Abstract
Histological and cell-level changes in the lumbar musculature in individuals with chronic lumbar spine degenerative conditions are not well characterized. Although prior literature supports evidence of changes in fiber type and size, little information exists describing the tissue quality and biology of pathological features of muscle in this population. The purpose of this study was to quantify multifidus tissue composition and structure, inflammation, vascularity, and degeneration in individuals with chronic degenerative lumbar spine pathology. Human multifidus biopsies were acquired from 22 consecutive patients undergoing surgery for chronic degenerative lumbar spine pathology. Relative fractions of muscle, adipose, and extracellular matrix were quantified along with muscle fiber type and cross-sectional area (CSA) and markers of inflammation, vascularity, satellite cell density, and muscle degeneration. On average, multifidus biopsies contained 48.5% muscle, 11.7% adipose tissue, and 26.1% collagen tissue. Elevated inflammatory cell counts (48.5 ± 30.0 macrophages/mm2) and decreased vascularity (275.6 ± 69.4 vessels/mm2) were also observed compared to normative values. Satellite cell densities were on average 13 ± 9 cells per every 100 muscle fibers. Large fiber CSA (3,996.0 ± 1,909.2 um2) and a predominance of type I fibers (61.8 ± 18.0%) were observed in addition to evidence of pathological degeneration-regeneration cycling (18.8 ± 9.4% centrally nucleated fibers, and 55.2 ± 24.2% of muscle regions containing degeneration). High levels of muscle degeneration, inflammation, and decreased vascularity were commonly seen in human multifidus biopsies of individuals with lumbar spine pathology in comparison to normative data. Evidence of active muscle degeneration suggests that changes in muscle tissue are more complex than simple atrophy.
Keywords: Low back pain, skeletal muscle, degeneration, atrophy, fatty infiltration, lumbar spine, histology, inflammation, surgery
INTRODUCTION
Low back pain (LBP) is a complex condition that affects 65–85% of the general population during their lifetime, and is highly correlated with chronic degenerative lumbar spine pathology (1–4). Although most LBP is considered self-limiting in nature, recent evidence suggests that a high proportion of individuals go on to develop recurrent symptoms leading to poor functional outcomes over time (5–7). In individuals who continue to experience recurrent or chronic symptoms, muscle tissue has been shown to be relatively unresponsive to traditional rehabilitative efforts, suggesting injured tissue is not recovering appropriately (8, 9). As such, understanding the structural changes in muscle in the presence of degenerative lumbar spine disease is essential to determining appropriate treatment strategies for optimal recovery.
The current body of evidence generally supports the notion that paraspinal muscle structural and energetic features are altered in the presence of LBP (10, 11). For example, fatty atrophic changes have been observed in whole muscle imaging studies of the multifidus in individuals with chronic degenerative lumbar spine pathology compared to healthy controls (12, 13). Similarly, decreased single fiber cross sectional areas (CSA’s) have been observed with histology, although there are conflicting reports, and the changes are small (10, 14). Furthermore, the majority of literature investigating histological features of LBP has focused on fiber type specific changes, which suggest selective atrophy of type II fibers and a shift in fiber type from oxidative to glycolytic (10, 15). Non-specific pathological changes such as core targetoid or moth-eaten features in muscle fibers have also been qualitatively described, but rarely quantified in this population (16, 17). Importantly, muscle atrophy, fatty infiltration, and pathological features are often interchangeably termed “muscle degeneration”, despite being biologically distinct processes (18–20). More quantitative and specific measures of muscle degeneration, including direct measures of membrane disruption, cell necrosis, and inflammatory myophagocytosis have not been performed in humans with degenerative lumbar spine pathology.
Comparing both macrostructural and microstructural properties of muscle tissue is necessary for characterizing atrophic and/or degenerative changes in muscle tissue as a function of disease severity. Distinguishing degeneration from atrophy is important because current rehabilitative paradigms aimed at reversing muscle atrophy do not seem to induce the expected hypertrophic adaptations seen in normal skeletal muscle, and may actually contribute to further tissue damage (8, 9). These structural and compositional features of muscle provide information regarding the underlying mechanisms of muscle loss, which is essential for understanding appropriate treatment guidelines and prognoses for individuals with this condition. Therefore, the purpose of this study was to characterize muscle structural features that are directly related to muscle function, such as tissue content, muscle fiber degeneration, CSA, regeneration, fiber type, vascularity, and inflammation.
