reflect changes in imaging technology and changes in radiopharmaceuticals used (see Villoing et al. 2017).
Thyroid doses from other nuclear medicine examinations are on the order of a few mGy to a few tenths or hundredths of a mGy. In these cases, dose to the thyroid is presumably a function of uptake of the radiopharmaceutical in structures near the thyroid (e.g., bone, lung, salivary glands), the magnitude of the administered activity, the average gamma-ray energy, and the half-time of the radionuclide in the body.
DISCUSSION
Estimated doses to the thyroid were substantial during 1930–1950 for most examinations, particularly for radiographic cervical spine, thoracic-cervical spine and neck, dental full-mouth series, fluoroscopic barium swallow and upper GI series. As expected, we found a clear downward trend of doses to the thyroid over time. Although we did not specifically study the possible causes of this trend, our literature review suggests that this trend was due to technological advances, and may also have reflected improved safety procedures and overall increased awareness of the potential dangers of medical radiation exposure.
For x-ray imaging modalities, it is clear that the major contributing factor to thyroid dose is whether the thyroid is within or close to the x-ray field. In the case of nuclear medicine procedures, if there is uptake of the radiopharmaceutical by the thyroid or organs near the thyroid, the dose to the thyroid is high. In comparison with the earliest applications of radiation for medical diagnosis, most procedures conducted today result in much smaller radiation doses to a patient’s thyroid.
In recent decades, newly developed digital techniques have become dominant in many of these x-ray imaging modalities. While a reduction in the exposure per image can be an advantage to radiation protection of the patient, this may be offset if more images are taken due to the increased convenience of acquiring the images.
Conventional Radiography
Cervical spine radiography, which yields the highest thyroid doses throughout the decades, requires an x-ray field that encompasses the thyroid. Cervical spine radiography results in both the highest direct dose to the thyroid and the highest amount of scattered radiation. Other radiographic examinations, such as radiography of the abdomen or pelvis, result in essentially no dose to the thyroid since the x-ray field is distant from the thyroid. Other factors with a direct relationship to thyroid dose include the number of projections (e.g., anterior-posterior, lateral) and current-time product (mAs). In general, mAs was reduced significantly between the 1930s and 2000s as a result of improvements in materials (film, intensifying screens) and technology. For example, the soft-tissue neck examination in the AP projection had a ten-fold reduction of mAs between 1930 and 2010 (Melo et al. 2016).
Skull radiography has had the largest reduction of thyroid dose, by a factor of over 50 between 1930 and 2009. For those radiographic examinations with the thyroid in or very close to the field, such as radiography of the skull, paranasal sinuses, cervical spine or clavicle, there appear to be three main eras of thyroid radiation dose: between 1930 and 1959, 1960 to 1989, and 1990 to 2009.
Some examination types produced higher doses in a more recent decade than in earlier ones. For example, shoulder radiography resulted in lower doses in the 1930s and 1940s than it did between 1960s and 1970s. While technological advances, in theory, should have decreased the dose, this was counterbalanced by an increase in the number of projections in those years.
Mammography
Starting in the 1970s, xeroradiography lowered the amount of radiation exposure to the patient, particularly to the breast, as compared to the film method in use at the time. Over the next two decades, new low-dose techniques and low dose screen-film combinations further reduced radiation exposures (Thierry-Chef et al. 2012), resulting in the relatively large abandonment of xeroradiography in the U.S. There were also substantial differences within the same decade, depending on the protocol used, as seen in the 1960s when the Egan protocol resulted in up to a five-fold higher breast dose than the Gershon-Cohen protocol, which subsequently also yielded a five-fold higher thyroid dose in our calculations.
Other factors affecting radiation exposure from mammography include the x-ray target material (i.e., tungsten or molybdenum), beam filtration, and breast composition and compressed thickness, exposure time, grid, image receptor, and distances (source-image, source-oject, object-image) (Thierry-Chef et al. 2012, NCRP 2008). One other potential factor of thyroid dose is the use of a thyroid collar, but such shielding is not recommended for mammography procedures (Sechopoulos and Hendrick 2012). It was assumed in this study that the breast composition consisted of 50 percent fibrograndular tissue and 50 percent adipose tissue (Thierry-Chef et al. 2012). While the median CBT is about 5 cm (Thierry-Chef et al. 2012), values range from less than 3 cm to more than 8 cm. Larger values of entrance air kerma are required for imaging breasts with larger CBT, resulting in the potential for greater scatter dose to the thyroid.
Often, doses to other tissues besides the breast are multiplied by two to account for the two standard views. One limitation in using a multiplication factor of two is the assumption that both mediolateral and craniocaudal views result in equal scatter doses. Some research has shown that lateral views tend to yield higher doses (Young and Burch 2000). There is also the possibility that some mammography examinations involved more than two views. Screening mammography routinely uses two views per breast, but diagnostic mammography, performed to evaluate a suspected abnormality, often require additional views.
Dental radiography
There have been many advances in film speed technology throughout the decades. When dental radiography was introduced in 1919, Kodak Regular dental film was used. By 1925, RadiaTized film became more commonly used, reducing the patient dose by 50 percent. Radiation dose to the patient was reduced to 25 percent of 1919 values with the introduction of Ultra-Speed film and newer RadiaTized film in the 1940s and 1950s (Farman and Farman 2000). By 1955, D Ultra-speed film was introduced and patient dose was just 4 percent of what it was in 1919 with the Kodak Regular dental film. In the 1980s and 1990s, speed group E films were used commercially, lowering total patient dose to 2 percent of 1919 values (Farman and Farman 2000). Most recently, speed group F films were put into practice and now radiation doses are just 1 percent of those in 1919 (Farman and Farman 2000). Thyroid doses from dental x-rays today are well below levels from procedures performed in the earliest decades not only due to better films, but also to the use of protective collars (Iannucci and Howerton 2016) to shield the gland.
Fluoroscopy
The barium swallow examination yielded much higher doses than the upper GI series. The reason for this difference is likely that the barium swallow examination focuses exclusively and in detail on the esophagus and swallowing mechanism. The upper GI series, while it includes examination of the esophagus, focuses on the stomach and duodenum. Barium swallow examinations yield relatively high doses to the thyroid compared to other procedures, but such doses are still almost 15 times lower than they were for doses we estimated (by extrapolation) in the earliest decades. Because of the assumption that fluoroscopic temporal trends mimicked that of radiographic trends, the three main dose eras (1930–1959, 1960–1989, 1990–2009) seen in radiography are also seen in estimates of dose for the barium swallow.
