Abstract
Background
We evaluated circulating levels of immunosuppressive regulatory T cells (Tregs) and other lymphocyte subsets in patients with newly diagnosed medulloblastoma (MBL) undergoing surgery compared to a control cohort of patients undergo craniectomy for correction of Chiari malformation (CM) and further determined the impact of standard irradiation and chemotherapy on this cell population.
Methods
Eligibility criteria for this biologic study included age 4–21 years, patients with CM undergoing craniectomy (as non-malignant surgical controls) and receiving dexamethasone for prevention of post-operative nausea, and those with newly diagnosed posterior fossa tumors (PFT) undergoing surgical resection and receiving dexamethasone as an anti-edema measure. Patients with confirmed MBL were also followed for longitudinal blood collection and analysis during radiotherapy and chemotherapy.
Results
A total of 54 subjects were enrolled on the study [22-CM, 18-MBL, and 14-PFT]. Absolute number and percentage Tregs (defined as CD4+CD25+FoxP3+CD127low/−) at baseline were decreased in MBL and PFT compared to CM [p = 0.0016 and 0.001, respectively). Patients with MBL and PFT had significantly reduced overall CD4+ T cell count (p = 0.0014 and 0.0054, respectively) compared to those with CM. Radiation and chemotherapy treatment in patients with MBL reduced overall lymphocyte counts; however, within the CD4+ T cell compartment, Tregs increased during treatment but gradually declined post therapy.
Conclusions
Our results demonstrate that patients with MBL and PFT exhibit overall reduced CD4+ T cell counts at diagnosis but not an elevated proportion of Tregs. Standard treatment exacerbates lymphopenia in those with MBL while enriching for immunosuppressive Tregs over time.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2051-6) contains supplementary material, which is available to authorized users.
Keywords: Medulloblastoma, Immunotherapy, Regulatory T cells, CD4, Posterior fossa tumors
Introduction
Medulloblastoma (MBL) is the most common malignant brain tumor in children [2]. While significant advances have been made in treating children with newly diagnosed disease and survival is excellent with surgery followed by irradiation and chemotherapy [3], the outcome for patients with recurrent tumor is dismal [4]. The cytotoxic therapies for this tumor also result in severe late sequelae. Immunotherapy has the potential to target tumor cells with minimal side effects and is a novel approach to treating brain tumors [5]. However, malignant brain tumors often engage various pathways to evade immune recognition and produce immune suppression [6]. We and others have previously shown that T-regulatory cells (Tregs) in patients with GBM and other cancers induce a severe state of immunosuppression and are likely one of the factors responsible for immune evasion [7]. These cells are a subset of CD4+ T lymphocytes, express high levels of CD25 [IL-2α receptor (IL-2Rα)] and Foxp3 transcription factor, and have a unique dependence on IL-2R⍺ signaling for survival [7]. As immunotherapy is emerging as a potential strategy to treat MBL, we wanted to evaluate the immunologic profile (including Tregs) in newly diagnosed patients with MBL and other posterior fossa tumors (PFT) just prior to surgical resection as compared to a control population of Chiari malformation (CM) patients undergoing a similar intradural posterior fossa surgical procedure and also receiving perioperative steroids (in the case of CM for control of anesthesia-related nausea and vomiting). We also wanted to evaluate the effect of cytotoxic therapy (irradiation and chemotherapy) on Tregs and other lymphocyte subsets.
Methods
The primary objectives
The primary objectives of the study were (1) to evaluate the fraction of peripherally circulating Tregs in patients with MBL and those with PFT as compared to levels in a control group of patients undergoing craniectomy for CM and (2) the longitudinal effects of standard cytotoxic therapy on the overall population of lymphocytes and fraction of Tregs in MBL patients. The study was approved by the Institutional Review Boards of the participating institutions and informed consent was obtained from patients or parents prior to surgery by one of the designated study personnel.