METHODS
This was a cross-sectional prospective observational study (Level I) of twenty-two patients scheduled for lumbar spine surgery for degenerative conditions who were consented for a multifidus muscle biopsy (Table 1). This study was performed in accordance with Declaration of Helsinki and under approval of University of California San Diego Institutional Review Board. Patients were included if they were undergoing a primary posterior-approach procedure, and did not have any diagnosed myopathy or systemic neurological condition. Preoperative clinical MRI’s were analyzed and graded by an orthopedic surgeon and separately by a research fellow in radiology with no conflicting grades across patients, according to muscle fatty infiltration grade (Kjaer grade), with grade 0 corresponding to <10% fatty infiltration, grade 1 corresponding to 10–50% fatty infiltration, and grade 2 corresponding to >50% fatty infiltration (13). Biopsies (approximately 100mg) were harvested from the multifidus muscle during surgical approach at the spinolaminar border of the affected vertebral level. For individuals with unilateral symptom presentations, biopsies were obtained on the affected side, and for those with bilateral symptoms, biopsies were obtained based on surgeon’s approach preference. Biopsies were immediately pinned at in-vivo length using a custom biopsy clamp (21) and flash frozen in liquid nitrogen-cooled isopentane and transported on dry ice back to the laboratory. Samples were stored at −80°C until processing.
Table 1.
Subject Demographic Characteristics
| Kjaer Grade | |||
|---|---|---|---|
| 0 (n=3) | 1 (n=13) | 2 (n=6) | |
| Age (years) | 37.7 (7.7) | 60.9 (14.2) | 73.8 (7.3) |
| Gender (M:F) | 3:0 | 9:4 | 4:2 |
| Surgical Procedure | |||
| Fusion (n) | 1 | 3 | 0 |
| Laminectomy (n) | 1 | 9 | 6 |
| Discectomy (n) | 1 | 3* | 0 |
| NPRS (points) | 4.3 (2.5) | 6.6 (2.5) | 5.4 (2.5) |
| ODI (%) | 34.2 (21.8) | 53.0 (22.9) | 42.9 (19.6) |
discectomies performed in conjunction with laminectomies
NPRS: Numeric Pain Rating Scale
ODI: Oswestry Disability Index
Ten-micron sections were obtained from OCT-embedded frozen samples using a Leica (CM3050S, Buffalo Grove, USA) cryostat. Hematoxylin and Eosin (H&E) and Gomori Trichrome stains were used to visualize gross muscle morphology and quantify tissue content (22). ImageJ (http://imagej.nih.gov/ij) was used to automatically quantify the relative fractions of muscle, adipose, loose collagen, and dense collagen in Trichrome-stained biopsy (23). Briefly, tissue type was determined by manual intensity thresholding of the red (muscle), green (loose collagen), and blue (dense collage) channels of whole-section RGB images, while adipose tissue was identified morphologically and traced (22, 24). Inflammatory cells were quantified by calculating the number of CD68+ macrophages (Novocastra, CD68-L-CE) per square millimeter of tissue in the whole biopsy cross section (25). Similarly, vascularity was quantified using the number of Von Willebrand Factor positive vessels (SigmaF3520) per square millimeter (26). Muscle satellite cells were quantified by calculating the number of Pax7+ cells (R&D Systems, MAB1675) per muscle fiber in 10 randomly chosen 20x fields from each biopsy cross section. Samples were counterstained with Laminin-111 or -211 to identify muscle basal laminar borders (LAMA1 (SigmaL9393) or LAMA2 (Vector VP-M648)) and 4′6-diamidino-2-phenylindole (DAPI) and coverslipped with Vectamount mounting medium (Vector H-5000). Numbers of centrally nucleated fibers were calculated based on presence of DAPI signal within muscle fiber areas in 6 randomly chosen fields from each biopsy cross section. Muscle degeneration was quantified by superimposing a 1mm2 grid over each biopsy cross-section and calculating the number of quadrants containing degenerative muscle fibers (myophagocytosis, core fibers, moth eaten fibers, or infiltration of cells throughout the myofiber (27)), divided by the total number of quadrants containing muscle fibers. Silver-stain gels were used to quantify relative myosin heavy chain (MyHC) isoform content for MyHC I, IIa, and IIx isoforms(28).
