Abstract
Alterations in arginine metabolism and accelerated formation of advanced glycation end-products (AGEs), crucial mechanisms in obesity-related asthma, can be modulated by glucagon-like peptide 1 (GLP-1). L-arginine dysregulation in obesity promotes inflammation and bronchoconstriction. Prolonged hyperglycemia, dyslipidemia, and oxidative stress leads to production of AGEs, that bind to their receptor (RAGE) further potentiating inflammation. By binding to its widely distributed receptor, GLP-1 blunts the effects of RAGE activation and arginine dysregulation. The GLP-1 pathway, while comprehensively studied in the endocrine and cardiovascular literature, is under-recognized in pulmonary research. Insights into GLP-1 and the lung may lead to novel treatments for obesity-related asthma.
Keywords: Glucagon-like peptide 1, advanced glycation end products, arginine, obesity, asthma
Obesity-related asthma
While traditionally considered to be an allergic disease of reversible airway obstruction, asthma is more aptly described as a syndrome, presenting with many different subtypes. Characterization of asthma phenotypes continues to be an active area of research, but some categories include age of onset (childhood or adult), allergic (T-helper type 2 cell inflammatory responses, Th2-high), or non-allergic (Th2-low). Th2-high asthma is generally responsive to the accepted treatment paradigms of corticosteroids, bronchodilators, and allergy control.
However, asthma afflicts over 20 million people in the United States and approximately 50% have uncontrolled disease, which is associated with a decreased quality of life and increased health care system (Centers for Disease Control, 2017). While the underlying pathobiology is diverse and complicated, the most advanced asthma therapies are directed solely towards Th2-high asthma, with biologics that inhibit immunoglobulin E or interleukin-5. These therapies are expensive and may be ineffective for Th2-low asthma. Although there are many non-pharmacologic factors that contribute to uncontrolled asthma, novel therapies are urgently needed.
In the United States, approximately 35% of all adults are obese, and 39% of adults with asthma are obese (Centers for Disease Control, 2017 ; Ogden et al., 2015). Obesity and metabolic syndrome are associated with an increased risk of coincident asthma and may represent distinct asthma phenotypes (Brumpton et al., 2013; Camargo et al., 1999). Some have traditional Th2-high asthma, but others have an increasingly recognized Th2-low asthma that tends to be more common in women, later in onset, and difficult to treat (Dixon and Poynter, 2016).
Despite strong epidemiologic data linking obesity and asthma, the underlying mechanisms of this relationship are complicated and incompletely understood. Suggested links include chronic inflammation, mitochondrial dysfunction,, Th17-induced neutrophilia, macrophage dysregulation, hormonal changes, lipid metabolism, insulin resistance, and body mechanics (Baffi et al., 2016; Chesne et al., 2014; Shore and Cho, 2016). Other well-described abnormalities in obesity and metabolic syndrome, accelerated formation of advanced glycation end products (AGEs) and alterations in arginine metabolism may also play a crucial role in asthma pathogenesis and may be modulated by the anti-inflammatory incretin, GLP-1 (Figure 1) (Holguin, 2013; Milutinovic et al., 2012; Ojima et al., 2013; Singh et al., 2015; Uribarri et al., 2015).
Figure 1.
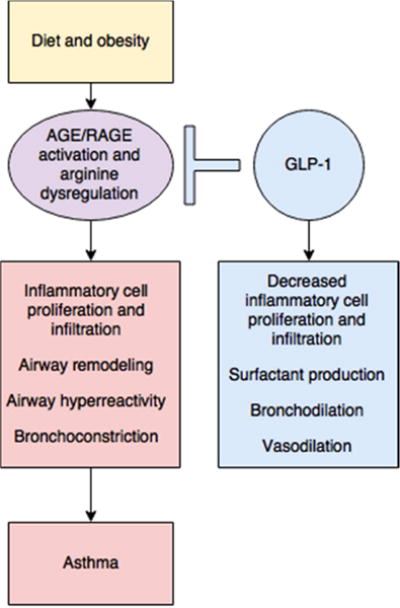
Diet and obesity may lead to dysregulated arginine metabolism and increase the production of advanced glycation end products (AGE) and subsequent activation of their receptor (RAGE), contributing to inflammation and asthma. The GLP-1 pathway may be critical to attenuating this inflammation.