Fluoroscopy is an examination in which patient doses, and therefore thyroid doses, are particularly affected by the technique of the fluoroscopist and the complexity of the medical condition. This is evident from the wide ranges in thyroid doses for the upper GI examination seen in Table 4. Even examinations performed by the same physician can yield a wide range of doses, as these examinations are tailored for individual patients to adequately evaluate any abnormalities that are seen. Data taken from Suleiman et al. (1991) were from procedures conducted by two different physicians; the number of spot radiographs ranged from 10 to 22. Fluoroscopy time can also differ widely. In general, the dose from the fluoroscopy portion of the procedures was higher than the dose from the radiographic portion, but in some cases, even with the same operating physician, the radiographic portion of the examination could yield a higher proportion of total dose. As such, there can be large uncertainties. This is evident when we included data that might sometimes be classified as outlier data.
Computed Tomography (CT)
Large thyroid doses were estimated for examinations that included the thyroid in the field, such as the chest/abdomen/pelvis (CAP) examination, cervical spine (neck), and chest examinations (Figure 3). The thyroid gland in females receives slightly greater dose than in males because of smaller body size, which results in less attenuation of the x-rays. Since organ dose is proportional to CTDIvol, the thyroid dose follows the same trend as CTDIvol. For example, the thyroid dose from CT of the cervical spine in the 2000s (34 mGy), is twice that in the 1970s (17 mGy).
Figure 3.
Temporal trends of gender-averaged mean thyroid doses for five different computed tomography (CT) examination types.
The rising prominence of automatic exposure control (AEC) has also helped contribute to lower radiation doses (Söderberg and Gunnarsson 2010). However, the overall radiation dose even with the use of AEC still relies on proper patient centering in the gantry (Gudjonsdottir et al. 2009).
Nuclear Medicine
With respect to radiation dose to the thyroid, two main NM examinations are particularly relevant: thyroid scan (imaging examinations) and thyroid uptake (physiologic studies). In terms of radiation doses, the highest doses to the thyroid were, on average, due to these examinations. For both of those examinations 131I-sodium iodide was the main radiopharmaceutical in use before the mid-1960s. The administered activities of this radiopharmaceutical at that time were 1.85 and 0.185 MBq, respectively (Drozdovitch et al., 2015). Two other radiopharmaceuticals were used after the mid-1960s: 99mTc- pertechnetate and 123I-sodium iodide (in the 1970’s). The use of 99mTc-pertechnetate increased rapidly to 62% of thyroid scans by the late 1970s, before decreasing to 36% in late 2000s. Its use remained infrequent for thyroid uptake studies, with a maximum of 11% of use between the mid-1970s and late-1980s. Use of 123I-sodium iodide for thyroid uptake studies gradually increased to 56% by the late 2000s. The administered activities of these three radiopharmaceuticals all increased over time in thyroid scans, whereas they remained constant for 99mTc-pertechnetate and 123I-sodium iodide in thyroid uptake studies (and increased by a factor of 1.4 for 131I-sodium iodide).
High doses (about 670 mGy) from thyroid scans in the 1960s decreased substantially over time due to changes in radiopharmaceuticals. When 99mTc-pertechnetate and 123I-sodium iodide began to replace 131I-sodium iodide, a rapid decrease in average dose was observed, about a factor of 3.0 over the two first decades. A 36 mGy increase was subsequently observed in the early 1980s, when all administered activities were increased (by a factor of 2 for 99mTc-pertechnetate and 123I-sodium iodide). Administered activities decreased by a factor of 1.9 between the 1980s and 2000s, when the use of 131I-sodium iodide again became more frequent. An increase in administered activities of 99mTc- pertechnetate and 123I-sodium iodide also occurred in mid 1990s.
Thyroid uptake studies changed much less over time compared to thyroid scans, decreasing from about 64 mGy in the early years to about 48 mGy between the 1960s to 1999, as 131I-sodium iodide was replaced by 99mTc-pertechnetate and then 123I-sodium iodide. An increase in thyroid dose was observed when the use of 99mTc-pertechnetate was stopped. In the most recent time period that we evaluated, 131I-sodium iodide was used for 45% of the thyroid uptake studies.
A third diagnostic NM examination, tumor localization scans, delivered high radiation doses to thyroid in the early years (78 mGy), due to the use of 131I-labeled human serum albumin (HSA). After this 131I-labeled radiopharmaceutical was replaced by less irradiating radiopharmaceuticals such as 67Ga-citrate and 18F-FDG, absorbed doses to the thyroid substantially decreased to 7.7 mGy in the 2000s. Other procedures that yield relatively high doses to the thyroid are brain scans, brain circulation studies and pancreas scans. The use of a thyroid blocking agent for 99mTc-pertechnetate with brain scans and brain circulation studies was effective in reducing radiation to the thyroid. NM brain scans, brain circulation studies and pancreas scans have now been replaced by CT and magnetic resonance imaging (MRI).
Uncertainty and limitations
Because of the uniqueness of each modality and the different limitations of the available data for each, it was not possible to develop and implement a single estimation strategy. Each modality required unique assumptions to estimate typical thyroid doses and trends over time. The dose estimates presented here were derived from many publications, sources of data, and estimation methods. For that reason, the estimates of thyroid dose presented here do not all have the same level of uncertainty associated with them. Uncertainty is difficult to quantitatively determine from many of the historical publications because of lack of relevant information. All dose estimates are presented in this work with a maximum of two significant digits (and sometimes fewer) to reflect the limited state of knowledge. Most important here is the caveat that the presented doses are estimates for typical patients in a given time period and not to any identified individual. The exception to this is for nuclear medicine doses, which are not for typical patients but are weighted average estimates over the different radiopharmaceuticals used for each particular examination type.
There are also attributes of each individual patient that result in uncertainty when considering the applicability of the data presented here. For example, patient size varies and the dose required for a given modality will usually reflect differences in exposure as a consequence of difference in body size. An example is the relationship of patient size and organ mass. Data indicates that persons with a larger body-mass-index (BMI) tended to have a larger thyroid volume (Wesche et al. 1998). However, how that might affect the dose can vary. Generally, for radiographic imaging, a larger air kerma is needed to visualize internal structures for larger size patients and can lead to larger doses. On the other hand, a person with a body mass and larger thyroid, but administered a standard radionuclide dosage, might receive a smaller organ dose if the radionuclide were distributed within a larger tissue mass. Such complex issues underscore why the presented doses should be viewed as typical for the examination type, but not applicable to individuals.
A significant limitation in the methods we used is that most of the estimates involved some degree of extrapolation. Quantitative uncertainty estimates are very difficult to achieve when extrapolation methods are used because of the absence of the error terms needed for conventional error propagation strategies. Nonetheless, it seems likely that the doses provided here which, again, are intended to represent typical exposures, are not in error for individuals by more than two-fold in either direction. The error might often be significantly less.