STATISTICAL ANALYSIS
For fiber area, inflammation, vascularity, centrally nucleated fibers, and degeneration, comparisons across Kjaer grades were made using one-way Analyses of Variance with Tukey post-hoc tests when appropriate. Tissue content fractions were compared across Kjaer grades with a two-way Analysis of Variance with a post-hoc Tukey test when appropriate. All statistical analyses were performed using Prism (GraphPad, CA). The p-value for significance was set at 0.05, and trends were defined as p-values of <0.1. Data are reported as mean (standard deviation).
RESULTS
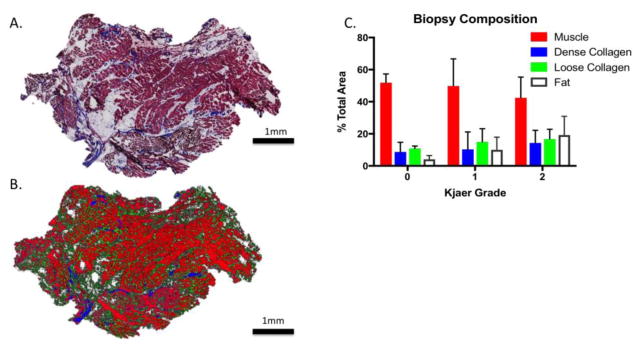
Of the 22 patients, 3 were categorized as Kjaer grade 0, 13 were categorized as Kjaer grade 1, and 6 were categorized as Kjaer grade 2, indicating that the majority of surgical patients demonstrated between 10–50% multifidus fatty infiltration levels (Table 1). Interestingly, the individuals in the Kjaer grade 1 category also demonstrated highest levels of pain on the Numeric Pain Rating Scale and LBP related disability on the Oswestry Disability Index. Age increased significantly across Kjaer grade categories (p= 0.0002), and there were more men than women in all categories. The majority (60%) of individuals participating in the study were undergoing laminectomy procedures, with 16% undergoing instrumented fusion, and 16% undergoing discectomies. Three individuals underwent both a laminectomy and discectomy in the same procedure. All but 4 individuals underwent multi-level surgical procedures to the lumbar spine. On average biopsies were composed of 48.5 (14.8)% muscle, 11.7 (9.7)% adipose tissue, 11.2 (9.5)% dense connective tissue, and 14.9 (7.1)% loose connective tissue. There were no differences in tissue composition across Kjaer grades (p=0.43) (Figure 1).
Figure 1.
(A) Gomori trichrome stained biopsy section demonstrating regions of dense collagen, loose collagen, and muscle. (B) Tissue fractions as classified by ImageJ, with muscle in red, dense collagen in blue, and loose collagen in green. (C) Biopsy composition grouped by Kjaer grade. Bar plots are mean and error bars are SD.
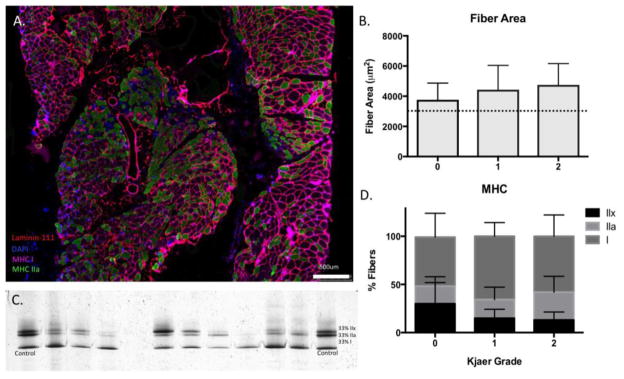
Muscle fibers had CSA’s averaging 3,996 (1,909) μm2, which are larger than previously published normative values in the human multifidus muscle of 3,100 (1,800) μm2 (29), however CSA’s were not different across Kjaer grades (p=0.69). Additionally, muscle fibers largely consisted of type I MyHC) isoforms at 61.6 (18.0)%, followed by 21.9 (13.5)% type IIa fibers, and 16.6 (13.2)% type IIx fibers; which is largely consistent with prior reports of fiber type proportions in this population(15) (Figure 2).
Figure 2.
(A) Myosin Heavy Chain- stained overlay image demonstrating myofibers containing type I isoforms (magenta) and type IIa isoforms (green). Myofiber membranes are stained with laminin-111 and nuclei are indicated with DAPI (blue). (B) Fiber areas quantified using ImageJ across Kjaer grades, with dotted lines indicating mean (black) ranges from previously reported normative values for multifidus myofibers (29). (C) Representative silver stain gel for determination of MHC isoform proportions as compared to a brachioradialis control muscle (indicated by 33% lines at each end of gel). (D) Mean (SD) percentage of type I (black), type IIa (light grey), and type IIx (dark grey) fiber types across Kjaer grades.
