Abstract
Background
Renevia is a hyaluronin-gelatin crosslinked matrix scaffold that has been studied as an alternative to adipose transfer in soft tissue reconstruction. It is designed to emulate the native extracellular matrix environment by supporting stromal vascular fraction (SVF) cell attachment, survival, and proliferation, thus promoting cell-based volume restoration. However, the concentration of incorporated cells for a clinically relevant result has yet to be determined.
Methods
Five experimental groups of seven CD-1 nude immunodeficient mice were given 250 uL grafts of the following composition: 1 million human SVF cells per mL of Renevia scaffold, 6 million human SVF cells per mL scaffold, 12 million human SVF cells per mL scaffold, Renevia scaffold alone or human adipose tissue alone. Volumetric analysis was conducted at discrete time points over 16 weeks using three-dimensional ultrasound, after which time the grafts were explanted for histologic analysis.
Results
At the conclusion of the study at week 16, the Renevia scaffold group incorporating the highest concentration of human SVF cells (12 million cells per mL scaffold) had significantly greater volume retention compared to the two lower concentrations, scaffold alone, and fat alone groups. Histology of the 12 million scaffold group revealed abundant adipocyte formation within the scaffold, exceeding that observed in the 6 million, 1 million and scaffold alone groups. The 12 million group also demonstrated significantly increased vascularity per CD31 staining.
Conclusions
Stromal vascular fraction cells coupled with Renevia hydrogel scaffold can enhance soft tissue volume reconstruction. In this study, we observed the greatest effect with 12 million cells per mL. From the perspective of volume retention, incorporation of higher concentrations of SVF cells with Renevia may be an alternative to conventional adipose tissue grafting.
Keywords: soft tissue reconstruction, hydrogel, scaffold, BioTime, Renevia, hyaluronic acid, stromal vascular fraction, graft
Introduction
Human soft tissue defects exact a tremendous medical and emotional burden on patients1. Their etiologic footprint is expansive, spanning rare congenital soft tissue syndromes to defects acquired secondary to trauma, oncologic resection, or to diseases2. Disordered soft tissue development manifests in several rare but debilitating congenital syndromes, including craniofacial microsomia and Treacher Collins syndrome3. Beyond congenital etiologies, soft tissue deficits are acquired either with degenerative conditions such as Parry-Romberg syndrome, or operatively, such as those resulting from oncologic resections of head and neck malignancies. Finally, disease processes can lead to soft tissue loss, as seen with HIV lipodystrophy, a condition affecting 40 to 50% of HIV patients undergoing highly active anti-retroviral therapy (HAART) with first-generation thymidine nucleoside analog reverse-transcriptase inhibitors (NRTI)4. The disease manifests with atrophy of malar and temporal soft tissue, as well as with peripheral fat wasting in the limbs and buttocks4.
A multitude of strategies have been advanced to address soft tissue reconstruction, and they center on the broader themes of de novo adipose tissue formation, autologous fat transfer, cell-based therapy and non-biologic injectable fillers. De novo tissue formation refers to the guided differentiation of cells towards an adipogenic lineage. Several commercially available cell lines have demonstrated the ability to differentiate into fat cells, in vitro and in vivo, with and without exogenous inductive signaling from adipogenic growth factors5–8. Perhaps more relevant to the in vivo biology of adipogenesis, primary cells also have been successfully induced to fat differentiation, the most studied of which being human mesenchymal stem cells (MSC)9,10, including bone marrow stem cells (bMSC)11 and adipose-derived stromal cells (ASC)12. As previously alluded to, some of this differentiation towards the adipogenic fate is attributable to the inclusion of growth factors well known to potentiate adipogenic differentiation – fibroblast growth factor (FGF)-213, insulin-like growth factor (IGF)-114, matrix metalloproteinases (MMP)15, and platelet-derived growth factor (PDGF)16.
In contrast to the nascent promise of de novo adipogenesis, autologous fat grafting has emerged over the past two decades as the preeminent technique for managing soft tissue defects. However, despite its ubiquity, the employment of fat grafting has been plagued by highly variable clinical results, with some studies reporting retention rates ranging from 10 to 80%17,18. Given the complications and unpredictability of adipose tissue grafting, alternative synthetic fillers – most notably hyaluronic acid-based dermal fillers – have been popularly employed in soft tissue reconstruction. Hyaluronic acid-based injectable fillers largely avoid the variability of adipose tissue quality, while also minimizing donor site morbidity19. In general, patients report satisfaction with the cosmetic results of HA dermal fillers and endorse an increase in quality of life secondary to the therapy20. However, the rapidly absorbent nature of the hyaluronic acid-based injectable fillers greatly concede the durability of their desirable effects. Thus, the challenge remains to develop a synthetic vehicle for a cell-based approach capable of replacing human adipose tissue.