There are few relevant publications for many of these examinations. Because of the need to extrapolate data in this study, assumptions, sometimes for different purposes, had to be made in regard to when, and to what degree, technological changes were implemented. The actual year when changes in technology would have taken place differed by hospital and medical center. Hence, the temporal trends demonstrated here represent an overall nationwide change but cannot represent any specific medical facility.
CONCLUSIONS
The dose estimates presented in this study are the result of a comprehensive, international literature search spanning eight decades. Six major imaging modalities were further separated into specific exams commonly performed in each decade with accounting of temporal trends of evolving safety and imaging trends. Significant strengths in this research include an extensive historical and international literature search and review dating back from the early 20th century to the present.
The highest thyroid doses in our analysis came from nuclear medicine thyroid scans (>600 mGy) in the 1960s. Nuclear medicine thyroid scans today still contribute the largest dose received from the examinations we investigated. Historical nuclear medicine doses are difficult to summarize because of the changes in technology and radiopharmaceuticals used. For that reason, we presented a weighted average of thyroid doses over all nuclear medicine patients who received thyroid exams that considers the fractional usage of each pharmaceutical available in each time period as presented by Drozdovitch et al. (2015). In the 21st century, the highest thyroid doses are still from nuclear medicine thyroid scans and uptake examinations (130 and 56 mGy, respectively). Moderately large thyroid doses today are also associated with chest/abdomen/pelvis CT scans (18 and 19 mGy for male and females, respectively). The largest thyroid doses from conventional radiography arise from cervical spine and skull examinations where the gland is clearly in the field to be imaged.
Also of note were high doses to the thyroid from full-mouth series dental radiography (390 mGy) in the early years of the use of x-rays in dentistry (1930s). Dentistry today clearly contributes very small doses to the thyroid gland, on the order of a few hundredths of a mGy to a few tenths of a mGy, and can be even lower if protective thyroid collars are used.
Thyroid doses from mammography (which began in the 1960s) were generally estimated to be a fraction of a mGy but depend on the thickness of the breast to be imaged since the entrance air kerma must be greater for larger CBT.
There are considerable uncertainties associated with the presented doses, particularly for the purpose of characterizing exposures of individual identified patients. The dose estimates presented here represent typical exposures (except for weighted average dose from nuclear medicine) and are not intended to represent the dose to any specific patient. Estimates are presented with a maximum of one to two significant digits to reflect the limits in the state-of-knowledge.
The tables provided here are the only comprehensive report on the estimation of typical radiation doses to the thyroid gland from medical diagnostic procedures over eight decades (1930–2010) of which we are aware. These data are intended as a resource for epidemiologic studies that include exposure to radiation for medical examinations, either as the primary or a secondary source of exposure to the thyroid gland.
References
- Al-Okshi A, Nilsson M, Petersson A, Wiese M, Lindh C. Using GafChromic film to estimate the effective dose from dental cone beam CT and panoramic radiography. Dentomaxillofacial Radiology. 2013;42(7) doi: 10.1259/dmfr.20120343. DOI: http://dx.doi.org/10.1259/dmfr.20120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcox RW, Jameson WR. Patient exposures from intraoral radiographic examinations. The Journal of the American Dental Association. 1974;88(3):568–579. doi: 10.14219/jada.archive.1974.0128. DOI: http://dx.doi.org/10.14219/jada.archive.2004.0322. [DOI] [PubMed] [Google Scholar]
- American Thyroid Association. Policy Statement on Thyroid Shielding During Diagnostic Medical and Dental Radiology [online] 2013. [Accessed 16 March 2016]; Available at: http://www.thyroid.org/wp-content/uploads/statements/ABS1223_policy_statement.pdf.
- Angelopoulos C, Bedard A, Katz JO, Karamanis S, Parissis N. Digital panoramic radiography: An overview. Paper presented at: Seminars in Orthodontics. 2004 [Google Scholar]
- Antoku S, Kihara T, Russell WJ, Beach DR. Doses to critical organs from dental radiography. Oral Surgery, Oral Medicine, Oral Pathology. 1976;41(2):251–260. doi: 10.1016/0030-4220(76)90237-1. DOI: http://dx.doi.org/10.1016/0030-4220(89)90369-1. [DOI] [PubMed] [Google Scholar]
- Aroua A, Buchillier-Decka I, Dula K, et al. Radiation exposure in dental radiology: a 1998 nationwide survey in Switzerland. Dentomaxillofacial Radiology. 2014;33:211–219. doi: 10.1259/dmfr/26126766. DOI: http://dx.doi.org/10.1259/dmfr/26126766. [DOI] [PubMed] [Google Scholar]
- Avendanio B, Frederiksen NL, Benson BW, Sokolowski TW. Effective dose and risk assessment from detailed narrow beam radiography. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1996;82(6):713–719. doi: 10.1016/s1079-2104(96)80448-3. DOI: http://dx.doi.org/10.1016/S1079-2104(96)80448-3. [DOI] [PubMed] [Google Scholar]
- Bahadori A, Miglioretti D, Kruger R, et al. Calculation of Organ Doses for a Large Number of Patients Undergoing CT Examinations. American Journal of Roentgenology. 2015;205(4):827–833. doi: 10.2214/AJR.14.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baily NA. Patient exposure to ionizing radiation in dental radiography. Radiology. 1957;69(1):42–45. doi: 10.1148/69.1.42. [DOI] [PubMed] [Google Scholar]
- Bankvall G, Owman T. Patient radiation doses in upper GI examinations: A comparison between conventional and double-contrast techniques. Gastrointestinal radiology. 1982;7(1):231–234. doi: 10.1007/BF01887644. [DOI] [PubMed] [Google Scholar]
- Bengtsson G, Blomgren P-G, Bergman K, Åberg L. Patient exposures and radiation risks in Swedish diagnostic radiology. Acta radiologica: oncology, radiation, physics, biology. 1978;17(2):81–105. doi: 10.3109/02841867809127910. DOI: http://dx.doi.org/10.3109/02841867809127910. [DOI] [PubMed] [Google Scholar]