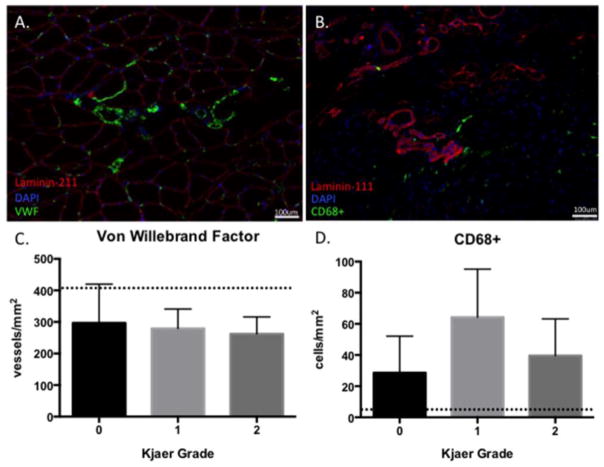
Decreased vascularity was prominent in biopsies as compared to previously published normative values for healthy human skeletal muscle (26, 30), with an average vessel density of 275.6 (69.4) vessels/mm2 (Figure 3A). Conversely, there were high levels of inflammation as measured by CD68+ cell density, with an average of 48.5 (30.0) cells/mm2, which is over four times higher than macrophage densities reported for healthy muscle (31, 32) (Figure 3B). There were no significant differences in vascularity (p=0.76) across Kjaer grades, although there was a trend for higher inflammatory cell densities in the Kjaer grade I group (p=0.08).
Figure 3.
(A) Laminin-211, DAPI, and Von Willebrand Factor overlay image used to quantify vascularity, with positive vessels stained in green. (B) Laminin-111, DAPI, and CD68+ overlay image used to quantify inflammation, with CD68+ cells stained in green. (C) Average vessel density across Kjaer grades, with the dotted line representing average vessel densities reported for healthy muscle using similar methods (25). (D) Density of macrophages across Kjaer grade, with dotted line representing average macrophage densities reported for healthy muscle (30).
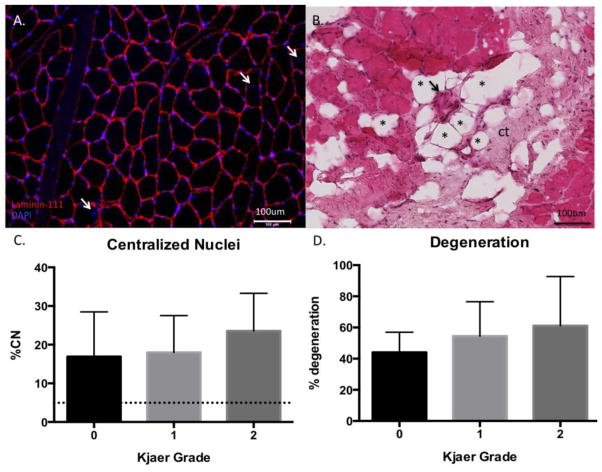
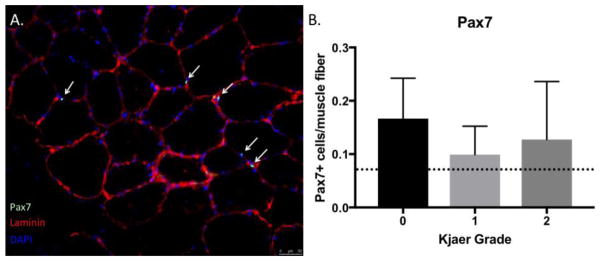
Approximately 85% of all section regions contained muscle fibers when analyzed for signs of degeneration. Within the muscle-containing regions, a majority contained signs of degeneration (55.2 (24.2)%) (Figure 4A), and 60.5 (27.0)% contained evidence of peri-or intra-muscular adipocyte infiltration. Satellite cell densities were consistent with, or slightly higher than previously reported norms in human muscle (33) at 13(9) Pax7+ cells/100 muscle fibers with no differences in satellite cell number across Kjaer grade (p=0.42) (Figure 5A,B). Similarly, the percentage of centrally nucleated myofibers was significantly higher (18.9 (9.6)%)) than the threshold that is considered normal (3%) in healthy skeletal muscle (34) (Figure 4B). Importantly, because the quantified regions of degeneration were high, and evidence of muscle loss (i.e. infiltration of other tissue types) was present throughout the biopsy regions, this indicates that regeneration (centrally nucleated fibers) was not sufficient to maintain or restore a normal muscle phenotype. There were no differences in percentage of centrally nucleated myofibers (p=0.57) or degenerated regions (p=0.61) across Kjaer grades.