Renevia (BioTime, Inc; Alameda, CA) is a hyaluronin-gelatin crosslinked matrix scaffold that is marketed as an alternative to adipose transfer in soft tissue reconstruction. It is designed to emulate the native extracellular matrix (ECM) environment, and by doing so, supports SVF cell attachment, survival, and proliferation, thus promoting durable cell-based volume restoration. Currently, the Renevia scaffold is undergoing a Phase III pivotal clinical trial in Europe, in which its use in correcting subcutaneous facial lipoatrophy attributable to HIV is being evaluated. In that trial, the Renevia scaffold is supplemented with SVF cells at concentrations ranging from 3 to 8 million cells per microliter of scaffold. However, the threshold concentration for a clinically relevant result with cell-based therapy has yet to be determined. This present study employs a titration experiment to evaluate multiple concentrations of SVF cells, the results of which enhance our understanding of Renevia cell-based therapy for soft tissue reconstruction.
Methods
Fat Harvesting/SVF cell isolation
In accordance with the Stanford University Institutional Review Board Guidelines, human lipoaspirate was obtained from a single female donor (age 47, BMI 26) with no significant medical comorbidities undergoing suction assisted liposuction. Immediately upon receipt from the operating room, the lipoaspirate was twice washed with phosphate-buffered saline (Thermo Fisher Scientific; Waltham, MA) and centrifuged at 350 × g for five minutes, thus permitting separation and removal of excess blood and oil. A fraction of the lipoaspirate designated for grafting was set aside and refrigerated at 4°C. The remainder of the lipoaspirate was digested with collagenase (Sigma-Aldrich; St. Louis, MO) at 37°C for 30 minutes under moderate agitation on an orbital shaker. The digested product was then centrifuged at 350 × g for 10 minutes at 4°C. The resultant pellet, containing the stromal vascular fraction (SVF) cells, was suspended in conventional Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific; Waltham, MA) cell culture media, processed through a 100-micron filter (BD Falcon; Franklin Lakes, NJ), and re-suspended in Red Blood Cell Lysis Buffer (Sigma-Aldrich; St. Louis, MO). Upon removal of the red blood cells, the product was gently pipetted atop Histopaque 1077 (Sigma-Aldrich; St. Louis, MO) and centrifuged at 400 × g for 30 minutes at room temperature. This stratified the heterogeneous mixture into distinct layers, thus enabling precise isolation and removal of the SVF cell layer. The total SVF cell yield was calculated via hemocytometer.
Scaffold reconstitution and supplementation
The Renevia scaffold was prepared in accordance with the manufacturer’s protocol. Briefly, the individual vials of Glycosil, Gelin, and Extralink lyophilized solids were separately reconstituted with sterile water. The Glycosil and Gelin solutions were combined, and this mix was added to the Extralink vial. After fifteen minutes at room temperature, the combined solution attained sufficient viscosity to enable grafting into the unsupplemented scaffold mouse group. The SVF cell-supplemented grafts were prepared by incorporating the appropriate quantity of SVF cells into the Glycosil/Gelin solution, prior to delivery into the Extralink. Three concentrations of cell-supplemented scaffold were created: 1 million human SVF cells per mL of Renevia scaffold, 6 million human SVF cells per mL scaffold and 12 million human SVF cells per mL scaffold. Separate 1 mL syringes were prepared for the three SVF concentrations and for scaffold only. A fifth syringe was prepared containing adipose tissue, only.
Grafting
Thirty-five immunodeficient Crl:CD1-Foxn1nu CD-1® Nude mice (Charles River Laboratories, Inc; Wilmington, MA) were segregated into five experimental groups: 1 million SVF cells per mL Renevia scaffold, 6 million SVF cells per mL scaffold, 12 million SVF cells per mL scaffold, Renevia scaffold alone, and adipose tissue alone (N = 7 animals per group). The mice were anesthetized with 2.5% isoflurane gas delivered through a mask. Two hundred-fifty microliter (250 uL) Renevia grafts both with and without SVF cells were placed subcutaneously into the scalps of the CD-1 mice. Two hundred-fifty microliter fat-alone grafts also were placed, as a control. All injections were performed using a 20-gauge needle, and delivered in retrograde fashion as previously described by Chung et al.17.
Volumetric assessment
Volumetric analysis was conducted at discrete time points (day 0, 1, 3; week 1, 2, 4, 6, 8, 10, 13, and 16) using three-dimensional ultrasound. Images were captured with the FujiFilm VisualSonics Vevo 2100 high-frequency digital imaging platform (FujiFilm VisualSonics Inc.; Toronto, Ontario, Canada) utilizing a 40 MHz ultrasound transducer. The mice were anesthetized with 2.5% isoflurane gas delivered through a mask. Cardiac status was monitored via stage-integrated electrocardiogram pads. Image capture was gated to respiratory cycle to minimize motion artifact. Volume reconstruction and quantification was accomplished on the Vevo LAB software suite (Figure 1).