Advanced glycation end-products and their receptor
AGEs are highly reactive, non-enzymatically glycated proteins or lipids implicated in the modulation of inflammatory responses. All proteins have some amount of AGE modification, but AGE formation is typically slow under normal healthy conditions (Kellow and Coughlan, 2015). Persistent hyperglycemia, dyslipidemia, and oxidative stress may accelerate AGE production. AGEs can also be consumed from foods prepared with intense heat (such as baking or frying). Serum AGEs are markers of insulin resistance and inflammation, and elevated levels may distinguish metabolic syndrome from obesity (Uribarri et al., 2015).
Interactions between AGEs and their receptor (RAGE) generate oxidative stress and perpetuate inflammatory, thrombogenic, and fibrotic reactions (Yamagishi et al., 2015). RAGE is a type of immunoglobulin cell surface marker, expressed constitutively and ubiquitously at low levels (Buckley and Ehrhardt, 2010; Demling et al., 2006). It can bind to a diverse number of ligands in addition to AGEs, such as high mobility group box 1 protein (HMGB1), amyloid fibrils, and S100 proteins. Upregulation of RAGE or its ligands results in a pro-inflammatory cascade activating NF-κB, TNF-α, IL-1β and IL-8, and is seen in diseases ranging from Alzheimer’s to atherosclerosis (Bierhaus et al., 2005; Tobon-Velasc et al., 2014). Yet, there are also soluble isoforms of RAGE in the serum, whose roles are incompletely understood, but may act to scavenge RAGE ligands before they activate the membrane-bound receptor.
Lung tissues have high levels of RAGE expression. Located on type I alveolar cells, it may promote spreading of adherent cells on collagen IV, helping to ensure effective gas exchange. However, RAGE over-activation may be deleterious and central in asthma pathogenesis. Asthmatics may have greater levels of neutrophils, HMGB1, and RAGE in their sputum. Moreover, HMGB1 seems to correlate with asthma severity and might serve as a novel biomarker of disease (Watanabe et al., 2011; Zhou et al., 2012).
Experimentally, RAGE knockout mice exhibit decreases in airway hypersensitivity, remodeling, and both Th1 and Th2 cytokines (Akirav et al., 2014; Milutinovic et al., 2012; Taniguchi et al., 2015). Taniguchi et al. found that RAGE on lung structural cells but not on hematopoietic cells resulted in allergic inflammation, while RAGE on hematopoietic cells and absent in the lung showed reduced inflammation. Interestingly, the absence of RAGE on lung structural cells also resulted in increased levels of IL-33 and enhanced innate airway hyperresponsiveness (Taniguchi et al., 2015). The mechanisms behind this are unclear, but allude to its complex role in regulating airway inflammatory responses.
Undoubtedly, these experimental links provide clues as to why AGE-RAGE inflammation in obesity and diabetes could potentiate asthma. This may then be directly attenuated by GLP-1, which reduces RAGE expression, downstream RAGE signaling, and inflammatory chemokines (Ojima et al., 2013; Yamagishi et al., 2015). These effects were seen in experiments with human kidney tubular cells and a rat model of diabetes. Furthermore, co-existing with RAGE-mediated inflammation, dysregulated arginine metabolism may be another important pathway that can be addressed through GLP-1 (Ojima et al., 2013).
Arginine metabolism
L-Arginine is a substrate in a number of diverse metabolic pathways, including the synthesis of nitric oxide (NO), and it stimulates the production of GLP-1. Arginine converts to nitric oxide primarily via nitric oxide synthase (NOS), which can create both beneficial and inflammatory reactive species of nitric oxide. Arginine can also be hydrolyzed through arginase, which catalyzes the formation of L-ornithine, urea, and L-proline. Furthermore, it can undergo methylation and generate asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor. Dysregulation of arginine metabolism occurs in obesity, metabolic syndrome, and asthma, resulting in depletion of L-arginine through increased ADMA production and arginase activity (Kenyon et al., 2011; Linderholm et al., 2014).
