Abstract
The retinal vasculature is affected in a number of clinically important retinopathies, including diabetic retinopathy. There has been a considerable amount of research into the pathogenesis of retinal microvascular diseases, but the potential contribution of the most abundant cell population in the retina, photoreceptor cells, has been largely overlooked. This review summarizes ongoing research suggesting that photoreceptor cells play a critical role in the development of retinal vascular disease in diabetic retinopathy and in models of photoreceptor degeneration.
Keywords: retina, photoreceptors, microvasculature, opsin
Introduction
The vasculature of the retina is adversely affected in a number of retinopathies, including diabetic retinopathy, retinopathy of prematurity, and also in retinal degenerations. In retinopathies where the vasculature is regarded as the central site of pathology (such as diabetic retinopathy (DR)), a central research focus to explain the visual dysfunction has been on the vasculature itself, but the potential contribution of photoreceptor cells (which account for most of the mass and metabolic activity of the retina) to the vascular disease has been largely overlooked. In genetic diseases that cause photoreceptor degeneration, in contrast, there has been great focus on the photoreceptor damage, but little interest in the retinal vasculature, even though the photoreceptor degeneration causes retinal capillaries to become damaged or degenerate [1-7]. In recent years, evidence has begun to accumulate implicating retinal photoreceptor cells in the pathogenesis of retinal vascular degeneration. The goal of this review is to summarize recent evidence suggesting that photoreceptor cells play a critical role in the development of retinal vascular disease in mouse models by comparing DR (regarded clinically as a vascular disease) and models of photoreceptor degeneration. The discussion will focus primarily on rods, since rod photoreceptor cells are more prevalent than cones in the mammalian retina.
Retinal photoreceptor cells
Photoreceptors are the most abundant cell-type in the retina [8], and are the most metabolically active neuron in the central nervous system [9], and contain at least 75% of total retinal mitochondria [10-14]. They serve a unique function in the body, absorbing light and turning it into electrical energy that results in sight. When photoreceptors are struck by a photon of light, 11-cis retinal undergoes photoisomerization to all-trans retinal, initiating a signaling cascade that results in the closing of cGMP-gated ion channels, and hyperpolarization of the photoreceptor cell. Even though it is clearly recognized that these cells are metabolically active in light, they are active also in darkness, using ATP to maintain ion gradients. The oxygen demand in rods has been calculated to more than double during dark adaptation [15].
Photoreceptor cells and the retinal vasculature
Absorption of light by photoreceptors clearly affects the retinal vasculature under normal physiologic conditions. This is easily demonstrated by the rapid change in retinal vascular diameter after turning a light on and off (flicker) [16]. This neurovascular coupling likely shows the response of the vasculature to changes in metabolic demand by photoreceptors and other retinal cells in the presence and absence of light, although there is evidence that light can directly influence the vasculature [17].
The effect of photoreceptors on the development of vascular diseases in the retina has been less obvious. Two pieces of evidence, however, suggest an important role of retinal photoreceptor cells in the development and progression of retinal vascular disease:
i. Beneficial effects of photocoagulation
Laser photocoagulation has been known for many years to inhibit progression of advanced retinopathies including DR and age-related macular degeneration [18,19]. It has long been accepted that one mechanism by which this beneficial effect is mediated in ischemic retinas is at least in part by destroying significant numbers of photoreceptors and RPE cells, thus resulting in reduced oxygen consumption due to fewer photoreceptor cells. As a result, there is increased availability of oxygen for use by the remaining retinal cells. Since acute destruction of photoreceptor cells can inhibit the progression of these retinal vascular diseases, the data strongly suggests that photoreceptors are important in at least advanced stages of those diseases. Evidence from the ETDRS (Early Treatment of Diabetic Retinopathy Study) [20] indicates that photocoagulation has benefit also in somewhat earlier stages of DR, but how much earlier in the development of the retinopathy that photoreceptors contribute is not clear.
ii. Degeneration of the retinal vasculature in photoreceptor-specific diseases
The retinal vasculature is attenuated in genetic diseases that affect only photoreceptors, including retinitis pigmentosa [21], and degeneration of the retinal microvasculature has been detected also in mouse models of photoreceptor degeneration [2,4,7,22]. Since rhodopsin expression is unique for the photoreceptor cells, the studies in opsin-deficient or opsin-mutant animals clearly illustrate that photoreceptors can initiate dysfunction and degeneration of retinal capillaries (Fig 1). The vascular loss in retinal degenerations has escaped widespread recognition, likely because photoreceptor degeneration is more serious under those circumstances.