Figure 1.
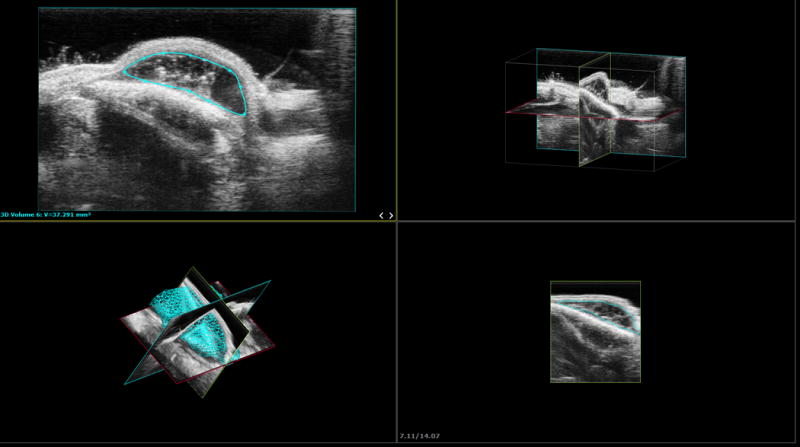
Ultrasound experimental configuration and three-dimensional image reproduction. 3D reconstruction of stacked planar ultrasound images.
Histology
Mice were sacrificed at week sixteen for histologic analysis. Grafts were explanted in their entirety through a dorsal incision made into the overlying scalp. Tissue samples were fixed in formalin, embedded in paraffin and then sectioned for hematoxylin and eosin (H&E) staining to evaluate general architecture, neovascularization, adipocyte formation, and extracellular matrix (ECM) formation. Additional sections were treated with CD31 (PECAM-1) antibody-coupled stain (Ab 18613, 1:200; BioLegend, San Diego, CA) for detailed analysis of vascularity. Slides were then digitalized and imported into ImageJ (National Institutes of Health; Bethesda, MD) for quantification of CD31 positivity.
Statistics
Statistical analysis was performed on Prism (GraphPad Software, Inc.; La Jolla, CA). Intergroup variance was evaluated using one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered statistically significant.
Results
All grafts were placed successfully into the experimental animals, with no evidence of acute infection or rejection. The surgeries were well tolerated by the mice. At the conclusion of the study, the Renevia scaffold group supplemented with the highest concentration of human SVF cells (12 million cells per mL scaffold) had significantly greater percentage volume retention (34.97%, SD 5.55) when compared to the adipose alone group (24.63%, SD 2.85), the one million cells per mL scaffold group (10.31%, SD 5.70), the six million cells per mL scaffold group (9.80%, SD 1.30), and the scaffold alone group (16.76%, SD 0.41) at week 16 (ANOVA, *p < 0.05) (Figure 2). Following injection, the adipose alone group had significantly greater mean volume retention compared to all other groups beginning at day 3 and continuing through week 4 measurements (*p < 0.05). This trend continued up to week 10, at which point an average of 44.58% was noted (SD 18.43), however this value was not statistically significant compared to the average of the next closest group, the 12 million scaffold group (34.85%, SD 7.65, p = 0.722). After week 14, the 12 million group surpassed the adipose alone group and thereafter became the highest percentage retention group for the remainder of the study. The one million and six million SVF cells per mL scaffold groups performed similarly to the scaffold alone group throughout the experiment. Taken together, the four cohorts employing the Renevia scaffold all experienced a precipitous drop in volume in the initial segment of the experiment, losing approximately half their volume in the first week after surgery.
Figure 2.
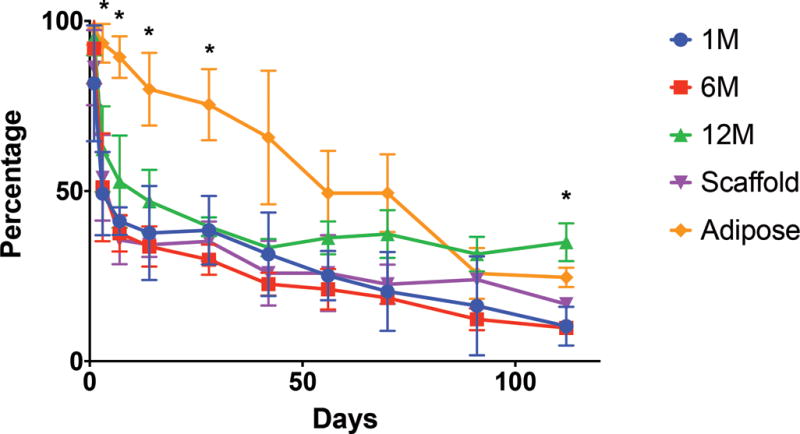
Graft volume retention over time. Percentage retention of the original graft volume over sixteen weeks.