A recent study found that asthma-like changes with dysregulated arginine metabolism occurred in lungs of mice with diet-induced metabolic syndrome (Singh et al., 2015). Metabolic syndrome correlated with increased airway hyperresponsiveness to methacholine and reduced arginine bioavailability, with inhibition of NOS associated with ADMA and arginase levels in the lung.
Analysis of lung tissue of asthmatic subjects confirms that increased arginase expression correlated with increased serum arginase activity and decreased plasma L-arginine (Morris et al., 2004; North et al., 2009). The downstream products of arginase in obese asthmatics may contribute to airway remodeling and cellular proliferation (Linderholm et al., 2014). Obese, non-atopic asthmatics also have significantly increased levels of plasma ADMA and a reduced L-arginine:ADMA ratio (Eid et al., 2004).
L-arginine supplementation may be therapeutic, bolstering the L-arginine:ADMA ratio and outcompeting the NOS inhibitor molecule, ADMA. In murine models of asthma, L-arginine supplementation has been associated with decreased airway inflammation and hyperresponsiveness, bronchoalveolar lavage fluid eosinophilia, Th2 cytokines, and arginase activity (Mabalirajan et al., 2010; Yang et al., 2006; Zhang et al., 2015).
L-arginine supplementation also increases production of GLP-1 (Clemmensen et al., 2013). In examining the effects of GLP-1 on RAGE and arginine-mediated inflammation in streptozotocin-induced diabetic rats, Ojima et al. found that the GLP-1 analog, exendin-4, inhibited renal RAGE gene expression and decreased ADMA generation (Ojima et al., 2013). GLP-1 further inhibited AGE-induced RAGE gene expression, reactive oxygen species generation, and decreased ADMA in cultured human proximal tubular cells. This study provides a mechanistic link by which disordered arginine metabolism and activation of RAGE seen in obesity and asthma may be affected by the GLP-1 pathway (Figure 2).
Figure 2.
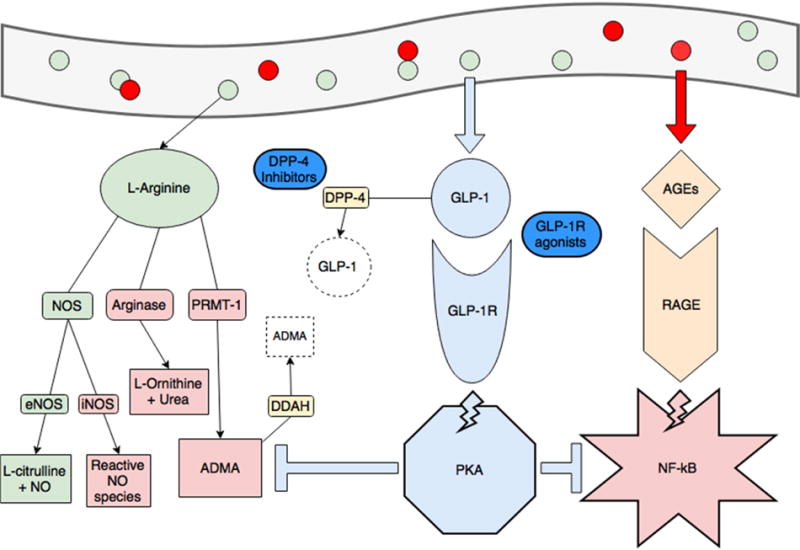
Obesity and consumption of foods high in advanced glycation end-products (red circles, AGEs) creates a pro-inflammatory state through dysregulated arginine metabolism (increasing arginase activity and production of ADMA, in red) and activating RAGE-mediated, NF-kB inflammation (pink star). ADMA also inhibits endothelial NOS (eNOS) and increases NF-kB activity. GLP-1 production is spurred by consumption of L-arginine (green circles) and when binding its receptor, activates protein kinase A (blue octagon). This activity blunts RAGE-mediated inflammation and production of ADMA (blue T-lines). The GLP-1 pathway is also the current target of treatments for diabetes and obesity. GLP-1 is rapidly degraded by DPP-4, and DPP-4 inhibitors (gliptins) are used to increase GLP-1. GLP-1 receptor agonists (exenatide and liraglutide) are also available.