Fig 1.

Photomicrographs illustrating degeneration of the retinal vasculature in response to a molecular defect that occurs only in photoreceptor cells (P23H mutation of opsin). Diabetes likewise causes capillary degeneration in wildtype animals, but at a much slower pace. Samples collected at 10 months of age (8 months of diabetes). Representative degenerated retinal capillaries are identified by arrows.
The mechanism by which the absence or degeneration of retinal photoreceptor cells in diseases could cause degeneration of the retinal vasculature is only beginning to be considered. The studies by de Gooyer et al [4] showed that opsin-deficient animals (whose photoreceptors had undergone degeneration as a consequence of the opsin deficiency) developed a significant reduction in vascular density, consistent with vaso-obliteration. Liu et al [7] compared two mouse models of photoreceptor degeneration (opsin−/− and RhoP23H/P23H) and control C57Bl/5J mice, and showed that retinal capillary degeneration in the two opsin mutants was substantial while photoreceptors were still present, but slowed after the photoreceptors degenerated. Photoreceptor degeneration in the opsin mutants studied happened quickly (essentially all photoreceptors were lost by 3-4 months of age), and capillary degeneration likewise ensued rapidly in these animals, with a doubling of the number of degenerate capillaries within the first month of life [7]. Thus, it was not the absence of photoreceptors that damaged the retinal vasculature, but the presence of “sick” or stressed photoreceptors that damaged the vasculature, likely by release of soluble factors from the stressed photoreceptor cells that directly or indirectly led to the capillary degeneration.
Both of the opsin mutants developed retinal oxidative stress and activation of leukocytes, which might have contributed to the development of the retinal vascular disease. Activated leukocytes have been shown by us to cause cytotoxicity to retinal endothelial cells [23-31].
Do retinal photoreceptors contribute to vascular lesions of DR?
Merely because photoreceptor cells play a role in dysfunction and degeneration of retinal capillaries in severe degenerative retinopathies like those mentioned above, it is not necessarily certain that similar mechanisms cause vascular lesions in DR. Nevertheless, it has been recognized for years that the retinal vasculature is especially sensitive to diabetes, even in comparison to the vasculature of the embryologically similar brain (grey matter) [32]. This raises a possibility that something unique to the retina plays an important role in the pathogenesis of the spectrum of lesions regarded as characteristic of DR. Consistent with this, there is data specifically related to DR that suggests that photoreceptors play an important role in the development of at least early stages of DR.
i. Influence of photoreceptor degeneration on retinal vessel disease in diabetes
Compared to nondiabetic Rho−/− mice, de Gooyer et al [6] reported that experimentally diabetic Rho−/− mice showed a significant survival of the retinal vasculature in both the peripheral and central retina. The authors concluded that loss of the outer retina reduced the severity of DR in that model. Feng et al likewise reported that diabetes (in a ciliopathy-induced retinal degeneration rat) inhibited the vasoregression that occurred secondary to the photoreceptor degeneration [33]. In contrast, other investigators studying the effects of diabetes on the opsin-deficient mouse, as well as a different mouse having mutant opsin knocked in (RhoP23H/P23H), found that diabetes did not protect against the photoreceptor-mediated degeneration of the retinal microvasculature [7].
ii. Absence of DR in diabetic patients having also retinitis pigmentosa
Results of a survey sent to a group of diabetic patients who also had retinitis pigmentosa [34] suggested that diabetics who also had retinitis pigmentosa (and therefore, photoreceptor degeneration) were protected from the development of DR. This study lacked the photographic documentation or systematic quantitation of retinopathy that is found in clinical trials, but it does provide supportive evidence for the hypothesis that photoreceptor cells play an important role in the development of DR. The postulated mechanism for this effect is that the dark current in rods accounts for considerable utilization of energy and oxygen in the dark, making the retina borderline hypoxic at night. Therefore, elimination of some rods (as happens in retinitis pigmentosa) should reduce this metabolic stress, and thereby reduce the metabolic abnormalities that contribute to the retinopathy. Supplementing with oxygen has been reported to reduce diabetic macular edema and improve aspects of visual function in some diabetic patients [35], suggesting that oxygen insufficiency is impacting the retina in these patients [36]). Whether or not oxygen insufficiency contributes to the characteristic vascular pathology that develops early in DR is not known.