Note significantly greater volume retention early with the adipose along group (*p < 0.05). However, by week 16 the group with 12 million cells per mL scaffold demonstrated significantly higher volume retention compared to all other groups (*p < 0.05).
Histology specimens procured at week sixteen grossly demonstrated elements of fibrous connective tissue bridging, neovascularization, and adipocyte formation that were variable in distribution and magnitude across all samples.
Hematoxylin and eosin histology of the 12 million scaffold group revealed abundant adipocyte formation within the scaffold, itself (Figure 3A). The degree of adipocyte formation in the 12 million group grossly exceeded that of the 6 million (Figure 3B), 1 million (Figure 3C), and scaffold alone (Figure 3D) groups. As anticipated, the greatest number of adipocytes was observed in the H&E preparations of the adipose tissue alone group (Figure 3E). Among the Renevia experimental groups, the 12 million cohort also demonstrated increased formation of fibrous connective tissue integrated throughout the scaffold (Figure 3F) as compared to the scaffold only group.
Figure 3.
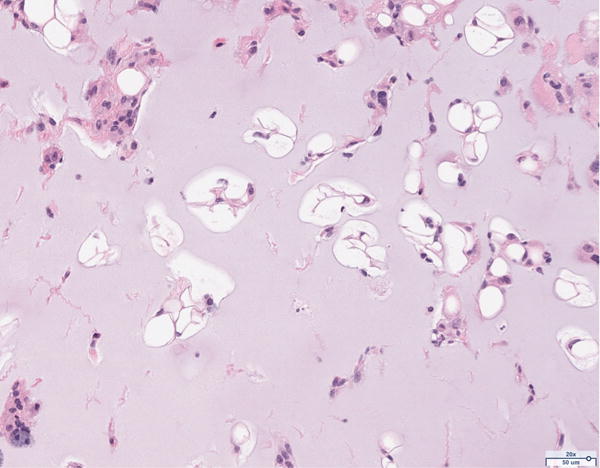
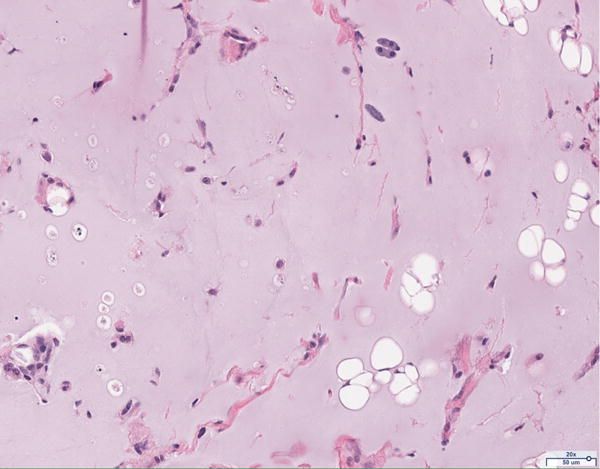
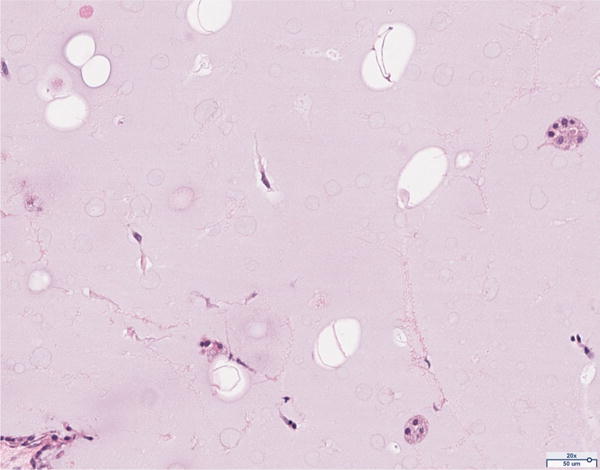
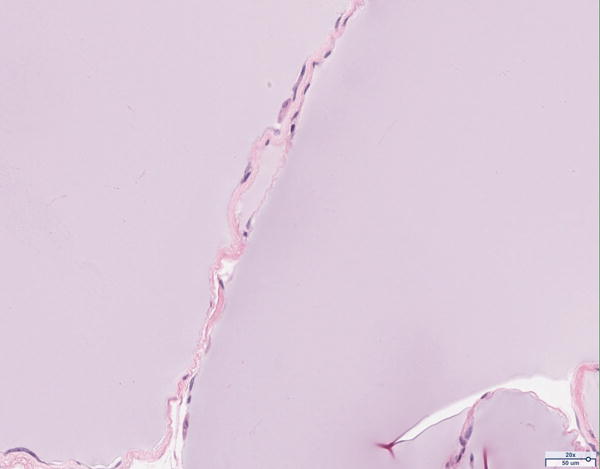
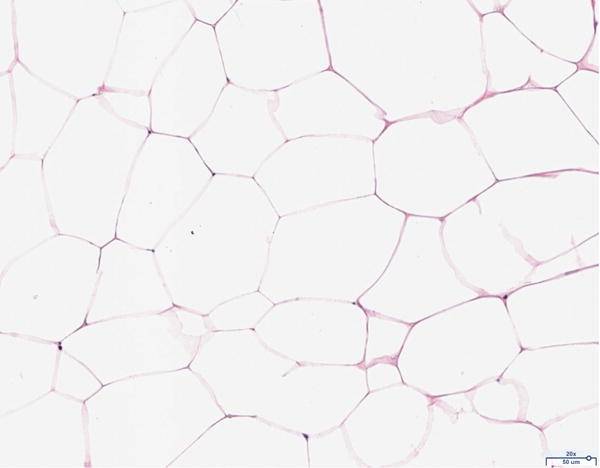
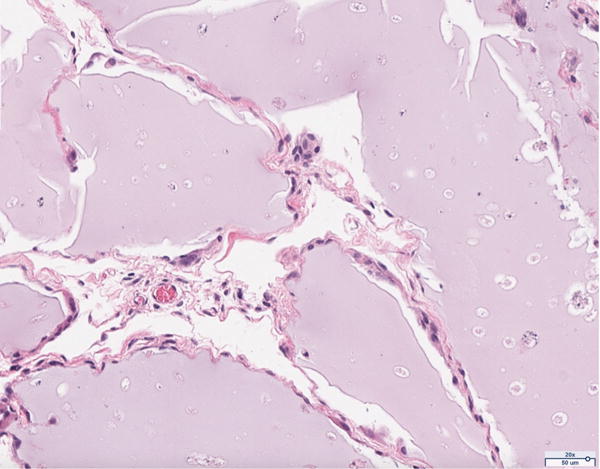
Hematoxylin and eosin (H&E) staining of explanted grafts. Grossly, the (a) 12 million cells per mL scaffold group demonstrated a greater degree of intra-scaffold adipocyte formation compared to the (b) 6 million cells per mL scaffold, (c) 1 million cells per mL scaffold, and (d) scaffold alone groups. Not surprisingly, the (e) fat alone samples displayed the greatest number of adipocytes. The 12 million group demonstrated increased formation of new ECM, fibrous tissue, and blood vessels (f).
The presence of vascularity was determined by CD31 positive staining, as quantified by ImageJ software. The pattern of staining was largely peripheral to the mature adipocytes; however, the presence of endothelial cells was observed to a limited extent in close proximity to the adipocytes of the scaffold in the 12 million group (Figure 4A). The 6 million (Figure 4B), 1 million (Figure 4C), and scaffold alone (Figure 4D) groups together demonstrate the least CD31 staining of all groups considered; the differences in CD31 positivity between these three groups was not statistically significant. Staining in the fat alone group illustrated the presence of endothelial cells homogenously distributed within the intra-lobular septations between adipocytes (Figure 4E). The 12 million group had the greatest magnitude of CD31 staining of all experimental groups, followed by the fat alone group (*p < 0.05, Figure 4F).
Figure 4.
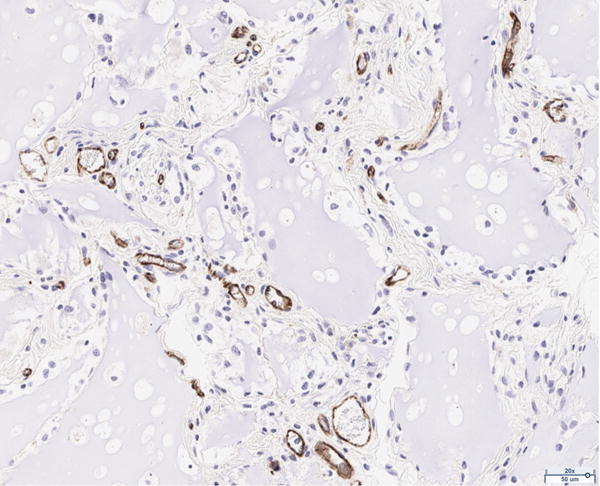
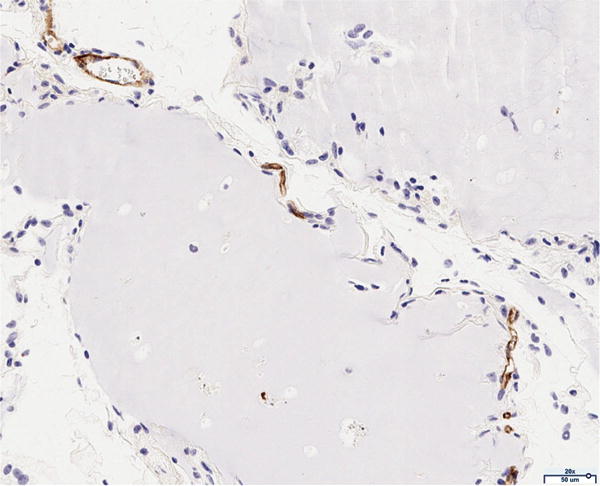
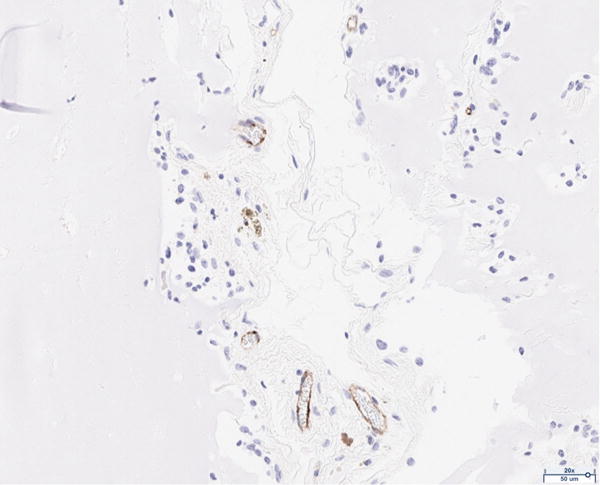
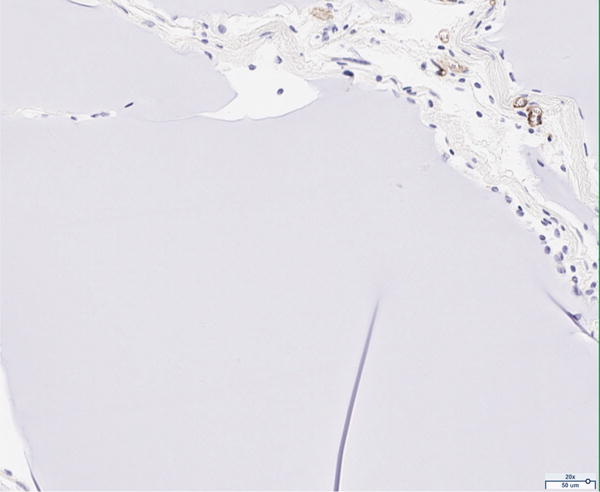
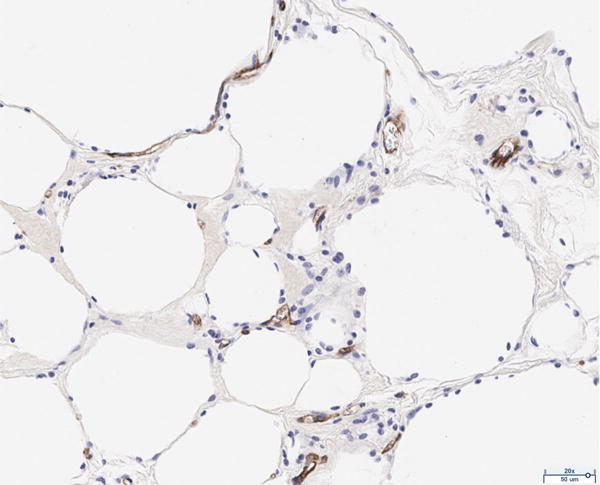
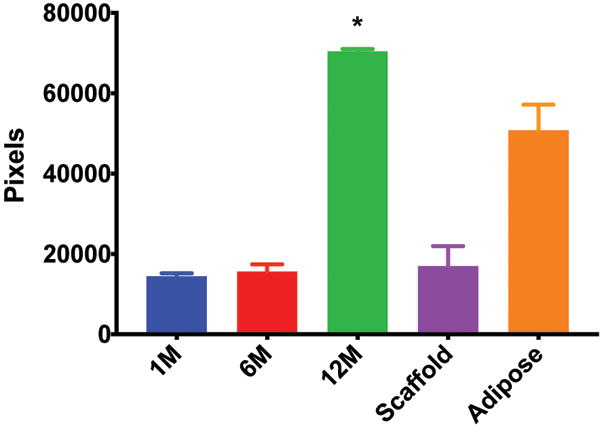
CD31 staining of explanted grafts. Representative images of the (a) 12 million cells per mL scaffold group reveal higher CD31 stain positivity compared to the (b) 6 million cells per mL scaffold, (c) 1 million cells per mL scaffold, (d) scaffold alone groups, and (e) fat alone groups. The increased (f) average pixel count in the 12 million group was statistically significant (*p < 0.05).