Glucagon-like peptide 1 and the lung
Synthesized by enteroendocrine L cells in the distal small intestine, GLP-1 production is increased by oral intake of glucose, amino acids, and fatty acids as well as by vagal stimulation. Despite rapid inactivation by dipeptidyl peptidase-4 (DPP-4), GLP-1’s incretin activity occurs after binding to its widely distributed receptor (GLP-1R). GLP-1 secretion is reduced in obesity and type 2 diabetes (Baggio and Drucker, 2007). Furthermore, hyperglycemia may cause receptor down-regulation and contribute to insulin resistance (Xu et al., 2007). But beyond its endocrine activity, GLP-1 activity and metabolism may be crucial in lung homeostasis.
GLP-1 receptors are abundant in the lung, yet their function is poorly understood. The highest relative levels of the receptor reside within the lung, followed by intestines, and brain (Viby et al., 2013). GLP-1R seems to be widely distributed– within the alveoli, septa, airways, and vascular smooth muscle (Korner et al., 2007; Rogliani et al., 2016).
Although there is no evidence of GLP-1 production within the lung, it appears to be in much higher concentrations within bronchoalveolar fluid as compared to the serum in one study of healthy adults (Mendivil et al., 2015; Richter et al., 1993). It is not known how these levels might change in unhealthy conditions, but reductions in GLP-1 production with obesity and diabetes likely are paralleled within the lung and may contribute to dysfunction.
Functionally, GLP-1 stimulates vasodilation, surfactant production, and bronchodilation (Richter et al., 1993; Rogliani et al., 2016; Vara et al., 2001). These effects may occur via NO through phosphorylation of endothelial NOS and increase of cAMP concentrations (Chai et al., 2012; Golpon et al., 2001; Vara et al., 2001). Rogliani et al. evaluated the effects of exendin-4 on hyperglycemia-induced bronchial hyperresponsiveness ex vivo (Rogliani et al., 2016). Exendin-4 caused bronchodilation that was inhibited by either GLP1-R or cAMP-PKA antagonism. Additionally, a significant increase in GLP-1R expression was seen with passive sensitization using sera derived from asthmatics, with exendin-4 preventing over-expression. This may suggest a negative feedback loop mechanism to avoid over-activation of GLP-1R signaling.
GLP-1 degradation by DPP-4 may also be an important facet in lung homeostasis, as DPP-4 is amply expressed in the lung. A pro-inflammatory upregulation can be seen after allergen exposure within the lung (Nieto-Fontarigo et al., 2016). Bronchial epithelial cells from untreated human asthmatics express high levels of DPP-4 that are further enhanced by IL-13, correlating with elevated exhaled nitric oxide levels and proliferation of lung fibroblasts and bronchial smooth muscle (Shiobara et al., 2016). DPP-4 appears to activate pro-inflammatory pathways (MAPK and NF-κB) and may also increase generation of reactive oxygen species, AGEs, and RAGE gene expression (Ishibashi et al., 2013; Wronkowitz et al., 2014). Although upregulated DPP-4 may act to downregulate GLP-1’s benefits, the evidence for the use of DPP-4 inhibition in asthma is conflicting (Nader, 2015; Nieto-Fontarigo et al., 2016).
Therapeutic benefits of GLP-1 in asthma
The role of the GLP-1 pathway has been explored in various mouse models of asthma. Most recently, it has been demonstrated that GLP-1 may inhibit aeroallergen-induced IL-33 release and further reduce group 2 innate lymphoid cell activation (Toki et al., 2017). In another murine study, GLP-1R agonists, liraglutide and exenatide, improved survival and pulmonary function (Viby et al., 2013). Liraglutide has also been found to reduce airway inflammation, mucus secretion, and inflammatory cell counts in bronchoalveolar lavage fluid (Zhu et al., 2015). Furthermore, there was a reduction in activation of the pro-inflammatory transcription factor, NF-κB. All these effects were attenuated in the setting of a protein kinase A inhibitor, suggesting protein kinase A is a key downstream effector of GLP-1. GLP-1 stimulation of endothelial NOS may also be an important beneficial effect in asthma (Chai et al., 2012; Ten Broeke et al., 2006).