iii. Inhibitory effects of light on DR
Since there is overwhelming evidence that photoreceptor cells absorb light, studies using light to inhibit or reverse vascular lesions of DR [31,37-42] strongly suggest that photoreceptor cells are involved in the development of those retinopathies. Nevertheless, those studies do not directly demonstrate a role of photoreceptors in development of the retinopathy. Arden et al [37,43] has studied diabetic patients to determine if sleeping without total darkness (to reduce rod dark current) could improve diabetes-induced abnormalities in retinal function and retinal thickness. Patients with mild non-proliferative diabetic retinopathy and early, untreated non-sight-threatening macular edema slept for 6 months wearing masks that illuminated the eyelid of one closed eye with 505 nm light of sufficient intensity to suppress dark current in that eye. At 6 months, the number of treated eyes showing intraretinal cysts was reduced by about one third, whereas untreated fellow eyes showed almost a doubling of eyes showing intraretinal cysts. In the treated eyes, retinal thickness was significantly reduced, and visual acuity, achromatic contrast sensitivity, and microperimetric thresholds improved significantly. The authors concluded that sleeping in dim light that keeps rods light-adapted has clinical benefit. A larger study of this approach is ongoing [40]. An animal study investigating whether retinal neuronal and glial abnormalities in early stages of diabetes were alleviated by preventing the rods from dark-adapting failed to find a benefit in short-term studies [44], and the authors concluded that at least those abnormalities were not exacerbated by rod photoreceptor O2 consumption in the dark.
Kern and colleagues have been using a similar light-based approach (referred to as photobiomodulation) to inhibit early DR, except that they have used far-red light (670 nm) for only 3-4 minutes per day. Animal studies using albino rats and pigmented mice showed that whole-body photobiomodulation inhibited diabetes-induced abnormalities in retinal oxidative stress, inflammation, and electroretinogram [31,38], and very effectively inhibited diabetes-induced increases in capillary degeneration and accumulation of albumin in neural retina (unpublished). In a case report series of diabetic patients having non-center involved retinal edema, application of the same wavelength of light for an equally short interval daily showed beneficial effects to reverse the edema in the treated eye [39].
A general conclusion of the studies described above is that metabolic stress that develops within photoreceptors in certain diseases can contribute to the damage or degeneration of retinal capillaries, and that the absence of photoreceptors can inhibit this capillary damage. In contrast, evidence has been reported recently that comes to the opposite conclusion [45,46]. Using Optical Coherence Tomography-Angiography on a small number of patients with diabetic retinopathy, investigators found that macular photoreceptor disruption corresponded with areas of capillary nonperfusion in the deep retinal vasculature. The authors interpreted this as indicating that disruption of the deep retinal vasculature might contribute to photoreceptor degeneration. Why the deep retinal vasculature would have such a strong effect on photoreceptor survival seems puzzling, since available evidence has indicated that the choriocapillaris, and not the retinal vasculature, provides photoreceptors with most their oxygen and nutrients.
How might photoreceptor cells contribute to vascular damage in the retina?