Inclusion criteria
Inclusion criteria for this study included newly diagnosed patients (over 3 years of age) with posterior fossa tumors (who routinely receive dexamethasone for edema control) scheduled to undergo sub occipital craniotomy for tumor resection and patients with known CM scheduled to have a craniectomy and receive steroids peri-operatively to decrease risk of post-anesthesia nausea and emesis. Those with unexplained febrile illness, active infections, autoimmune, or immunosuppressive disorders were excluded.
Blood sample procurement and processing
Blood samples in patients with MBL were obtained pre- and post-operatively, prior to RT, post-RT (±1 week), prior to (or within 72 h) and 3 weeks (or ±72 h) after the first cycle of standard chemotherapy following RT. In patients with CM, blood samples were obtained pre-and post-operatively only. Those with PFT had a pre-operative blood sample only. Samples were processed immediately (within an hour) upon arrival in lab. Samples were divided into 100 μL aliquots and surface stained at room temperature for 15 min with the following antibodies: CD4-FITC (RPA-T4), CD127-PE (hIL-7R-M21), CD39-PE (TU66), CD49d-PE (9F10), HLA-DR-PE (L243), CD8-PE (RPA-T8), CD45RO-PE (UCHL-1), CD25-APC (M-A251), and msIgG-PE (MOPC-21). Samples were fixed for 15 min in the dark with 1 ml Fix/Permeabilization (eBioscience, 00-5523 kit). After addition of 3 ml PBS, samples were centrifuged at 600×g for 5 min at 10 °C and carefully decanted and 2 ml permeabilizing buffer (eBioscience, 00-5523 kit) was added. Tubes were centrifuged at 800×g for 5 min followed by careful decantation. Fox-p3-PerCPcy5.5 (eBioscience, PCH101), ratIgG2a-PerCPcy5.5 (R35-95), Ki67-PE (B56), and msIgG-PE were added to appropriate tubes, and incubated in the dark for 15 min. After washing twice with Permeabilization buffer, samples were re-suspended in 300 µL PBS containing 0.5% formalin and analyzed by flow cytometry on a BD FAC Calibur.
Statistical considerations
Evaluable patients were those who had obtained all samples as required by protocol. Based on the report from Fecci et al. on Tregs in GBM as compared to healthy controls [8], a total of 30 patients were to be enrolled in the MBL and CM groups, respectively, to detect a difference of >6% in Treg fraction between the two groups. Enrollment in the PFT group would continue until 30 patients were enrolled into the MBL group. Only baseline Tregs levels were analyzed in patients with PFT. Due to slow accrual in the MBL and CM cohort, a decision was made to close the study before planned enrollment was completed. Descriptive statistics were provided for all protocol variables. To compare the distribution of a given marker across multiple subgroups of patients, we used Kruskal–Wallis test, which is a non-parametric counterpart of one-way ANOVA. To investigate the significance of change in a given marker between two time points, we used Wilcoxon signed-rank test, which is a non-parametric counterpart of the paired t test. We modeled the change of a given marker overtime through random coefficient models to assess linear and quadratic change overtime; the models assumed different intercept and slope for each patient. The p values reported in the “Results” section were not adjusted for multiplicity.