In the latest human study, Mitchell et al. evaluated GLP-1R expression and GLP-1 effects on eosinophils and neutrophils of normal and allergic asthmatic subjects (Mitchell et al., 2016). While both cell types expressed the receptor, expression was significantly higher in controls compared to asthmatics. Although receptor expression did not change with allergen challenge, a GLP-1 analog attenuated lipopolysaccharide-stimulated eosinophil activation ex vivo and decreased production of IL-4, 8, and 13.
Indeed, the GLP-1 pathway may be a potent modulator of inflammation that has been understudied in asthma and lung disease. With the advent of GLP-1 receptor agonists, future clinical trials may reveal a novel use for these medications.
Conclusion
We propose a metabolic pathway by which obesity and asthma are related, connecting known overlapping alterations in a way that is plausible, previously undescribed, and relatively easily studied. Obesity and metabolic syndrome result in a pro-inflammatory state caused by arginine dysregulation and activation of the RAGE pathway. Although these are widely accepted as mediators of cardiovascular disease, they likely play key roles in the pathogenesis of lung disease, particularly asthma.
Most recently, two large randomized controlled trials showed reduced death from cardiovascular causes as well as cardiovascular complications in patients with type-2 diabetes mellitus using GLP-1 receptor agonists liraglutide and semaglutide (Marso et al., 2016a; Marso, et al., 2016b). The consequences of diabetes and obesity on respiratory health need better characterization, and GLP-1 receptor agonists are safe, ready-to-use agents in both laboratory and clinical research.
Further research is necessary to deepen our understanding of the intersection between endocrine dysfunction, the asthma syndrome, and lung disease. Provocative studies have shown the potential of metformin, DPP-4 inhibitors, and statins in treating asthma (Huang et al., 2011; Li et al., 2016; Nader, 2015; Zeki et al., 2013). And while the GLP-1 pathway may also be effective, it is unclear which phenotypes of asthma it may help. But perhaps by treating the underlying metabolic syndrome, we can also treat the related metabolic asthma.
Acknowledgments
Funding sources:
This research was funded by the United States Department of Health and Human Services, National Institutes of Health/National Heart, Lung, and Blood Institute Grants T32 HL07013 and 4R01HL105573-05, and the Veterans Affairs Northern California Healthcare System.
Abbreviations
- AGEs
Advanced glycation end products
- ADMA
Asymmetric dimethylarginine
- cAMP
Cyclic adenosine monophosphate
- DPP-4
Dipeptidyl peptidase 4
- GLP-1
Glucagon-like peptide 1
- GLP-1R
Glucagon-like peptide 1 receptor
- HMGB-1
High mobility group box 1 protein
- IL
Interleukin
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PKA
Protein kinase A
- RAGE
Receptor for advanced glycation end products
- Th1
T-helper type 1 cell
- Th2
T-helper type 2 cell
- TNF-α
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
This is an original review that has not been published and is not under consideration for publication elsewhere.
Conflicts of Interest:
The authors declare that there are no conflicts of interest.
References
- Akirav EM, Henegariu O, Preston-Hurlburt P, Schmidt AM, Clynes R, Herold KC. The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma. PLoS One. 2014;9(4):e95678. doi: 10.1371/journal.pone.0095678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffi CW, Wood L, Winnica D, Strollo PJ, Gladwin MT, Que LG, Holguin F. Metabolic Syndrome and the Lung. Chest. 2016 doi: 10.1016/j.chest.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83(11):876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Brumpton BM, Camargo CA, Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42(6):1495–1502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159(21):2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. National Center of Health Statistics - AsthmaStats. https://www.cdc.gov/asthma/asthma_stats/default.htm March 6, 2017.
- Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61(4):888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Smajilovic S, Smith EP, Woods SC, Brauner-Osborne H, Seeley RJ, et al. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology. 2013;154(11):3978–3983. doi: 10.1210/en.2013-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323(3):475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- Dixon AE, Poynter ME. Mechanisms of Asthma in Obesity. Pleiotropic Aspects of Obesity Produce Distinct Asthma Phenotypes. Am J Respir Cell Mol Biol. 2016;54(5):601–608. doi: 10.1165/rcmb.2016-0017PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid HMA, Arnesen H, Hjerkinn EM, Lyberg T, Seljeflot I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism-Clinical and Experimental. 2004;53(12):1574–1579. doi: 10.1016/j.metabol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102(2–3):81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- Holguin F. Arginine and nitric oxide pathways in obesity-associated asthma. J Allergy (Cairo) 2013;2013:714595. doi: 10.1155/2013/714595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, et al. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest. 2011;41(5):507–512. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013;12:125. doi: 10.1186/1475-2840-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellow NJ, Coughlan MT. Effect of diet-derived advanced glycation end products on inflammation. NutrRev. 2015;73(11):737–759. doi: 10.1093/nutrit/nuv030. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Last M, Bratt JM, Kwan VW, O’Roark E, Linderholm A. L-Arginine Supplementation and Metabolism in Asthma. Pharmaceuticals. 2011;4(12):187–201. doi: 10.3390/ph4010187. [DOI] [Google Scholar]
- Korner M, Stockli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48(5):736–743. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology. 2016;21(7):1210–1218. doi: 10.1111/resp.12818. [DOI] [PubMed] [Google Scholar]
- Linderholm AL, Bratt JM, Schuster GU, Zeki AA, Kenyon NJ. Novel therapeutic strategies for adult obese asthmatics. Immunol Allergy Clin North Am. 2014;34(4):809–823. doi: 10.1016/j.iac.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabalirajan U, Ahmad T, Leishangthem GD, Joseph DA, Dinda AK, Agrawal A, Ghosh B. Beneficial effects of high dose of L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 2010;125(3):626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016b doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016a;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendivil CO, Koziel H, Brain JD. Metabolic hormones, apolipoproteins, adipokines, and cytokines in the alveolar lining fluid of healthy adults: compartmentalization and physiological correlates. PLoS One. 2015;10(4):e0123344. doi: 10.1371/journal.pone.0123344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinovic PS, Alcorn JF, Englert JM, Crum LT, Oury TD. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. Am J Pathol. 2012;181(4):1215–1225. doi: 10.1016/j.ajpath.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PD, Salter BM, Oliveria JP, El-Gammal A, Tworek D, Smith SG, et al. Glucagon Like Peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin Exp Allergy. 2016 doi: 10.1111/cea.12860. [DOI] [PubMed] [Google Scholar]
- Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170(2):148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- Nader MA. Inhibition of airway inflammation and remodeling by sitagliptin in murine chronic asthma. Int Immunopharmacol. 2015;29(2):761–769. doi: 10.1016/j.intimp.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Nieto-Fontarigo JJ, Gonzalez-Barcala FJ, San Jose E, Arias P, Nogueira M, Salgado FJ. CD26 and Asthma: a Comprehensive Review. Clin Rev Allergy Immunol. 2016 doi: 10.1007/s12016-016-8578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L911–920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief no 219. 2015 [PubMed] [Google Scholar]
- Ojima A, Ishibashi Y, Matsui T, Maeda S, Nishino Y, Takeuchi M, et al. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol. 2013;182(1):132–141. doi: 10.1016/j.ajpath.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Richter G, Feddersen O, Wagner U, Barth P, Goke R, Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol. 1993;265(4 Pt 1):L374–381. doi: 10.1152/ajplung.1993.265.4.L374. [DOI] [PubMed] [Google Scholar]