- Block AJ, Goepp RA, Mason EW. Thyroid radiation dose during panoramic and cephalometric dental x-ray examinations. The Angle orthodontist. 1977;47(1):17–24. doi: 10.1043/0003-3219(1977)047<0017:TRDDPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bohay RN, Kogon SL, Stephens RG. A survey of radiographic techniques and equipment used by a sample of general dental practitioners. Oral Surgery, Oral Medicine, Oral Pathology. 1994;78(6):806–810. doi: 10.1016/0030-4220(94)90100-7. DOI: http://dx.doi.org/10.1016/S0300-5712(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Bontrager KL. Textbook of radiographic positioning and related anatomy. 3. St. Louis: Mosby-Year Book Inc.; 1993. [Google Scholar]
- Bontrager KL. Textbook of radiographic positioning and related anatomy. 4. St. Louis: Mosby-Year Book Inc.; 1997. [Google Scholar]
- Bontrager KL. Textbook of radiographic positioning and related anatomy. 5. St. Louis: Mobys-Year Book Inc.; 2001. [Google Scholar]
- Bristow RG, Wood RE, Clark GM. Thyroid dose distribution in dental radiography. Oral surgery, oral medicine, oral pathology. 1989;68(4):482–487. doi: 10.1016/0030-4220(89)90150-3. [DOI] [PubMed] [Google Scholar]
- Brand JW, Kuba RK, Aeppli DM, Johnson JC. Radiation dosimetry in specific area radiography. Oral Surgery, Oral Medicine, Oral Pathology. 1989;67(3):347–353. doi: 10.1016/0030-4220(89)90368-x. [DOI] [PubMed] [Google Scholar]
- Budowsky J, Piro JD, Zegarelli EV, Kutscher AH, Barnett A. Radiation exposure to the head and abdomen during oral roentgenography. The Journal of the American Dental Association. 1956;52(5):555–559. doi: 10.14219/jada.archive.1956.0086. DOI: http://dx.doi.org/10.14219/jada.archive.1956.0086. [DOI] [PubMed] [Google Scholar]
- Button TM, Moore WC, Goren AD. Causes of excessive bitewing exposure: results of a survey regarding radiographic equipment in New York. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1999;87(4):513–517. doi: 10.1016/S1079-2104(99)70254-4. [DOI] [PubMed] [Google Scholar]
- Cederberg RA, Frederiksen NL, Benson BW, Sokolowski TW. Effect of the geometry of the intraoral position-indicating device on effective dose. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1997;84(1):101–109. doi: 10.1016/s1079-2104(97)90304-8. [DOI] [PubMed] [Google Scholar]
- Chetlen AL, Brown KL, King SH, et al. JOURNAL CLUB: Scatter Radiation Dose From Digital Screening Mammography Measured in a Representative Patient Population. American Journal of Roentgenology. 2016;206(2):359–365. doi: 10.2214/AJR.15.14921. [DOI] [PubMed] [Google Scholar]
- Clark KC. Positioning in radiography. 1. London: William Heinemann Medical Books, Ltd.; 1939. [Google Scholar]
- Clark KC. Positioning in radiography. 5. London: William Heinemann Medical Books, Ltda.; 1949. (revised) ed. [Google Scholar]
- Clark KC. Positioning in radiography. 7. London: William Heinemann Medical Books, Ltda.; 1956. (revised) ed. [Google Scholar]
- Clark KC. Positioning in radiography. 8. London: William Heinemann Medical Books, Ltda; 1967. (revised) ed. [Google Scholar]
- Clark KC, James . Positioning in radiography. 9. London: William Heineman Medical Books, Ltda; 1973. [Google Scholar]
- Clark KC, James . Positioning in radiography. 11. London: William Heineman Medical Books, Ltda; 1986. [Google Scholar]
- Cohnen M, Kemper J, Möbes O, Pawelzik J, Mödder U. Radiation dose in dental radiology. European radiology. 2002;12(3):634–637. doi: 10.1007/s003300100928. [DOI] [PubMed] [Google Scholar]
- Collett WK. Diagnostic radiation exposures and doses in dentistry: II. The Journal of the American Dental Association. 1968;77(5):1104–1108. doi: 10.14219/jada.archive.1968.0343. DOI: http://dx.doi.org/10.14219/jada.archive.1968.0343. [DOI] [PubMed] [Google Scholar]
- Conference of Radiation Control Program Directors and United States Food and Drug Administration. Nationawide Evaluation of X-Ray Trends (NEXT): 1996 Upper G.I. Fluoroscopy Survey; 1996. [Google Scholar]
- Conference of Radiation Control Programs Director, Inc. and United States Food and Drug Administration. Nationawide Evaluation of X-Ray Trends (NEXT): 2003 Upper G.I. Fluoroscopy Survey; 2003. [Google Scholar]
- Conway BJ, McCrohan JL, Antonsen RG, Rueter FG, Slayton R, Suleiman OH. Average radiation dose in standard CT examinations of the head: results of the 1990 NEXT survey. Radiology. 1992;184(1):135–140. doi: 10.1148/radiology.184.1.1609069. [DOI] [PubMed] [Google Scholar]
- Crawley MT, Savage P, Oakley F. Patient and operator dose during fluoroscopic examination of swallow mechanism. The British Journal of Radiology. 2004;77(920):654–656. doi: 10.1259/bjr/22832251. DOI: http://dx.doi.org/10.1259/bjr/22832251. [DOI] [PubMed] [Google Scholar]
- Danforth RA, Clark DE. Effective dose from radiation absorbed during a panoramic examination with a new generation machine. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2000;89(2):236–243. doi: 10.1067/moe.2000.103526. [DOI] [PubMed] [Google Scholar]
- Dauer LT, Branets I, Stabulas-Savage J, et al. Optimising radiographic bitewing examination to adult and juvenile patients through the use of anthropomorphic phantoms. Radiation protection dosimetry. 2014;158(1):51–58. doi: 10.1093/rpd/nct196. [DOI] [PubMed] [Google Scholar]
- Davies L, Welch HG. Current Thyroid Cancer Trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- Drozdovitch V, Brill AB, Callahan RJ, Clanton JA, DePietro A, Goldsmith SJ, Greenspan BS, Gross MD, Hays MT, Moore SC, Ponto JA, Shreeve WW, Melo DR, Linet MS, Simon SL. Use of Radiopharmaceuticals in Diagnostic Nuclear Medicine in the United States: 1960–2010. Health Physics. 2015;108(5):520–537. doi: 10.1097/HP.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dula K, Sanderink G, van der Stelt PF, Mini R, Buser D. Effects of dose reduction on the detectability of standardized radiolucent lesions in digital panoramic radiography. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1998;86(2):227–233. doi: 10.1016/S1079-2104(98)90130-5. [DOI] [PubMed] [Google Scholar]
- Endo A, Katoh T, Kobayashi I, Joshi R, Sur J, Okano T. Characterization of optically stimulated luminescence dosemeters to measure organ doses in diagnostic radiology. Dentomaxillofacial Radiology. 2014;41(3):211–216. doi: 10.1259/dmfr/98708146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekestubbe A, Thilander-Klang A, Lith A, Gröndahl H-G. Effective and organ doses from scanography and zonography: a comparison with periapical radiography. Dentomaxillofacial Radiology. 2004;33(2):87–92. doi: 10.1259/dmfr/24877187. [DOI] [PubMed] [Google Scholar]