Figure 4.
(A) Laminin-DAPI overlay image used to quantify centralized nuclei (white arrows). (B) H&E image of muscle fibers demonstrating signs of degeneration (black arrow), fatty replacement represented by adipocytes (examples marked with *), and connective tissue (ct). (C) Percentage of centrally-nucleated myofibers within a biopsy across Kjaer grades, demonstrating pathological elevation compared to the clinical standard for pathology (dotted line). (D) Quantification of degeneration (percentage of muscle-containing regions presenting with signs of degeneration as described in the methods section) across Kjaer grades.
Figure 5.
(A) Laminin-DAPI overlay image used to quantify Pax7+ cells in cyan (white arrows). (B) Number of Pax7+cells/muscle fiber across Kjaer grades, demonstrating elevation compared to prior literature in muscle(33).
DISCUSSION
This data represents novel information on quantitative measures of tissue composition, vascularity, inflammation, and satellite cell density in individuals undergoing surgery for chronic lumbar spine pathology. An additional novel finding is the profound extent to which this patient population demonstrates multifidus muscle degeneration, in conjunction with elevated inflammation, fiber size, and large proportions of adipose and connective tissue. Additionally, the muscle is predominantly composed of type I fibers, and demonstrates decreased vascularity. This indicates muscle degeneration without concurrent signs of atrophy at the individual fiber level, and is generally stable across levels of whole-muscle fatty infiltration as measured by Kjaer grade. Importantly, this is the first study in humans to quantify tissue composition to this depth and breadth; to date similar studies of histological changes involving measures of inflammation and tissue composition have only been performed in animal models of disc herniation (14, 35, 36). While many studies have investigated fiber type and diameters of multifidus muscles, only one study has investigated vascularity of the multifidus in healthy humans (30), and no previous study has reported or quantified the effects of degenerative lumbar spine pathology on multifidus vascularity, inflammation, or satellite cell density.
Fiber Type and Area
Our data is consistent with prior literature describing the predominance of type I fibers in the multifidus muscle, regardless of presence of pathology. Prior literature reports ranges between 54–77% for proportion of type I fibers in the multifidus muscle (10, 11, 37) and our data are in the middle of that range (62%). However, a larger proportion of type IIx fibers (16.6 (13.2)%) was observed when compared to healthy individuals (9–10%) (15, 16, 38). Interestingly, although muscle fiber proportions were consistent with prior literature, muscle fiber CSA in our biopsy samples tended to be slightly larger than previously reported norms for healthy multifidus fibers, and did not differ across fiber types (29, 39). While absence of multifidus fiber atrophy in degenerative spinal pathology has been demonstrated in a recent animal experiment (14), this data represents the first human evidence that fiber atrophy may not be the primary mechanism of muscle volume loss in chronic lumbar spine pathology.
Vascularity and Inflammation
Measurements of local inflammation and vascularity in human multifidus muscles in individuals with chronic degenerative lumbar spine pathology have not been directly investigated in previous studies. Normative values for vascular density using similar methods have been determined in other healthy human skeletal muscles such as the vastus lateralis (26) and erector spinae (30). Our measurements demonstrated a significant deficit in capillary density compared to the previously reported norms, which may have implications for efficiency of metabolite clearance and uptake, or could be reflective of the diminished proportion of healthy muscle in the biopsy. These densities should be interpreted with caution as blood vessel quantification from cross-sections may include multiple branches of vessels from a single capillary bed (40). Additionally, vascular densities across different muscle groups and regions are likely to be variable. Conversely, the density of inflammatory cells was over 4-fold higher than previously reported norms in healthy skeletal muscle (1–10 macrophages/mm2) (31). These increases were most profound in those with Kjaer grade I fatty infiltration. This suggests that inflammation may play an important role in the progression of muscle degeneration, as well as potentially contributing to painful symptoms.