- Rogliani P, Calzetta L, Capuani B, Facciolo F, Cazzola M, Lauro D, Matera MG. GLP1-R: a Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol. 2016 doi: 10.1165/rcmb.2015-0311OC. [DOI] [PubMed] [Google Scholar]
- Shiobara T, Chibana K, Watanabe T, Arai R, Horigane Y, Nakamura Y, et al. Dipeptidyl peptidase-4 is highly expressed in bronchial epithelial cells of untreated asthma and it increases cell proliferation along with fibronectin production in airway constitutive cells. Respir Res. 2016;17:28. doi: 10.1186/s12931-016-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SA, Cho Y. Obesity and Asthma: Microbiome-Metabolome Interactions. Am J Respir Cell Mol Biol. 2016;54(5):609–617. doi: 10.1165/rcmb.2016-0052PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Aggarwal R, Singh S, Banik A, Ahmad T, Patnaik BR, et al. Metabolic Syndrome Is Associated with Increased Oxo-Nitrative Stress and Asthma-Like Changes in Lungs. PLoS One. 2015;10(6):e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi A, Miyahara N, Waseda K, Kurimoto E, Fujii U, Tanimoto Y, et al. Contrasting roles for the receptor for advanced glycation end-products on structural cells in allergic airway inflammation vs. airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L789–800. doi: 10.1152/ajplung.00087.2015. [DOI] [PubMed] [Google Scholar]
- Ten Broeke R, De Crom R, Van Haperen R, Verweij V, Leusink-Muis T, Van Ark I, et al. Overexpression of endothelial nitric oxide synthase suppresses features of allergic asthma in mice. Respir Res. 2006;7:58. doi: 10.1186/1465-9921-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as Mediator of NF-kB Pathway Activation in Neuroinflammation and Oxidative Stress. Cns and Neurological Disorders-Drug Targets. 2014;13(9):1615–1626. doi: 10.2174/1871527313666140806144831. [DOI] [PubMed] [Google Scholar]
- Toki S, Goleniewska K, Reiss S, Zhang J, Bloodworth MH, Stier MT, et al. Glucagon-like Peptide 1 Receptor (GLP-1R) Signaling Inhibits Aeroallergen-Induced IL-33 Release and Reduces Group 2 Innate Lymphoid Cell (ILC2) Activation In Vivo. Journal of Allergy and Clinical Immunology. 2017;139(2):AB81. doi: 10.1016/j.jaci.2016.12.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J Clin Endocrinol Metab. 2015;100(5):1957–1966. doi: 10.1210/jc.2014-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara E, Arias-Diaz J, Garcia C, Balibrea JL, Blazquez E. Glucagon-like peptide-1(7–36) amide stimulates surfactant secretion in human type II pneumocytes. Am J Respir Crit Care Med. 2001;163(4):840–846. doi: 10.1164/ajrccm.163.4.9912132. [DOI] [PubMed] [Google Scholar]
- Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154(12):4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105(4):519–525. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Wronkowitz N, Gorgens SW, Romacho T, Villalobos LA, Sanchez-Ferrer CF, Peiro C, et al. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim Biophys Acta. 2014;1842(9):1613–1621. doi: 10.1016/j.bbadis.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56(6):1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced Glycation End Products: A Molecular Target for Vascular Complications in Diabetes. Mol Med. 2015;21(Suppl 1):S32–40. doi: 10.2119/molmed.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Rangasamy D, Matthaei KI, Frew AJ, Zimmmermann N, Mahalingam S, et al. Inhibition of Arginase I Activity by RNA Interference Attenuates IL-13-Induced Airways Hyperresponsiveness. The Journal of Immunology. 2006;177(8):5595–5603. doi: 10.4049/jimmunol.177.8.5595. [DOI] [PubMed] [Google Scholar]
- Zeki AA, Oldham J, Wilson M, Fortenko O, Goyal V, Last M, et al. Statin use and asthma control in patients with severe asthma. BMJ Open. 2013;3(8) doi: 10.1136/bmjopen-2013-003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Kubo M, Murakami I, Setiawan H, Takemoto K, Inoue K, et al. l-Arginine administration attenuates airway inflammation by altering l-arginine metabolism in an NC/Nga mouse model of asthma. J Clin Biochem Nutr. 2015;56(3):201–207. doi: 10.3164/jcbn.14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang YQ, Wang WX, Zhou ZX, Wang YG, Yang L, Ji YL. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum Immunol. 2012;73(11):1171–1174. doi: 10.1016/j.humimm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Zhu T, Wu XL, Zhang W, Xiao M. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-kappaB (NF-kappaB) Signaling Pathway in Mice. Int J Mol Sci. 2015;16(9):20195–20211. doi: 10.3390/ijms160920195. [DOI] [PMC free article] [PubMed] [Google Scholar]