- Farman TT, Farman AG. Evaluation of a new F speed dental X-Ray film. The effect of processing solutions and a comparison with D and E speed films. Dentomaxillofacial Radiology. 2000;29:41–45. doi: 10.1038/sj/dmfr/4600499. [DOI] [PubMed] [Google Scholar]
- Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ, Nallamothu BK. Exposure to low-dose ionizing radiation from medical imaging procedures. The New England Journal of Medicine. 2009;361(9):849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Vaccarella S. Thyroid Cancer: an epidemic of disesase or an epidemic of diagnosis? Int. J Cancer. 2015;136(11):2738–2739. doi: 10.1002/ijc.29311. [DOI] [PubMed] [Google Scholar]
- Freeman JP, Brand JW. Radiation doses of commonly used dental radiographic surveys. Oral surgery, oral medicine, oral pathology. 1994;77(3):285–289. doi: 10.1016/0030-4220(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Frey NW, Wuehrmann AH. Radiation dosimetry and intraoral radiographic techniques: II. Internal and external dose measurements. Oral Surgery, Oral Medicine, Oral Pathology. 1974;38(4):639–652. doi: 10.1016/0030-4220(74)90098-x. DOI: http://dx.doi.org/10.1016/0030-4220(93)90022-V. [DOI] [PubMed] [Google Scholar]
- Gavala S, Donta C, Tsiklakis K, Boziari A, Kamenopoulou V, Stamatakis HC. Radiation dose reduction in direct digital panoramic radiography. European Journal of Radiology. 2009;71(1):42–48. doi: 10.1016/j.ejrad.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Geist JR, Katz JO. Radiation dose-reduction techniques in North American dental schools. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2002;93(4):496–505. doi: 10.1067/moe.2002.121387. [DOI] [PubMed] [Google Scholar]
- Gijbels F, Jacobs R, Sanderink G, et al. A comparison of the effective dose from scanography with periapical radiography. Dentomaxillofacial Radiology. 2002;31(3):159–163. doi: 10.1038/sj/dmfr/4600683. [DOI] [PubMed] [Google Scholar]
- Gijbels F, Sanderink G, Bou Serhal C, Pauwels H, Jacobs R. Organ doses and subjective image quality of indirect digital panoramic radiography. Dentomaxillofacial Radiology. 2001;30(6):308–313. doi: 10.1038/sj/dmfr/4600640. [DOI] [PubMed] [Google Scholar]
- Gilda JE, Maillie HD. Dosimetry of absorbed radiation in radiographic cephalometry. Oral Surgery, Oral Medicine, Oral Pathology. 1992;73(5):638–643. doi: 10.1016/0030-4220(92)90113-5. [DOI] [PubMed] [Google Scholar]
- Gold RH, Bassett LW, Widoff BE. Highlights from the history of mammography. Radiographics. 1990;10(6):1111–1131. doi: 10.1148/radiographics.10.6.2259767. [DOI] [PubMed] [Google Scholar]
- Greer DF. Determination and analysis of absorbed doses resulting from various intraoral radiographic techniques. Oral Surgery, Oral Medicine, Oral Pathology. 1972;34(1):146–162. doi: 10.1016/0030-4220(72)90283-6. [DOI] [PubMed] [Google Scholar]
- Gudjonsdottir J, Svensson JR, Campling S, Brennan PC, Jonsdottir Efficient Use of Automatic Exposure Control Systems in Computed Tompgraphy Requires Correct Patient Positioning. Acta Radiol. 2009;50(9):1035–1041. doi: 10.3109/02841850903147053. [DOI] [PubMed] [Google Scholar]
- Hallikainen D. History of panoramic radiography. Acta radiologica. 1996;37(3P2):441–445. doi: 10.1177/02841851960373P207. [DOI] [PubMed] [Google Scholar]
- Han G, Cheng J, Li G, Ma X. Shielding effect of thyroid collar for digital panoramic radiography. Dentomaxillofacial Radiology. 2013;42(9) doi: 10.1259/dmfr.20130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Fujimori H, Kuroyanagi K. Absorbed doses with intraoral radiography: Function of various technical parameters. Oral Surgery, Oral Medicine, Oral Pathology. 1993;76(4):519–524. doi: 10.1016/0030-4220(93)90022-v. [DOI] [PubMed] [Google Scholar]
- Horner K, Hirschmann P. Dose reduction in dental radiography. Journal of Dentistry. 1990;18(4):171–184. doi: 10.1016/0300-5712(90)90106-o. [DOI] [PubMed] [Google Scholar]
- Iannucci JM, Howerton LJ. Dental Radiography Principles and Techniques. 5. St. Louis. Missouri: Elsevier/Saunders; 2016. [Google Scholar]
- Hudson D, Kumpula J. Ionization chambers for radiation data during dental x-ray exposure. United States Armed Forces Medical Journal. 1955;6(8):1131. [PubMed] [Google Scholar]
- Hujoel P, Hollender L, Bollen A-M, et al. Thyroid shields and neck exposures in cephalometric radiography. BMC Medical Imaging. 2006;6:6. doi: 10.1186/1471-2342-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hujoel P, Hollender L, Bollen A-M, Young JD, McGee M, Grosso A. Head-and-neck organ doses from an episode of orthodontic care. American Journal of Orthodontics and Dentofacial Orthopedics. 2008;133(2):210–217. doi: 10.1016/j.ajodo.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Ice R, Updegrave W, Bogucki E. Influence of dental radiographic cones on radiation exposure. The Journal of the American Dental Association. 1971;83(6):1297–1302. doi: 10.14219/jada.archive.1971.0468. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiation Units and Measurements. Tissue Substitutes in Radiation Dosimetry and Measurement (Report 44) 1989 [Google Scholar]
- International Commission on Radiation Units and Measurements. Managing Patient Dose in Digital Radiology (Report 93) 2004 [Google Scholar]
- Jadu F, Yaffe M, Lam E. A comparative study of the effective radiation doses from cone beam computed tomography and plain radiography for sialography. Dentomaxillofacial Radiology. 2014;39(5):257–263. doi: 10.1259/dmfr/62878962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman AC, Kinsley EL, Morris CR. Absorbed radiation from panoramic plus bitewing exposures vs full-mouth periapical plus bitewing exposures. The Journal of the American Dental Association. 1973;86(2):420–423. doi: 10.14219/jada.archive.1973.0058. [DOI] [PubMed] [Google Scholar]
- Jung T. Gonadal doses resulting from panoramic x-ray examinations of the teeth. Oral Surgery, Oral Medicine, Oral Pathology. 1965;19(6):745–753. doi: 10.1016/0030-4220(65)90343-9. [DOI] [PubMed] [Google Scholar]
- Kanal KM, Butler PF, Sengupta D, Bhargavan-Chatfield M, Coombs LP, Morin RL. U.S. Diagnostic Reference Levels and Acheivable Doses for 10 Adult CT Examinations. Radiology. 2017 doi: 10.1148/radio.2017161911. [DOI] [PubMed] [Google Scholar]