Results
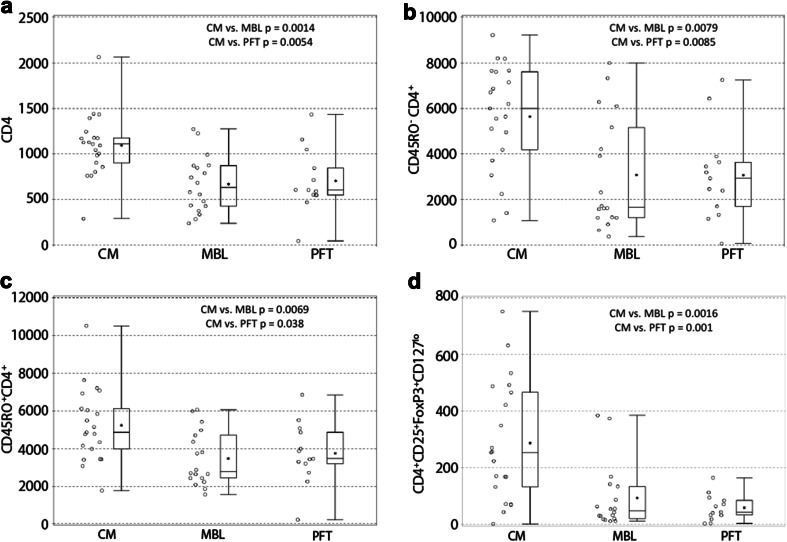
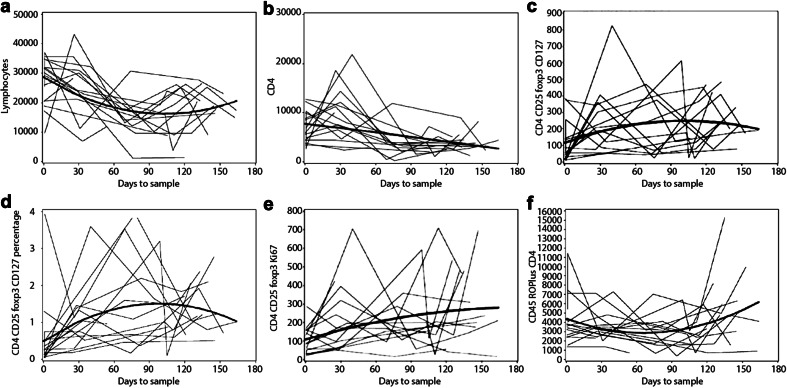
Between April 2010 and August 2014, 54 evaluable patients from nine participating centers were enrolled on the study (22-CM, 18-MBL, 14-PFT, Table 1). We could not obtain baseline samples from one patient each with CM and PFT, respectively. Hence, evaluation of samples at baseline was performed in 21, 18, and 13 patients in CM, MBL, and PFT cohorts, respectively. Absolute number (median 48 vs. 254) (p = 0.0016) and percentage (median 0.18 vs. 0.73%) (p = 0.0015) of baseline activated Tregs (CD4+CD25+FoxP3+CD127lo/−) were decreased in MBL as compared to CM (Table 2; Figs. 1 and 2d). Similarly, both absolute number (median 43 vs. 254) (p = 0.001) and percentage (median 0.17 vs. 0.73%) (p = 0.0015) of baseline activated Tregs (CD4+CD25+FoxP3+CD127lo/−) were decreased in PFT as compared to CM (Table 2; Fig. 2d). Those with MBL and PFT were also characterized by having a significantly reduced overall CD4+ T cell count compared to patients with CM (median 6347.5 and 6073 vs. 11,114) (p = 0.0014 and 0.0054 for MBL and PFT, respectively) (Table 2; Fig. 2a), which was also observed for the non-memory and memory T lymphocytes (CD45RO− and CD45RO+CD4+) (Table 2; Fig. 2b, c). However, the percentage of baseline non-memory CD8+ T lymphocytes (CD45RO−CD8+) but not absolute numbers were elevated in MBL and PFT as compared to CM (median 57.8 and 61.5 vs. 44.0%) (p < 0.0001). Since absolute numbers of these cells were not significantly different, this increased proportion of CD8+ T cells is likely explained by the lower CD4+ T cell count in these patients. Radiation and chemotherapy treatment reduced overall lymphocyte counts (Fig. 3a, b). In contrast, however, within the CD4+ T cell compartment, Tregs increased during therapy (Fig. 3c, d, p = 0.0628 and 0.0070, respectively). There was also a trend toward increased numbers of actively dividing Tregs during therapy (Fig. 3e). The absolute and percentage central memory CD8+ T cell population (CD45RO+CD8 lymphocytes) decreased during therapy but rebounded soon thereafter (Fig. 3f).