i. Hypoxia
It is well known that photoreceptors account for much of the oxygen consumed by the retina, and that this metabolism is increased during the dark [47,48], when the rod dark current becomes maximal [49-51]. In the dark, oxygen consumption is greater than any other cell in the retina [52]. Arden and coworkers provided evidence that the metabolic activity of photoreceptors substantially affects the health and function of nearby retinal cells, especially in the dark [36,37,53], and reported that the oxygen supply of the healthy retina is barely adequate in the dark. Diseases might further impair oxygenation or health of the retina by any of a variety of mechanisms. One potential mechanism might involve expression of hypoxia-inducible factor (HIF)1α, which is a known master transcriptional regulator of a variety of relevant cellular processes including vascularization and angiogenesis, energy metabolism, cell survival, as well as tumor invasion. de Gooyer et al [4] showed that hypoxia and expression of HIF1α and VEGF were significantly reduced in opsin−/− mice compared to wildtype mice, consistent with the postulate that the appreciable metabolism of photoreceptors under wildtype conditions can induce retinal hypoxia, and the absence of those photoreceptors reduced local oxygen consumption (and thus hypoxia and factors induced by it). The extent to which the hypothesis that hypoxia drives the development also of early DR (before the vasculature is compromised) requires additional study [54], but it is interesting that the extensive capillary degeneration observed opsin-deficient animals by both de Gooyer [6] and Liu [7] developed despite the apparent absence of hypoxia [4].
ii. Oxidative stress and induction of inflammatory proteins
Oxidative stress is a characteristic molecular abnormality in of the retina in diabetes, and inhibition of that oxidative stress by feeding antioxidants or overexpressing antioxidant enzymes has been shown to inhibit development of local retinal inflammation and subsequent vascular lesions of early DR [55-58]. Several years ago, we reported that photoreceptor cells are the major site of that oxidative stress in the retina in diabetes [59], and experimental or genetic degeneration of those photoreceptors inhibited the diabetes-induced oxidative stress and upregulation of pro-inflammatory proteins. Consistent with the theories of Arden and colleagues [37,41,53], the presence or absence of light affects the retinal oxidative stress, resulting in the retinal oxidative stress caused by diabetes being worse in the dark [59]. Photoreceptors express NADPH oxidase [60] and contain most of the mitochondria found in the retina [61], and both of these can be sources of reactive oxygen species [59,62,63].
Inflammation processes likewise have been implicated in the development of vascular lesions of early DR [23-27,64]. With regard to contribution of photoreceptor cells to the induction of pro-inflammatory cells in the retina in diabetes, laser microdissection to isolate photoreceptor cells away from remaining retinal cells showed that diabetes increased mRNA for inflammatory proteins such as iNOS and COX2 only in photoreceptors (and not in the inner retina), demonstrating that induction of at least some inflammatory proteins within the retina in diabetes occurs in photoreceptors. Expression of ICAM-1, in contrast, was induced by diabetes in both the inner retina and photoreceptor cells [65].
iii. Release of soluble factors that activate nearby cells to damage the vasculature
Accumulating in vitro and ex vivo evidence demonstrates that products released from photoreceptors under diabetes-like conditions can alter nearby cells. In vitro studies showed that incubation of photoreceptor-like (661W) cells in elevated glucose led to the release of soluble factors that caused induction of TNFα production by leukocytes and endothelial cells [65]. Likewise, using freshly isolated sheets of retinal photoreceptor cells [65], we are finding that photoreceptors from diabetic mice (and Rho−/− mice) release numerous cytokines and other products into the media in greater amounts than do photoreceptors from normal control mice (unpublished). We postulate that the metabolic stress of diabetes (or other abnormalities) causes retinal photoreceptor cells to the release soluble factors which contribute to the damage of retinal microvessels, in part by activating nearby cells such as endothelial cells and circulating leukocytes. We have previously reported that leukocytes from diabetic patients or mice or from opsin mutant mice become activated to kill retinal endothelial cells [7,23,26,28,29], and diabetic animals in whom photoreceptors degenerated many months before no longer show the leukocyte-mediated killing of retinal endothelial cells (Fig 2).
Fig 2.