- Kircos LT, Angin LL, Lorton L. Order of magnitude dose reduction in intraoral radiography. Journal of the American Dental Association. 1987;144(3):344–347. doi: 10.14219/jada.archive.1987.0085. DOI: http://dx.doi.org/10.14219/jada.archive.1987.0085. [DOI] [PubMed] [Google Scholar]
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. doi: 10.1038/nrendo.2016.110. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kite OW, Swanson LT, Levin S, Bradbury E. Radiation and image distortion in the panorex x-ray unit. Oral Surgery, Oral Medicine, Oral Pathology. 1962;15(10):1201–1210. doi: 10.1016/0030-4220(62)90155-x. [DOI] [PubMed] [Google Scholar]
- Kuba RK, Beck JO. Radiation dosimetry in Panorex roentgenography: Part III. Radiation dose measurements. Oral Surgery, Oral Medicine, Oral Pathology. 1968;25(3):393–404. doi: 10.1016/0030-4220(68)90014-5. [DOI] [PubMed] [Google Scholar]
- Kumpula JW. Present status of panoramic roentgenography. The Journal of the American Dental Association. 1961;63(2):194–200. doi: 10.14219/jada.archive.1961.0180. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertucci P, Levi F, Negri E. Thyroid cancer mortality and incidence: A global overview. Int J Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- Lambrecht JT, Roth J, Kiefer H. Dose exposition from intra-and extraoral dental radiography. International Congress Series. 2004;1268:1147–1151. [Google Scholar]
- Lecomber A, Yoneyama Y, Lovelock D, Hosoi T, Adams A. Comparison of patient dose from imaging protocols for dental implant planning using conventional radiography and computed tomography. Dentomaxillofacial Radiology. 2001;30(5):255–259. doi: 10.1038/sj/dmfr/4600627. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim KP, Bolch WE, Moroz BE, Folio L. NCICT: a computational solution to estimate organ doses for pediatric and adult patients undergoing CT scans. Journal of Radiological Protection. 2015;35(4):891–909. doi: 10.1088/0952-4746/35/4/891. [DOI] [PubMed] [Google Scholar]
- Lee C, Lodwick D, Hurtado J, Pafundi D, Williams JL, Bolch WE. The UF family of reference hybrid phantoms for computational radiation dosimetry. Physics in Medicine and Biology. 2009;55(2):339–363. doi: 10.1088/0031-9155/55/2/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. Comparative radiation doses in dental radiography. Oral Surgery, Oral Medicine, Oral Pathology. 1974;37(6):962–968. doi: 10.1016/0030-4220(74)90449-6. [DOI] [PubMed] [Google Scholar]
- Linet MS, Slovis TL, Miller DL, Kleinerman R, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012;62(2):75–100. doi: 10.3322/caac.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow JB, Davies-Ludlow LE, White SC. Patient risk related to common dental radiographic examinations: the impact of 2007 International Commission on Radiological Protection recommendations regarding dose calculation. The Journal of the American Dental Association. 2008;139(9):1237–1243. doi: 10.14219/jada.archive.2008.0339. [DOI] [PubMed] [Google Scholar]
- Mah JK, Danforth RA, Bumann A, Hatcher D. Radiation absorbed in maxillofacial imaging with a new dental computed tomography device. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2003;96(4):508–513. doi: 10.1016/S1079210403003500. [DOI] [PubMed] [Google Scholar]
- McCrohan JL, Patterson JF, Gagne R, Goldstein H. Average radiation doses in a standard head examination for 250 CT systems. Radiology. 1987;163(1):263–268. doi: 10.1148/radiology.163.1.3823446. [DOI] [PubMed] [Google Scholar]
- McCullough EC, Payne JT. Patient Dosage in Computed Tomography. Radiology. 1978;129(2):457–463. doi: 10.1148/129.2.457. [DOI] [PubMed] [Google Scholar]
- McCullough EC, Payne JT, Baker HL, Jr, Hattery RR, Sheedy PF, Stephens DH, Gedgaudus E. Performance Evaluation and Quality Assurance of Computed Tomography Scanners, with Illustrations from the EMI, ACTA, and Delta Scanners. Radiology. 1976;120(1):173–188. doi: 10.1148/120.1.173. [DOI] [PubMed] [Google Scholar]
- McNitt-Gray MF. AAPM/RSNA Physics Tutorial for Residents: Topics in CT: Radiation Dose in CT. Radiographics. 2002;22(6):1541–1553. doi: 10.1148/rg.226025128. [DOI] [PubMed] [Google Scholar]
- Melo DR, Miller DL, Chang L, Moroz B, Linet MS, Simon SL. Organ Doses From Diagnostic Medical Radiography—Trends Over Eight Decades (1930 to 2010) Health Physics. 2016;111(3):235–255. doi: 10.1097/HP.0000000000000524. [DOI] [PubMed] [Google Scholar]
- Miller DL, Balter S, Cole PE, Lu HT, Schueler BA, Geisinger M, Berenstein A, Albert R, Georgia JD, Noonan PT, St George J, Cardella JF, Russell E, Malisch T, Vogelzang RL, Miller GL, Anderson J. Radiation doses in interventional radiology procedures: The RAD-IR study: part I: overall measures of dose. Journal of Vascular and Interventional Radiology. 2003;14(6):711–727. doi: 10.1097/01.rvi.0000079980.80153.4b. [DOI] [PubMed] [Google Scholar]
- Mitchell LD., Jr Panoramic Roentgenography a Clinical Evaluation. The Journal of the American Dental Association. 1963;66(6):777–786. DOI: http://dx.doi.org/10.14219/jada.archive.1963.0193. [Google Scholar]
- Myers DR, Shoaf HK, Wege WR, Carlton WH, Gilbert MA. Radiation exposure during panoramic radiography in children. Oral Surgery, Oral Medicine, Oral Pathology. 1978;46(4):588–593. doi: 10.1016/0030-4220(78)90390-0. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. A Snapshot of Thyroid Cancer. [Accessed 16 March 2016];2014 < http://www.cancer.gov/research/progress/snapshots/thyroid>.
- National Council on Radiation Protection and Measurements (NCRP) Report No. 93, Ionizing Radiation Exposure of the Population of the United States. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 1987. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP) Report No. 149, A Guide to Mammography and Other Breast Imaging Procedures. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2004. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP) Report No. 159, Risk to the Thyroid from Ionizing Radiation. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2008. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP) Report No. 160, Ionizing radiation exposure of the population of the United States. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2009. [Google Scholar]
- National Institute of Standards and Technolgy. X-ray mass attenuation coefficient database. [Accessed 16 March 2016];Version 1.4. 2004 < https://www.nist.gov/pml/x-ray-mass-attenuation-coefficients>.