Table 1.
Demographics of 54 patients with either Chiari malformation or posterior fossa tumors
| Chiari malformation (N = 22) | Posterior fossa tumor: medulloblastoma (N = 18) | Posterior fossa tumor: non-medulloblastoma (N = 14) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Sex | ||||||
| Males | 7 | 31.8 | 13 | 72.2 | 7 | 50.0 |
| Females | 15 | 68.2 | 5 | 27.8 | 7 | 50.0 |
| Ethnicity | ||||||
| Hispanic or Latino | 1 | 4.5 | 3 | 16.7 | 3 | 21.4 |
| Non-Hispanic | 20 | 90.9 | 15 | 83.3 | 9 | 64.3 |
| Unknown | 1 | 4.5 | 0 | 0.0 | 2 | 14.3 |
| Race | ||||||
| Black | 2 | 9.1 | 5 | 27.8 | 1 | 7.1 |
| More than one race | 1 | 4.5 | 0 | 0.0 | 0 | 0.0 |
| Unknown | 0 | 0.0 | 1 | 5.6 | 2 | 14.3 |
| White, non-Hispanic | 19 | 86.4 | 12 | 66.7 | 11 | 78.6 |
Table 2.
Lymphocyte subsets in patients with Chiari malformation and posterior fossa tumors
| Marker | Group | N | Absolute no. (%*) median | Range (%*) | p value |
|---|---|---|---|---|---|
| Total lymphocytes | CM | 21 | 29,876 | 21246–128,913 | |
| MBL | 18 | 29,775 | 9594–37,085 | ||
| PFT | 13 | 28,456 | 3002–50,537 | ||
| CD4 | CM | 21 | 11,114 | 2917–20,658 | |
| MBL | 18 | 6347.5 | 2416–12,747 | 0.0014 (CM vs. MBL) | |
| PFT | 13 | 6073 | 433–14346 | 0.0054 (CM vs. PFT) | |
| CD45RO+−CD4+ | CM | 21 | 5999 (19.9) | 1090–9230 (6.14–27.2) | |
| MBL | 18 | 1677 (9.4) | 358–8002 (3.03–27.24) | 0.0079 (CM vs. MBL) | |
| PFT | 13 | 2942 (8.2) | 55–7257 (2.89–22.65) | 0.0085 (CM vs. PFT) | |
| CD45RO−CD4+ | CM | 21 | 4873 (19.1) | 1771–10,516 (7.71–25.57) | |
| MBL | 18 | 2794 (13.5) | 1570–6063 (6.55–26.38) | 0.0069 (CM vs. MBL) | |
| PFT | 13 | 3477 (13.4) | 195–6861 (7.17–24.37) | 0.038 (CM vs. PFT) | |
| CD4+CD25+FoxP3+CD127lo | CM | 21 | 254 (0.73) | 1–752 (0–12.4) | |
| MBL | 18 | 48 (0.18) | 11–385 (0.03–3.9) | 0.0016 (CM vs. MBL) | |
| PFT | 13 | 43 (0.17) | 3–165 (0.01–0.9) | 0.001 (CM vs. PFT) |
CM Chiari malformation, MBL medulloblastoma, PFT posterior fossa tumors
* As a percentage of total lymphocyte count
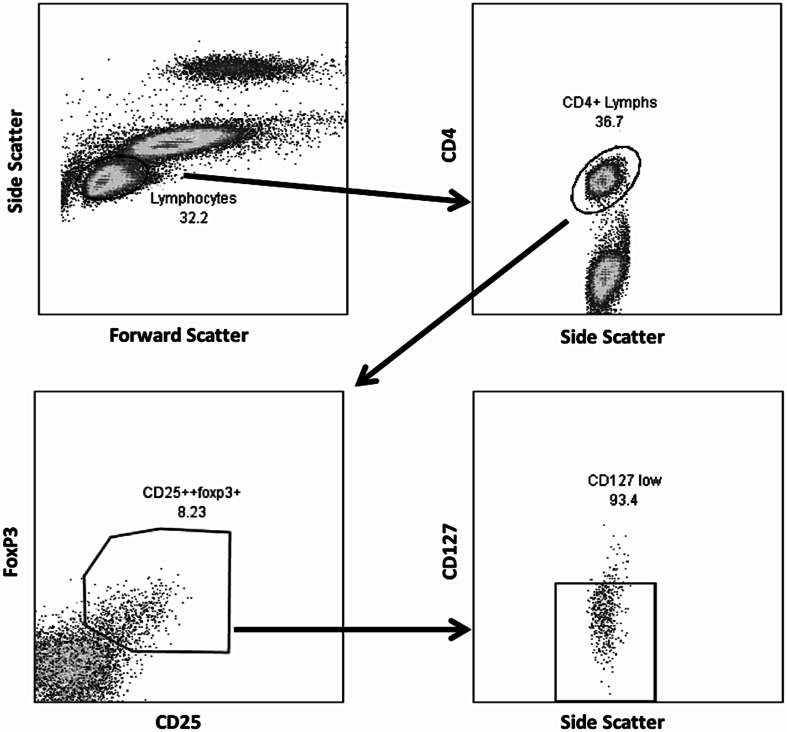
Fig. 1.
Treg lymphocyte population (CD4+CD25+Foxp3+CD127low) identified by flow cytometry
Fig. 2.
a–d Dot plots representing total CD4 (a), non-memory (CD45RO−CD4+) (b), memory (CD45RO+CD4+) (c), and regulatory (CD4+CD25+FoxP3+CD127lo) T cells (d) in patients with Chiari malformation (CM), medulloblastoma (MBL), and other posterior fossa tumors (PFT) demonstrating significantly lower counts of these lymphocyte subsets in patients with posterior fossa tumors as compared to control CM patients (p values are from Kruskal–Wallis test)
Fig. 3.
a–f Total lymphocyte count and subsets (absolute or percentage) prior to surgery, post-surgery, prior to RT (~30 days), post-RT (~72 days), pre-chemotherapy (~114 days), and 3 week post-chemotherapy in 18 patients with medulloblastoma. a, b Total lymphocyte and CD4 counts decreasing as expected following cytotoxic therapy. c, d Absolute and percentage regulatory T cells (CD4CD25FoxP3CD127lo) increasing during cytotoxic therapy only to decline thereafter. e CD4CD25FoxP3Ki-67 counts showing a corresponding increase, suggesting that increase in regulatory T cells during this period is due to actual proliferation of Tregs. f Decrease in effector T cells during cytotoxic chemotherapy with a slow rise thereafter. Dark lines in each panel represent the best model fit