Photoreceptor cells contribute to leukocyte-mediated killing of retinal endothelial cells. Leukocytes from wildtype (WT) mice diabetic for 8 months were co-cultured with mouse retinal endothelial cells. Leukocytes from WT diabetic mice caused more endothelial cell death than did leukocytes from WT nondiabetic animals. Leukocytes from opsin−/− animals lacking photoreceptor cells who were likewise diabetic for the same duration were protected from the diabetes-induced increase in leukocyte-mediated cytotoxicity to retinal endothelial cells.Reprinted without change from Tonade, Liu; and Kern (2016) Photoreceptor Cells Produce Inflammatory Mediators That Contribute to Endothelial Cell Death in Diabetes, Invest Ophthalmol Vis Sci, .57, 4264-4271. Creative Commons Attribution (CC BY) license
Photoreceptor cells and retinal neovascularization
Retinal photoreceptor cells have long been accepted to play a major role in retinal neovascularization in ischemic diseases such as DR and oxygen-induced retinopathy, as a result of their substantial metabolic activity consuming the limited amounts of oxygen. A recent study of Vldlr−/− mice showed that dysregulated lipid and glucose metabolism within photoreceptors leads to stabilization of HIF1α in the absence of hypoxia, with resulting induction of VEGF and invasion of the normally avascular photoreceptor layer by new vessels originating from the retinal vasculature [66]. Over-expression of VEGF in photoreceptor cells also causes neovascularization into the photoreceptor layer [67]. Thus, this data provides further evidence that retinal photoreceptor cells influence the retinal vasculature also in a stimulatory manner, potentially resulting in neovascularization in some diseases.
Retinal pigment epithelium
This review has focused on photoreceptors, but the RPE has an important influence on photoreceptors, thus potentially making the RPE a novel therapeutic target to inhibit photoreceptor-mediated damage to the retinal vasculature. Retinylamine is a retinoid that inhibits RPE65 in the RPE, and has been found to significantly inhibit the diabetes-induced oxidative stress, induction of inflammatory proteins, capillary permeability and capillary degeneration that are characteristic of DR [29]. Because RPE65 is part of the visual cycle that regenerates retinoids, this study raises a possibility also that the visual cycle might play a previously unappreciated role in the pathogenesis of DR. The possibility that retinylamine might interact also with retinoid receptors requires further study.
Future directions
Available evidence provides strong support for the concept that photoreceptors play an important role in the pathogenesis of retinal vascular pathology in a variety of different retinal diseases. The mechanisms known at present are summarized in Fig 3.
Fig 3.
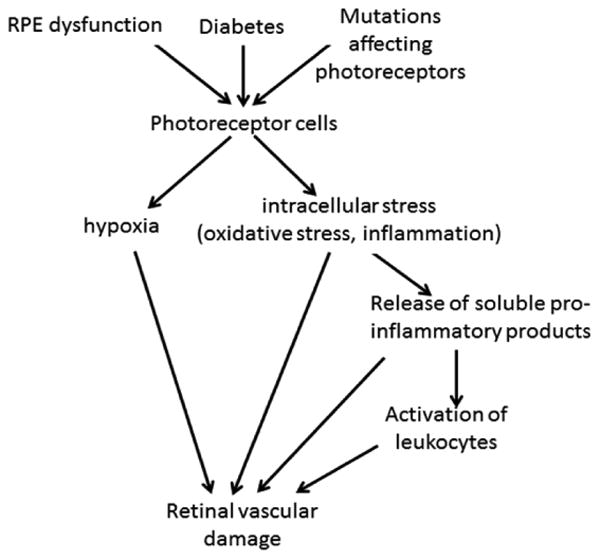
Summary of postulated roles of photoreceptors in the development of dysfunctional and degenerating retinal capillaries.
In order to more firmly establish the role of photoreceptors in the pathogenesis of retinal vascular diseases such as DR, modern molecular approaches, such as photoreceptor-specific gene knockouts of relevant enzymes will be useful. Very important for further investigation, however, are the therapeutic opportunities of using photoreceptor cells as targets at which to inhibit the development of diseases such as DR. Potential approaches include the use of light (photobiomodulation) or agents that affect photoreceptors by direct or indirect means, including retinylamine, and agonists and antagonists of G-protein coupled receptors [28] (which are known to be prevalent in photoreceptors). Administration of other retinaldehydes has corrected diabetes-induced photoreceptor dysfunction (including subnormal dark-adapted rod photoresponses, rod-dominated light-stimulated expansion of the subretinal space, and cone-dominated contrast sensitivity), potentially via an antioxidant mechanism [68]. The extent to which the visual cycle contribute to the retinopathy, and the possibility that light-initiated phototransduction or subsequent changes in photoreceptor ion channel activities also might contribute to the molecular processes leading to DR merit further investigation.