Discussion
Achieving durable soft tissue volume outcomes using a cell-based hyaluronic acid strategy has been challenging, thus far. Studies into non-injectable, spongy HA scaffolds supplemented with ASCs and implanted intact into a human subcutaneous in vivo model have shown proficiency for HA to serve as a cell carrier, but poor ability to promote de novo adipose tissue formation21. With respect to the commercially available, injectable hyaluronic acid-based fillers, complications arise in the mechanics of incorporating SVF cells into the gel. Due to the fact that these fillers arrive already crosslinked, their increased viscosity precludes sufficient homogenization after supplemented with stromal cells. To this point, Renevia may not only be amenable to cell supplementation, but may actually facilitate cell-based therapy, as evidenced by its biochemical dynamics. After the Glycosil and Gelin components are dissolved into solution, Extralink, a polyethylene glycol diacrylate cross-linker, is introduced to create the vital crosslinks that confer coherence and rigidity to the scaffold. Since this reaction requires eight to 15 minutes to complete, Renevia begins as a low viscosity gel that mixes readily with supplemented cells and delivers easily through a syringe prior to solidifying in the target area. Moreover, Renevia’s specificity for cell-based therapy is further enhanced by a synthetic architecture that emulates the natural ECM. This is exemplified by the two major constituents of the Renevia assembly; whereas the Glycosil component provides the hyaluronan backbone of the scaffold, Gelin contains the requisite amino acid sequences that facilitate cellular attachment and proliferation. By this mechanism, Renevia is designed to be incrementally resorbed and slowly replaced by endogenous ECM over its lifetime.
As anticipated, the fat alone group demonstrated the greatest early volume retention. At week eight, the average graft volume retention in the fat only group was over 49%. Prior studies utilizing the same murine scalp model have found approximately 60% survival in fat grafts at 8 weeks, although with 200 uL grafts17,22. The difference in results is in part attributable to graft size, as the adipose and scaffold grafts placed in this experiment were 250 uL in volume. It is also important to note that prior studies continued to demonstrate a negative trend in volume retention out to twelve weeks, though these differences were not statistically significant. In this present study, we followed volume retention to sixteen weeks, and observed continued volume loss with fat grafts out to our later time points. The well-regarded graft survival theories of Peer, and more recently Carpaneda, predict adipocyte survival on the basis of expeditious host anastomotic revascularization, or to the proximity of adipocytes to the zone of imbibition23,24. It is widely accepted that larger grafts, with their under-perfused cores, have a comparatively smaller surface area to volume ratio, and are thus more susceptible to hypoxic cell damage and adipocyte death.
Initially, we saw a significant drop in volume in the hydrogel experimental groups in the days following grafting. This rapid volume loss potentially may be attributable to mobilization of fluid from the mostly-water scaffold. It is possible that this was aggravated further by the intraoperative volume status of the mice during inhalational anesthesia, and reflects the fact that the mice were not receiving normal saline support. This factor can be remedied in the future through postoperative administration of a saline bolus.
The experimental arm incorporating the highest concentration of SVF cells ultimately outperformed every other group with respect to graft survival at sixteen weeks. The fact that the two lower concentration scaffold groups performed no better than scaffold alone – and tracked closely to it throughout the study – suggests that the concentration of 12 million cells per milliliter of scaffold exceeds a possible threshold value to produce a significant difference.
The increased vascularity in the 12 million group, as evidenced by image quantification of CD31 positivity, is consistent with our hypothesis of increased perfusion conferring an advantage in volume retention. Predictably, the scaffold only group performed more poorly in comparison to the fat only group, both in volume retention as well as in CD31 positivity. This potentially may be due to native stem cells within the adipose tissue grafts, endothelial cell differentiation, and the concomitant secretion of pro-angiogenic growth factors.
We observed replacement of the scaffold with new ECM in the 12 million group; this was most evident when compared to the scaffold only group. Histology of the two groups revealed a grossly lower proportion of continuous, intact scaffold in the 12 million group, along with increased segmentation by bridges of new ECM, fibrous tissue, and blood vessels. Additionally, the residual scaffold in the 12 million samples were studded with adipocytes, potential evidence of adipogenic differentiation of the SVF cells used for supplementation. Comparatively, the scaffold in the scaffold-only group remained broadly intact throughout the experiment, with less dissolution or replacement.
Conclusion
Stromal vascular fraction cells increase the percentage of retention of the Renevia hydrogel scaffold. While lower concentrations of SVF with Renevia performed no better than scaffold alone, the hydrogel scaffold with the highest concentration of human SVF cells performed significantly better than all other groups including fat alone. Thus, from the perspective of volume retention, incorporation of higher concentrations of SVF cells with Renevia may be an alternative to conventional adipose tissue grafting. In this study, we have discovered a threshold concentration of cell supplementation to observe this effect. Future investigations can focus on determining the optimum concentration of SVF cells to most efficiently accomplish the aims of cell-based therapy.