Discussion
Immune evasion is one of the hallmarks of cancer and can be due to active or passive conditions of tolerance that can inhibit natural immune responses against tumors [8, 9]. Passive mechanisms include downregulation of MHC class I expression on tumor cells, low antigenicity caused by immune editing and selective tumor growth, or depletion of tryptophan by indolamine 2,3-dioxygenase and inhibition of immune cells [8, 9]. Active mechanisms include suppression of anti-tumor responses by infiltrating Tregs, myeloid-derived suppressor cells, or tumor cells expressing inhibitory ligands and cytokines [9]. Tregs are a subgroup of CD4+ T lymphocytes and can be easily identified through co-expression of CD25 (IL-2 α receptor)(surface) and FoxP3 antigens (intracellular) [9]. Additional specificity is conferred by low or absent expression of the IL-7 receptor, CD127 (CD4+CD25+FoxP3CD127lo/−) [10]. These cells have been identified as one of the most significant inhibitors of anti-tumor immunity through suppressing T cell activation and proliferation, downregulating IL-2 production, and stimulating increase in TH2 cytokine secretion in the target cells that can in turn increase Tregs expansion [8, 11]. Tregs infiltrate the tumor mass in its early stages and inhibit local immune responses allowing tumor to continue to grow. Elevated fraction of peripheral Tregs has also been demonstrated in the peripheral blood in a wide variety of cancers including GBM, ovarian, and breast carcinomas [8]. Facets of the immunologic profile in patients with MBL have been previously described [12], including decreased class I HLA expression [13], increase in circulating TH-17 lymphocytes [14], PD-L1 up-regulation (via CDK5) [15], and expression of CD1d (target for NK cells) [16]. Our current study is the first to evaluate the immunologic profile in children with newly diagnosed MBL and PFT with a focus on circulating Tregs. We found significantly lower levels of CD4+ T cell count and Tregs (absolute counts and percentage of CD4 T cells) compared to a control population of patients with CM who also received perioperative corticosteroids. In contrast, a similar study in adults with GBM revealed that while absolute CD4+ T cell and Tregs counts were decreased compared to controls, the Tregs fraction was significantly elevated. These observations suggest that Tregs might play a greater role in the immunosuppressive phenotype in patients with GBM as compared to other primary CNS malignancies [8, 17]. The reason(s) for lower Treg fraction in children with medulloblastoma compared to the control cohort is unclear. A limitation of this study is that we did not evaluate the degree of Tregs infiltration in the corresponding tumor samples from enrolled patients. It is possible that markedly different Tregs proportions are represented within the tumor-infiltrating lymphocytes of MBL. However, MBLs are histologically characterized by rather sparse lymphocytic infiltrates overall, and a close correlation between circulating Tregs and that in the tumor tissue in adult patients with GBM has been observed, suggesting that peripheral blood levels may be indicative of intratumoral Tregs [8]. In addition, Tregs originate in the thymus and can migrate to lymph nodes and spleen in both healthy subjects and those with systemic cancers [18, 19]. It is possible that cells in these sites along with peripheral blood Tregs might also be instrumental in causing immunosuppression.