Muscle Degeneration
Previous studies have shown that, compared to healthy individuals, patients with LBP qualitatively display signs of muscle pathology including core and targetoid fibers, abnormally shaped fibers, and moth-eaten fibers ranging from 1–21% of histological specimens (16, 37, 41–43). Our study demonstrated more than double these levels of degeneration in the muscle (55% of muscle regions contained degenerative fibers) using more specific methodology for quantification. We also saw a concomitant increase in regenerative signs (6-fold increase in centrally nucleated fibers compared to normal thresholds (34). This high level of degeneration, without evidence that the muscle is returning to a healthy histological phenotype despite some regenerating fibers, suggests that there is an imbalance in degeneration and regeneration in this condition. Our data also demonstrate the existence of Pax7+ cells, suggesting that the regenerative machinery is present within the remaining muscle, despite high levels of degeneration. However, the presence of these cells in isolation does not provide information regarding whether these cells are activated or quiescent. Together, these data suggest that an imbalance in the degenerative- regenerative process in muscle may have a more profound influence on the progression of muscle changes in chronic degenerative lumbar spine pathology than previously suggested. However, it is difficult to interpret the relative magnitude of muscle degeneration since quantifications of degeneration in healthy muscle do not currently exist.
Age-related influences
It is well known that age is a common confounder in muscle-related investigations of chronic musculoskeletal conditions. This is not surprising, given that individuals with longer duration of symptoms are generally older, and therefore uncoupling the effects of age from pathology is difficult. Recent data support that ageing accounts for approximately 30% of the variance in fatty composition of the multifidus and erector spinae muscles in individuals with chronic lumbar spine pathology, however, there seems to be more fatty infiltration in individuals with disease compared to their healthy counterparts, regardless of age (12). It is possible that many of the pathological changes that are observed in the current study are due to the aging process since there was a significant increase in age concurrent with Kjaer grade. However, when the current analyses were adjusted for age, the results were identical, although a slightly stronger trend was observed for inflammatory cell densities across Kjaer grade (p=0.06 vs p=0.08).
LIMITATIONS/FUTURE DIRECTIONS
There were several limitations to this study. First, recruitment was based on convenience sampling from a surgical population, so equal stratification across Kjaer grades, specifically in the grade 0 category which had the lowest sample size, was not achieved. This may affect power to detect differences in markers of muscle health across the different grades of fatty infiltration. Similarly, although muscle biopsy locations were standardized, the histological measurements only represent a small portion of the multifidus muscle and may not reflect whole muscle biology. Due to heterogeneity in the location of spine pathology, samples and Kjaer grades were often obtained from different lumbar spine vertebral segments, which may increase variability associated with architectural or physiological differences along the lumbar spine. Despite these limitations, the quantification of vascular and inflammatory cell densities as well as markers of cellular degeneration and regeneration provides novel information on pathological multifidus muscles, and supports the notion that atrophy and degeneration do not appear interchangeable in chronic degenerative spine conditions. This information may provide insight and direct future research on possible mechanisms of muscle loss and replacement by fat and connective tissue, and ultimately provide information on how to prevent this process.
CONCLUSIONS
Multifidus muscles in individuals with lumbar spine pathology demonstrate profound levels of muscle loss via imbalanced degeneration/regeneration, increased inflammation, and decreased vascularity. For these reasons, treatments aimed at reversing simple atrophy (i.e. muscle overload) may not be appropriate. This framework is consistent with the finding of unresolved “atrophy” in these patients, despite postoperative improvements in pain, and rehabilitation efforts.
Acknowledgments
The authors would like to acknowledge Oliver Tannous MD, Scott Lee MD, and Emily Osbourne MD, for their assistance in obtaining multifidus biopsies during surgical procedures. This study was funded by NIH 1TL1TR001443 awarded to BS. The authors declare no conflict of interest with performing this study.
Footnotes
AUTHOR CONTRIBUTIONS:
BS developed research design, acquisition, analysis, and interpretation of data, and drafted the paper. JCH contributed to research design, acquisition, analysis and interpretation of data and revised the paper. MCG, SR contributed to analysis and interpretation of the data and revised the paper. VZ, RTA, and SRG contributed to acquisition and revised the paper. SRW contributed to research design, analysis, and interpretation, and revised the paper. All authors read and approved the final submission.