Acknowledgments
This work was supported by NIH grants EY00300, EY022938 and R24 EY024864, and a Merit grant from the Department of Veteran Affairs.
Literature cited
- 1.Heegaard S, Rosenberg T, Preising M, Prause JU, Bek T. An unusual retinal vascular morphology in connection with a novel AIPL1 mutation in Leber's congenital amaurosis. Br J Ophthalmol. 2003;87:980–983. doi: 10.1136/bjo.87.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn JS, Li S, Naash MI. Ambient hypoxia reverses retinal vascular attenuation in a transgenic mouse model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:4007–4013. [PubMed] [Google Scholar]
- 3.Fernandez-Sanchez L, Lax P, Esquiva G, Martin-Nieto J, Pinilla I, et al. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS One. 2012;7:e43074. doi: 10.1371/journal.pone.0043074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Curtis TM, et al. Rod photoreceptor loss in Rho−/− mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest Ophthalmol Vis Sci. 2006;47:5553–5560. doi: 10.1167/iovs.06-0646. [DOI] [PubMed] [Google Scholar]
- 5.Pennesi ME, Nishikawa S, Matthes MT, Yasumura D, LaVail MM. The relationship of photoreceptor degeneration to retinal vascular development and loss in mutant rhodopsin transgenic and RCS rats. Exp Eye Res. 2008;87:561–570. doi: 10.1016/j.exer.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, et al. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci. 2006;47:5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Tang J, Du Y, Saadane A, Tonade D, et al. Photoreceptor Cells Influence Retinal Vascular Degeneration in Mouse Models of Retinal Degeneration and Diabetes. Invest Ophthalmol Vis Sci. 2016;57:4272–4281. doi: 10.1167/iovs.16-19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 9.Ames A, 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demontis GC, Longoni B, Marchiafava PL. Molecular steps involved in light-induced oxidative damage to retinal rods. Invest Ophthalmol Vis Sci. 2002;43:2421–2427. [PubMed] [Google Scholar]
- 11.Yang JH, Basinger SF, Gross RL, Wu SM. Blue light-induced generation of reactive oxygen species in photoreceptor ellipsoids requires mitochondrial electron transport. Invest Ophthalmol Vis Sci. 2003;44:1312–1319. doi: 10.1167/iovs.02-0768. [DOI] [PubMed] [Google Scholar]
- 12.Krizaj D, Copenhagen DR. Calcium regulation in photoreceptors. Front Biosci. 2002;7:d2023–2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgans CW, El Far O, Berntson A, Wassle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. J Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JE, Jr, Perkins GA, Giddabasappa A, Chaney S, Xiao W, et al. Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol Vis. 2007;13:887–919. [PMC free article] [PubMed] [Google Scholar]
- 15.Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007;293:H1696–1704. doi: 10.1152/ajpheart.00221.2007. [DOI] [PubMed] [Google Scholar]
- 16.Kern TS. Interrelationships between the Retinal Neuroglia and Vasculature in Diabetes. Diabetes Metab J. 2014;38:163–170. doi: 10.4093/dmj.2014.38.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci U S A. 2014;111:17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham CE, Binz N, Shen WY, Constable IJ, Rakoczy EP. Laser photocoagulation: ocular research and therapy in diabetic retinopathy. Adv Exp Med Biol. 2006;572:195–200. doi: 10.1007/0-387-32442-9_29. [DOI] [PubMed] [Google Scholar]
- 19.Virgili G, Bini A. Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004763.pub2. CD004763. [DOI] [PubMed] [Google Scholar]
- 20.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:766–785. [PubMed] [Google Scholar]