As almost all newly diagnosed patients with brain tumors are routinely treated with dexamethasone (a glucocorticoid) prior to surgical resection, it is important to control for the impact of steroids on T cell subsets [20]. While our control patients with CM also received a single dose of dexamethasone pre-operatively, we were not able to control for differences in steroid doses and duration of exposure prior to surgery in the study population. However, high-dose methylprednisolone has been shown to actually increase both the naïve and effector Tregs several fold within 48 h after treatment possibly related to increased transcription of Foxp3 following glucocorticoid receptor stimulation, that return to baseline in a week [21]. It is, therefore, unlikely that both the reduced number and fraction of Tregs in our patients with MBL and PFT are due to higher doses of steroid exposure. Similarly, Fecci et al. did not observe differences in Tregs fractions between non-GBM brain tumor patients receiving perioperative steroids and healthy controls [8]. As expected, cytotoxic therapy in our patients caused further lymphopenia, but the Tregs fraction increased during such treatment and slowly declined thereafter, suggesting resistance of these cells to chemo/radiotherapy and a more rapid recovery phase as previously reported [22, 23]. This elevated Tregs fraction during standard therapy may impact potential immunotherapy approaches for these patients and methods to decrease this subpopulation of CD4+ lymphocytes might be warranted in conjunction with immunotherapy protocols [9, 17]. Overall, this study suggests that patients with MBL, similar to adult patients with GBM, are characterized at diagnosis by CD4 T cell lymphopenia; however, they do not reflect elevated peripheral blood Tregs as part of a spectrum of tumor-associated immunosuppression. Further functional analysis of immune deficits in this patient population warrants focus on other immunoregulatory populations of cells such as myeloid-derived suppressor cells and axis of immune checkpoint receptor–ligand interactions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CM
Chiari malformation
- GBM
Glioblastoma multiforme
- MBL
Medulloblastoma
- PFT
Posterior fossa tumors other than medulloblastoma
- RT
Radiotherapy
- TH
Helper T cells
- Tregs
Regulatory T cells
Compliance with ethical standards
Funding
This work was supported by Pediatric Brain Tumor Consortium Grant U01CA81457, National Center for Research Resources Grant M01RR00188, and the American Lebanese Syrian Associated Charities.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Gururangan S, Reap E, Reynolds R, Grant G, Onar-Thomas A, Kocak M, Baxter P, Pollack I, Phillips P, Boyett JM, Fouladi M, Mitchell DA. Immunologic profile of patients with newly-diagnosed medulloblastoma at initial diagnosis and during standard radiation and chemotherapy (PBTC-N11) Neuro Oncol. 2016;18(3):iii118. [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 4.Gururangan S, Krauser J, Watral MA, Driscoll T, Larrier N, Reardon DA, Rich JN, Quinn JA, Vredenburgh JJ, Desjardins A, McLendon RE, Fuchs H, Kurtzberg J, Friedman HS. Efficacy of high-dose chemotherapy or standard salvage therapy in patients with recurrent medulloblastoma. Neuro Oncol. 