References Cited
- 1.Collaborators UBoD. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–5. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 3.Maetzel A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res Clin Rheumatol. 2002;16(1):23–30. doi: 10.1053/berh.2001.0204. [DOI] [PubMed] [Google Scholar]
- 4.Albert HB, Briggs AM, Kent P, Byrhagen A, Hansen C, Kjaergaard K. The prevalence of MRI-defined spinal pathoanatomies and their association with modic changes in individuals seeking care for low back pain. Eur Spine J. 2011;20(8):1355–62. doi: 10.1007/s00586-011-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11(7):622–32. doi: 10.1016/j.spinee.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Carey TS, Garrett JM, Jackman A, Hadler N. Recurrence and care seeking after acute back pain: results of a long-term follow-up study. North Carolina Back Pain Project. Med Care. 1999;37(2):157–64. doi: 10.1097/00005650-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, Abdu WA, Hilibrand AS, Boden SD, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441–50. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Käser L, Mannion AF, Rhyner A, Weber E, Dvorak J, Müntener M. Active therapy for chronic low back pain: part 2. Effects on paraspinal muscle cross-sectional area, fiber type size, and distribution. Spine (Phila Pa 1976) 2001;26(8):909–19. doi: 10.1097/00007632-200104150-00014. [DOI] [PubMed] [Google Scholar]
- 9.Airaksinen O, Herno A, Kaukanen E, Saari T, Sihvonen T, Suomalainen O. Density of lumbar muscles 4 years after decompressive spinal surgery. Eur Spine J. 1996;5(3):193–7. doi: 10.1007/BF00395513. [DOI] [PubMed] [Google Scholar]
- 10.Ng JK, Richardson CA, Kippers V, Parnianpour M. Relationship between muscle fiber composition and functional capacity of back muscles in healthy subjects and patients with back pain. J Orthop Sports Phys Ther. 1998;27(6):389–402. doi: 10.2519/jospt.1998.27.6.389. [DOI] [PubMed] [Google Scholar]
- 11.Cagnie B, Dhooge F, Schumacher C, De Meulemeester K, Petrovic M, van Oosterwijck J, Danneels L. Fiber Typing of the Erector Spinae and Multifidus Muscles in Healthy Controls and Back Pain Patients: A Systematic Literature Review. J Manipulative Physiol Ther. 2015 doi: 10.1016/j.jmpt.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomislic V, Allen RT, Hughes-Austin J, Garfin S, Ward SR. Contribution of Lumbar Spine Pathology and age to Paraspinal Muscle Size and fatty Infiltration. Spine (Phila Pa 1976) 2016 doi: 10.1097/BRS.0000000000001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2. doi: 10.1186/1741-7015-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges PW, James G, Blomster L, Hall L, Schmid A, Shu C, Little C, Melrose J. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: Molecular and morphological evidence. Spine (Phila Pa 1976) 2015 doi: 10.1097/BRS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 15.Mannion AF. Fibre type characteristics and function of the human paraspinal muscles: normal values and changes in association with low back pain. J Electromyogr Kinesiol. 1999;9(6):363–77. doi: 10.1016/s1050-6411(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 16.Mannion AF, Weber BR, Dvorak J, Grob D, Müntener M. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Orthop Res. 1997;15(6):881–7. doi: 10.1002/jor.1100150614. [DOI] [PubMed] [Google Scholar]
- 17.Mannion AF, Käser L, Weber E, Rhyner A, Dvorak J, Müntener M. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J. 2000;9(4):273–81. doi: 10.1007/s005860000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6(1):25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- 20.Preedy V, Peters T. Skeletal muscle: pathology, diagnosis and management of disease. Cambridge University Press; 2002. [Google Scholar]
- 21.Ward SR, Takahashi M, Winters TM, Kwan A, Lieber RL. A novel muscle biopsy clamp yields accurate in vivo sarcomere length values. J Biomech. 2009;42(2):193–6. doi: 10.1016/j.jbiomech.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JL, Watkin KL, Chen MF. Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. J Speech Lang Hear Res. 2002;45(1):51–65. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- 23.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics international. 2004;11(7):36–42. [Google Scholar]