Acknowledgments
Source of Funding: This research was supported by BioTime, Inc. M.T.L. was supported by NIH grants R21 DE024230-02, U01 HL099776, R01 DE021683, the Oak Foundation, Hagey Laboratory for Pediatric Regenerative Medicine, and the Gunn/Olivier Fund. D.C.W. was supported by NIH grant 1K08 DE024269-01, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award. C.P.B. was supported by The Plastic Surgery Foundation (SPO 123069) and the Stanford Transplant and Tissue Engineering Fellowship Endowment Fund.
Footnotes
Conflicts of Interest: For the remaining authors, none were declared.
References
- 1.Rubin JP, Marra KG. Soft tissue reconstruction. Methods Mol Biol. 2011;702:395–400. doi: 10.1007/978-1-61737-960-4_28. [DOI] [PubMed] [Google Scholar]
- 2.Herlin C, Genevieve D, Vincent M, Chaput B, Captier G. Treacher Collins Syndrome: A Systematic Review of Evidence-Based Treatment and Recommendations. Plast Reconstr Surg. 2016;138(2):374e–376e. doi: 10.1097/PRS.0000000000002381. [DOI] [PubMed] [Google Scholar]
- 3.Tanna N, Broer PN, Roostaeian J, Bradley JP, Levine JP, Saadeh PB. Soft tissue correction of craniofacial microsomia and progressive hemifacial atrophy. J Craniofac Surg. 2012;23(7 Suppl 1):2024–2027. doi: 10.1097/SCS.0b013e31825d0594. [DOI] [PubMed] [Google Scholar]
- 4.Hester EK. HIV medications: an update and review of metabolic complications. Nutr Clin Pract. 2012;27(1):51–64. doi: 10.1177/0884533611431985. [DOI] [PubMed] [Google Scholar]
- 5.Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- 6.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 7.Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A. 1978;75(12):6107–6109. doi: 10.1073/pnas.75.12.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn L, Woodhouse KA. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4(4):228–235. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 11.Hong L, Peptan I, Clark P, Mao JJ. Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Ann Biomed Eng. 2005;33(4):511–517. doi: 10.1007/s10439-005-2510-7. [DOI] [PubMed] [Google Scholar]
- 12.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27(36):6052–6063. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Gabrielsson BG, Johansson JM, Jennische E, et al. Depot-specific expression of fibroblast growth factors in human adipose tissue. Obes Res. 2002;10(7):608–616. doi: 10.1038/oby.2002.83. [DOI] [PubMed] [Google Scholar]
- 14.Yuksel E, Weinfeld AB, Cleek R, et al. De novo adipose tissue generation through long-term, local delivery of insulin and insulin-like growth factor-1 by PLGA/PEG microspheres in an in vivo rat model: a novel concept and capability. Plast Reconstr Surg. 2000;105(5):1721–1729. doi: 10.1097/00006534-200004050-00018. [DOI] [PubMed] [Google Scholar]
- 15.Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50(9):2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 16.Virakul S, Dalm VA, Paridaens D, et al. Platelet-Derived Growth Factor-BB Enhances Adipogenesis in Orbital Fibroblasts. Invest Ophthalmol Vis Sci. 2015;56(9):5457–5464. doi: 10.1167/iovs.15-17001. [DOI] [PubMed] [Google Scholar]
- 17.Chung MT, Hyun JS, Lo DD, et al. Micro-computed tomography evaluation of human fat grafts in nude mice. Tissue Eng Part C Methods. 2013;19(3):227–232. doi: 10.1089/ten.tec.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez AM, Lobocki C, Kelly CP, Jackson IT. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg. 2007;120(1):285–294. doi: 10.1097/01.prs.0000264401.19469.ad. [DOI] [PubMed] [Google Scholar]
- 19.Ho D, Jagdeo J. Safety and Efficacy of a Volumizing Hyaluronic Acid Filler for Treatment of HIV-Associated Facial Lipoatrophy. JAMA Dermatol. 2017;153(1):61–65. doi: 10.1001/jamadermatol.2016.3827. [DOI] [PubMed] [Google Scholar]
- 20.Ho D, Jagdeo J. Patient Reported Outcomes from HIV Facial Lipoatrophy Treatment With a Volumizing Hyaluronic Acid Filler: A Prospective, Open-Label, Phase I and II Study. J Drugs Dermatol. 2016;15(9):1064–1069. [PubMed] [Google Scholar]
- 21.Stillaert FB, Di Bartolo C, Hunt JA, et al. Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials. 2008;29(29):3953–3959. doi: 10.1016/j.biomaterials.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Garza RM, Rennert RC, Paik KJ, et al. Studies in fat grafting: Part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plast Reconstr Surg. 2015;135(4):1045–1055. doi: 10.1097/PRS.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peer LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946) 1955;16(3):161–168. doi: 10.1097/00006534-195509000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Carpaneda CA, Ribeiro MT. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast Surg. 1994;18(1):17–19. doi: 10.1007/BF00444242. [DOI] [PubMed] [Google Scholar]