2008;10(5):745–751. doi: 10.1215/15228517-2008-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clin Cancer Res. 2014;20(14):3651–3659. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60(8):1161–1171. doi: 10.1007/s00262-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wainwright DA, Dey M, Chang A, Lesniak MS. Targeting tregs in malignant brain cancer: overcoming IDO. Front Immunol. 2013;4:116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 9.Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71(22):6915–6920. doi: 10.1158/0008-5472.CAN-11-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartigan-O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319(1–2):41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonabend AM, Ogden AT, Maier LM, Anderson DE, Canoll P, Bruce JN, Anderson RC. Medulloblasoma: challenges for effective immunotherapy. J Neurooncol. 2012;108(1):1–10. doi: 10.1007/s11060-011-0776-1. [DOI] [PubMed] [Google Scholar]
- 13.Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garre ML, Cama A, Basso G, Ferrone S, Gambini C, Pistoia V. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res. 2007;67(11):5471–5478. doi: 10.1158/0008-5472.CAN-06-4735. [DOI] [PubMed] [Google Scholar]
- 14.Zhou P, Sha H, Zhu J. The role of T-helper 17 (Th17) cells in patients with medulloblastoma. J Int Med Res. 2010;38(2):611–619. doi: 10.1177/147323001003800223. [DOI] [PubMed] [Google Scholar]
- 15.Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, Huang AY, Petrosiute A. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353(6297):399–403. doi: 10.1126/science.aae0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Song L, Brawley VS, Robison N, Wei J, Gao X, Tian G, Margol A, Ahmed N, Asgharzadeh S, Metelitsa LS. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin Immunol. 2013;149(1):55–64. doi: 10.1016/j.clim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimberger AB, Kong LY, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Wei J, Qiao W, Schmittling RJ, Archer GE, Sampson JH, Hiraoka N, Priebe W, Fuller GN, Sawaya R. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- 18.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108(2):426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider T, Kimpfler S, Warth A, Schnabel PA, Dienemann H, Schadendorf D, Hoffmann H, Umansky V. Foxp3(+) regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J Thorac Oncol. 2011;6(3):432–438. doi: 10.1097/JTO.0b013e31820b80ca. [DOI] [PubMed] [Google Scholar]
- 20.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 21.Mathian A, Jouenne R, Chader D, Cohen-Aubart F, Haroche J, Fadlallah J, Claer L, Musset L, Gorochov G, Amoura Z, Miyara M. Regulatory T cell responses to high-dose methylprednisolone in active systemic lupus erythematosus. PLoS One. 2015;10(12):e0143689. doi: 10.1371/journal.pone.0143689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15(1):927–943. doi: 10.3390/ijms15010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medler TR, Cotechini T, Coussens LM. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer. 2015;1(1):66–75. doi: 10.1016/j.trecan.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.