- Nilsson L, Rohlin M, Thapper K. Exposure distribution, absorbed doses, and energy imparted for panoramic radiography using Orthopantomograph model OP 5. Oral Surgery, Oral Medicine, Oral Pathology. 1985;59(2):212–219. doi: 10.1016/0030-4220(85)90021-0. [DOI] [PubMed] [Google Scholar]
- Nolan WE. Radiation hazards to the patient from oral roentgenography. The Journal of the American Dental Association. 1953;47(6):681–684. doi: 10.14219/jada.archive.1953.0228. [DOI] [PubMed] [Google Scholar]
- Paatero YV. Pantomography and orthopantomography. Oral Surgery, Oral Medicine, Oral Pathology. 1961;14(8):947–953. doi: 10.1016/0030-4220(61)90009-3. [DOI] [PubMed] [Google Scholar]
- Parry RA, Glaze SA, Archer BR. The AAPM/RSNA Physics Tutorial for Residents: Typical Patient Radiation Doses in Diagonostic Radiology. Radio Graphics. 1999;190:1289-–1302. doi: 10.1148/radiographics.19.5.g99se211289. [DOI] [PubMed] [Google Scholar]
- Payne JT. CT Radiation Dose and Image Quality. Radiologic Clinics of North America. 2005;43(6):953–962. doi: 10.1016/j.rcl.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Kato H, Yoshimoto K, Mason M. Radiation exposure and thyroid cancer incidence among Hiroshima and Nagasaki residents. National Cancer Institute Monograph. 1982;62:207–212. [PubMed] [Google Scholar]
- Preston-Martin S, White SC. Brain and salivary gland tumors related to prior dental radiography: implications for current practice. The Journal of the American Dental Association. 1990;120(2):151–158. doi: 10.14219/jada.archive.1990.0026. [DOI] [PubMed] [Google Scholar]
- Public Health Service. Population Dose From X-Rays U.S. 1969. U.S. Department of Health, Education and Welfare; p. 1964. [Google Scholar]
- Quinn B, Dauer Z, Pandit-Taskar N, Schoder H, Dauer LT. Radiation Dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med Imaging. 2016;16(1):41. doi: 10.1186/s12880-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G, Padmanabhan V. Radiation doses to patients from X-ray examinations involving fluoroscopy. Indian Journal of Radiology and Imaging. 2001;11(4):181–184. [Google Scholar]
- Richards AG. How hazardous is dental roentgenography? Oral Surgery, Oral Medicine, Oral Pathology. 1961;14(1):40–51. doi: 10.1016/0030-4220(61)90471-6. [DOI] [PubMed] [Google Scholar]
- Richards A, Webber R. Dental x-ray exposure of sites within the head and neck. Oral Surgery, Oral Medicine, Oral Pathology. 1964;18(6):752–756. doi: 10.1016/0030-4220(64)90474-8. [DOI] [PubMed] [Google Scholar]
- Richards AG, Colquitt WN. Reduction in dental x-ray exposures during the past 60 years. The Journal of the American Dental Association. 1981;103(5):713–718. doi: 10.14219/jada.archive.1981.0378. [DOI] [PubMed] [Google Scholar]
- Ron E. Cancer risks from medical radiation. Health Physics. 2003;85(1):47–59. doi: 10.1097/00004032-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD., Jr Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiation Research. 1995;141(3):259–277. [PubMed] [Google Scholar]
- Ron E, Modan B. Benign and malignant thyroid neoplasms after childhood irradiation for tinea capitis. Journal of the National Cancer Institute. 1980;65(1):7–11. [PubMed] [Google Scholar]
- Ron E, Kleinerman RA, Boice JD, Jr, LiVolsi VA, Flannery JT, Fraumeni JF., Jr A population-based case-control study of thyroid cancer. Journal of the National Cancer Institute. 1987;79(1):1–12. [PubMed] [Google Scholar]
- Rush E, Thompson N. Dental radiography technique and equipment: How they influence the radiation dose received at the level of the thyroid gland. Radiography. 2007;13(3):214–220. DOI: http://dx.doi.org/10.1016/j.radi.2006.03.002. [Google Scholar]
- Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, Anderson RN, Ma J, Ly KN, Cronin KA, Penberthy L, Kohler BA. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1327. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansare K, Khanna V, Karjodkar F. Utility of thyroid collars in cephalometric radiography. Dentomaxillofacial Radiology. 2014;40(8):471–475. doi: 10.1259/dmfr/25040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman T. Si, CdTe, CdZnTe radiation detectors for imaging applications (docorate dissertation at the University of Helsinki) Helsinki; 2006. [Google Scholar]
- Schulze D, Heiland M, Thurmann H, Adam G. Radiation exposure during midfacial imaging using 4- and 16-slice computed tomography, cone beam computed tomography systems and conventional radiography. Dentomaxillofacial Radiology. 2004;33:83–86. doi: 10.1259/dmfr/28403350. DOI: http://dx.doi.org/10.1259/dmfr/28403350. [DOI] [PubMed] [Google Scholar]
- Sechopoulos I, Hendrick RE. Mammography and the risk of thyroid cancer. AJR Am J Roentgenol. 2012;198(3):705–708. doi: 10.2214/AJR.11.7225. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Koshida K, Miyati T. Monte Carlo simulation analysis of backscatter factor for low-energy x-ray. KEK Proceedings. 2001:115–118. [Google Scholar]
- Shope TB, Morgan TJ, Showalter CK, Pentlow KS, Rothenberg LN, White DR, Speller RD. Radiation dosimetry survey of computed tomography systems from ten manufacturers. The British Journal of Radiology. 1982;55(649):60–69. doi: 10.1259/0007-1285-55-649-60. [DOI] [PubMed] [Google Scholar]
- Shultz AG, Rollo FD. A Method For Measuring Radioiodine Uptake Which Corrects for Thyroid Depth. J Nucl Med. 1970;11(8):508–513. [PubMed] [Google Scholar]
- Simon SL, Preston DL, Linet MS, Miller JS, Sigurdson AJ, Alexander BH, Kwon D, Yoder RC, Bhatti P, Little MP, Rajaraman P, Melo D, Drozdovitch V, Weinstock RM, Doody MM. Radiation organ doses received in a nationwide cohort of U.S. radiologic technologists: methods and findings. Radiation Research. 2014;182(5):507–528. doi: 10.1667/RR13542.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL. Organ-Specific External Dose Coefficients and Protective Apron Transmission Factors for Historical Dose Reconstruction for Medical Personnel. Health Physics. 2011;101(1):13–27. doi: 10.1097/HP.0b013e318204a60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott B, Ron E, Schneider AB. Exposing the Thyroid to Radiation: A Review of Its Current Extent, Risks, and Implications. Endocr Rev. 2010;31(5):756–773. doi: 10.1210/er.2010-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg M, Gunnarsson M. Automatic exposure control in computed tomography – an evaluation of systems from different manufacturers. Acta Radiol. 2010;51(6):625–634. doi: 10.3109/02841851003698206. [DOI] [PubMed] [Google Scholar]
- Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D, Solberg LI, Vanneman N, Weinmann S, Williams AE. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307(22):2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SH. Nationwide Evaluation of X-ray Trends (NEXT) tabulation and graphical summary of 2000. Survey of Computed Tomography. 2007 [Google Scholar]
- Suleiman OH, Anderson J, Jones B, Rao GU, Rosenstein M. Tissue doses in the upper gastrointestinal fluoroscopy examination. Radiology. 1991;178(3):653–658. doi: 10.1148/radiology.178.3.1994397. [DOI] [PubMed] [Google Scholar]
- Tapiovaara M, Lakkisto M, Servomaa A. PCXMC - A PC-based Monte Carlo program for calculating patient doses in medical X-ray examinations. 2. Helsinki, Finland: Radiation and Nuclear Safety Authority (STUK); 2008. [Google Scholar]
- Thierry-Chef I, Simon SL, Weinstock RM, Kwon D, Linet MS. Reconstruction of absorbed doses to fibroglandular tissue of the breast of women undergoing mammography (1960 to the present) Radiation Research. 2012;177(1):92–108. doi: 10.1667/rr2241.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierris CE, Yakoumakis EN, Bramis GN, Georgiou E. Dose area product reference levels in dental panoramic radiology. Radiation Protection Dosimetry. 2004;111(3):283–287. doi: 10.1093/rpd/nch341. [DOI] [PubMed] [Google Scholar]
- Toossi MTB, Akbari F, Roodi SB. Radiation exposure to critical organs in panoramic dental examination. Acta Med Iran. 2012;50(12):809. [PubMed] [Google Scholar]
- Underhill TE, Chilvarguer I, Kimura K, Langlais RP, McDavid WD, Preece JW, Barnwell G. Radiobiologic risk estimation from dental radiology. Part I. Absorbed doses to critical organs. Oral Surgery, Oral Medicine, Oral Pathology. 1988;66(1):111–120. doi: 10.1016/0030-4220(88)90077-1. [DOI] [PubMed] [Google Scholar]
- Updegrave W. The role of panoramic radiography in diagnosis. Oral Surgery, Oral Medicine, Oral Pathology. 1966;22(1):49–57. doi: 10.1016/0030-4220(66)90141-1. [DOI] [PubMed] [Google Scholar]
- Vaccarella S, Franceschi S, Bray F, Wild CP, Plujmmer M, Dal Maso L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. New England Journal of Medicine. 2016;375(7):614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- Velders X, Van Aken J, Van der Stelt P. Absorbed dose to organs in the head and neck from bitewing radiography. Dentomaxillofacial Radiology. 1991;20(3):161–165. doi: 10.1259/dmfr.20.3.1808001. [DOI] [PubMed] [Google Scholar]
- Velders X, Van Aken J, Van der Stelt P. Risk assessment from bitewing radiography. Dentomaxillofacial Radiology. 1991;20(4):209–213. doi: 10.1259/dmfr.20.4.1808009. [DOI] [PubMed] [Google Scholar]
- Villoing D, Drozdovitch V, Simon SL, Kitahara CM, Linet MS, Melo DR. Retrospective Estimated Organ Doses to Nuclear Medicine Patients Over Five Deaces: 1960 – 2010. 2017 doi: 10.1097/HP.0000000000000721. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright W. Filtration for lowest patient dose in dental radiography. Oral Surgery, Oral Medicine, Oral Pathology. 1963;16:561–571. doi: 10.1016/0030-4220(63)90145-2. [DOI] [PubMed] [Google Scholar]
- Webster GW. The Physiological Measurement Handbook. Boca Raton: CRC Press, Taylor & Francis Group; 2014. [Google Scholar]
- Weissman DD, Longhurst GE. Comparative absorbed doses in periapical radiography. II. Panorex. Oral Surgery, Oral Medicine, Oral Pathology. 1972;33(4):661–668. doi: 10.1016/0030-4220(72)90376-3. [DOI] [PubMed] [Google Scholar]
- Weissman DD, Sobkowski FJ. Comparative thermoluminescent dosimetry of intraoral periapical radiography. Oral Surgery, Oral Medicine, Oral Pathology. 1970;29(3):376–386. doi: 10.1016/0030-4220(70)90137-4. [DOI] [PubMed] [Google Scholar]
- Wesche MFT, Wiersinga WM, Smits NJ. Lean body mass as a determinent of thyroid size. Clinical Endocrinology. 1998;48:701–706. doi: 10.1046/j.1365-2265.1998.00400.x. [DOI] [PubMed] [Google Scholar]
- Whelan C, McLean D, Poulos A. Investigation of thyroid dose due to mammography. Australasian Radiology. 1999;43(3):307–310. doi: 10.1046/j.1440-1673.1999.433660.x. [DOI] [PubMed] [Google Scholar]
- Whitcher BL, Gratt BM, Sickles EA. Leaded shields for thyroid dose reduction in intraoral dental radiography. Oral Surgery, Oral Medicine, Oral Pathology. 1979;48(6):567–570. doi: 10.1016/0030-4220(79)90306-2. [DOI] [PubMed] [Google Scholar]
- White S, Mallya S. Update on the biological effects of ionizing radiation, relative dose factors and radiation hygiene. Australian Dental Journal. 2012;57(S1):2–8. doi: 10.1111/j.1834-7819.2011.01665.x. [DOI] [PubMed] [Google Scholar]
- White S. 1992 assessment of radiation risk from dental radiography. Dentomaxillofacial Radiology. 1992;21(3):118–126. doi: 10.1259/dmfr.21.3.1397466. [DOI] [PubMed] [Google Scholar]
- Winkler KG. Influence of rectangular collimation and intraoral shielding on radiation dose in dental radiography. The Journal of the American Dental Association. 1968;77(1):95–101. doi: 10.14219/jada.archive.1968.0228. [DOI] [PubMed] [Google Scholar]
- Wøhni T. Phantom measurements of absorbed doses in dental radiography. Acta Radiologica: Therapy, Physics, Biology. 1977;16(2):194–198. doi: 10.3109/02841867709134312. [DOI] [PubMed] [Google Scholar]
- Young KC, Burch A. Radiation doses received in the UK Breast Screening Programme in 1997 and 1998. British Journal of Radiology. 2000;73:278–287. doi: 10.1259/bjr.73.867.10817044. [DOI] [PubMed] [Google Scholar]