- 24.Gibbons MC, Singh A, Anakwenze O, Cheng T, Pomerantz M, Schenk S, Engler AJ, Ward SR. Histological Evidence of Muscle Degeneration in Advanced Human Rotator Cuff Disease. J Bone Joint Surg Am. 2017;99(3):190–9. doi: 10.2106/JBJS.16.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973–80. doi: 10.1093/intimm/2.10.973. [DOI] [PubMed] [Google Scholar]
- 26.Qu Z, Andersen JL, Zhou S. Visualisation of capillaries in human skeletal muscle. Histochem Cell Biol. 1997;107(2):169–74. doi: 10.1007/s004180050101. [DOI] [PubMed] [Google Scholar]
- 27.Dubowitz V, Sewry C, Oldfors A. Muscle Biopsy: A Practical Approach, Expert Consult: Elsevier Health Sciences. 2013. [Google Scholar]
- 28.Brown SH, Gregory DE, Carr JA, Ward SR, Masuda K, Lieber RL. ISSLS prize winner: Adaptations to the multifidus muscle in response to experimentally induced intervertebral disc degeneration. Spine (Phila Pa 1976) 2011;36(21):1728–36. doi: 10.1097/BRS.0b013e318212b44b. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann C, Kalepu R, Ponfick M, Reichel H, Cakir B, Zierz S, Gdynia HJ, Kassubek J, Ludolph AC, Rosenbohm A. Histological characterization and biochemical analysis of paraspinal muscles in neuromuscularly healthy subjects. Muscle Nerve. 2015;52(1):45–54. doi: 10.1002/mus.24490. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen K, Nicholaisen T, Kato M. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men. Significance for isometric endurance. Spine (Phila Pa 1976) 1993;18(11):1439–50. [PubMed] [Google Scholar]
- 31.Dorph C, Englund P, Nennesmo I, Lundberg IE. Signs of inflammation in both symptomatic and asymptomatic muscles from patients with polymyositis and dermatomyositis. Ann Rheum Dis. 2006;65(12):1565–71. doi: 10.1136/ard.2005.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88(4):489–95. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 33.Macaluso F, Brooks NE, van de Vyver M, Van Tubbergh K, Niesler CU, Myburgh KH. Satellite cell count, VO(2max), and p38 MAPK in inactive to moderately active young men. Scand J Med Sci Sports. 2012;22(4):e38–44. doi: 10.1111/j.1600-0838.2011.01389.x. Epub 2011/09/27. [DOI] [PubMed] [Google Scholar]
- 34.Sorarù G, D’Ascenzo C, Polo A, Palmieri A, Baggio L, Vergani L, Gellera C, Moretto G, Pegoraro E, Angelini C. Spinal and bulbar muscular atrophy: skeletal muscle pathology in male patients and heterozygous females. J Neurol Sci. 2008;264(1–2):100–5. doi: 10.1016/j.jns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 2006;31(25):2926–33. doi: 10.1097/01.brs.0000248453.51165.0b. [DOI] [PubMed] [Google Scholar]
- 36.Hodges PW, James G, Blomster L, Hall L, Schmid AB, Shu C, Little C, Melrose J. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine (Phila Pa 1976) 2014;39(13):1010–7. doi: 10.1097/BRS.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 37.Demoulin C, Crielaard J-M, Vanderthommen M. Spinal muscle evaluation in healthy individuals and low-back-pain patients: a literature review. Joint Bone Spine. 2007;74(1):9–13. doi: 10.1016/j.jbspin.2006.02.013. http://dx.doi.org/10.1016/j.jbspin.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat. 1997;190(Pt 4):505–13. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorstensson A, Carlson H. Fibre types in human lumbar back muscles. Acta Physiol Scand. 1987;131(2):195–202. doi: 10.1111/j.1748-1716.1987.tb08226.x. [DOI] [PubMed] [Google Scholar]
- 40.Latroche C, Gitiaux C, Chrétien F, Desguerre I, Mounier R, Chazaud B. Skeletal Muscle Microvasculature: A Highly Dynamic Lifeline. Physiology (Bethesda) 2015;30(6):417–27. doi: 10.1152/physiol.00026.2015. [DOI] [PubMed] [Google Scholar]
- 41.Mattila M, Hurme M, Alaranta H, Paljärvi L, Kalimo H, Falck B, Lehto M, Einola S, Järvinen M. The multifidus muscle in patients with lumbar disc herniation. A histochemical and morphometric analysis of intraoperative biopsies. Spine (Phila Pa 1976) 1986;11(7):732–8. doi: 10.1097/00007632-198609000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Zhao WP, Kawaguchi Y, Matsui H, Kanamori M, Kimura T. Histochemistry and morphology of the multifidus muscle in lumbar disc herniation: comparative study between diseased and normal sides. Spine (Phila Pa 1976) 2000;25(17):2191–9. doi: 10.1097/00007632-200009010-00009. [DOI] [PubMed] [Google Scholar]
- 43.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 2: Histologic and histochemical analyses in humans. Spine (Phila Pa 1976) 1994;19(22):2598–602. doi: 10.1097/00007632-199411001-00018. [DOI] [PubMed] [Google Scholar]