- 21.Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Wang Y, Yang Z, Wu L, Hoffmann S, et al. Chronic hyperglycemia inhibits vasoregression in a transgenic model of retinal degeneration. Acta Diabetol. 2014;51:211–218. doi: 10.1007/s00592-013-0488-4. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Veenstra AA, Talahalli RR, Wang X, Gubitosi-Klug RA, et al. Marrow-derived cells regulate the development of early diabetic retinopathy and tactile allodynia in mice. Diabetes. 2012;61:3294–3303. doi: 10.2337/db11-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talahalli R, Zarini S, Tang J, Li G, Murphy R, et al. Leukocytes regulate retinal capillary degeneration in the diabetic mouse via generation of leukotrienes. J Leukoc Biol. 2013;93:135–143. doi: 10.1189/jlb.0112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang J, Allen Lee C, Du Y, Sun Y, Pearlman E, et al. MyD88-dependent pathways in leukocytes affect the retina in diabetes. PLoS One. 2013;8:e68871. doi: 10.1371/journal.pone.0068871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian P, Ge H, Liu H, Kern T, Du L, et al. Leukocytes from diabetic patients kill retinal endothelial cells: effects of berberine. Mol Vision. 2013;19:2092–2105. [PMC free article] [PubMed] [Google Scholar]
- 27.Veenstra AA, Tang J, Kern TS. Antagonism of CD11b with Neutrophil Inhibitory Factor (NIF) inhibits vascular lesions in diabetic retinopathy. PLoS One. 2013;8:e78405. doi: 10.1371/journal.pone.0078405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y, Cramer M, Lee CA, Tang J, Muthusamy A, et al. Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability. FASEB J. 2015;29:2194–2204. doi: 10.1096/fj.14-269431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Tang J, Du Y, Lee CA, Golczak M, et al. Retinylamine Benefits Early Diabetic Retinopathy in Mice. J Biol Chem. 2015;290:21568–21579. doi: 10.1074/jbc.M115.655555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Tang J, Lee CA, Kern TS. Metanx and early stages of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:647–653. doi: 10.1167/iovs.14-15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saliba A, Du Y, Liu H, Patel S, Roberts R, et al. Photobiomodulation Mitigates Diabetes-Induced Retinopathy by Direct and Indirect Mechanisms: Evidence from Intervention Studies in Pigmented Mice. PLoS One. 2015;10:e0139003. doi: 10.1371/journal.pone.0139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kern TS, Engerman RL. Capillary lesions develop in retina rather than cerebral cortex in diabetes and experimental galactosemia. Arch Ophthalmol. 1996;114:306–310. doi: 10.1001/archopht.1996.01100130302013. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Wang Y, Stock O, Pfister F, Tanimoto N, et al. Vasoregression linked to neuronal damage in the rat with defect of polycystin-2. PLoS One. 2009;4:e7328. doi: 10.1371/journal.pone.0007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arden GB. The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. Br J Ophthalmol. 2001;85:366–370. doi: 10.1136/bjo.85.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, et al. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004;45:617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 36.Arden GB, Sivaprasad S. The pathogenesis of early retinal changes of diabetic retinopathy. Doc Ophthalmol. 2012;124:15–26. doi: 10.1007/s10633-011-9305-y. [DOI] [PubMed] [Google Scholar]
- 37.Arden GB, Jyothi S, Hogg CH, Lee YF, Sivaprasad S. Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye (Lond) 2011;25:1546–1554. doi: 10.1038/eye.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang J, Du Y, Lee CA, Talahalli R, Eells JT, et al. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Invest Ophthalmol Vis Sci. 2013;54:3681–3690. doi: 10.1167/iovs.12-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, Herda AA, Kern TS. Photobiomodulation in the treatment of patients with non-center- involving diabetic macular oedema. Br J Ophthalmol. 2014 doi: 10.1136/bjophthalmol-2013-304477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sivaprasad S, Arden G, Prevost AT, Crosby-Nwaobi R, Holmes H, et al. A multicentre phase III randomised controlled single-masked clinical trial evaluating the clinical efficacy and safety of light-masks at preventing dark-adaptation in the treatment of early diabetic macular oedema (CLEOPATRA): study protocol for a randomised controlled trial. Trials. 2014;15:458. doi: 10.1186/1745-6215-15-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivaprasad S, Arden G. Spare the rods and spoil the retina: revisited. Eye (Lond) 2015 doi: 10.1038/eye.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsey DJ, Arden GB. Hypoxia and Dark Adaptation in Diabetic Retinopathy: Interactions, Consequences, and Therapy. Curr Diab Rep. 2015;15:118. doi: 10.1007/s11892-015-0686-2. [DOI] [PubMed] [Google Scholar]
- 43.Arden GB, Gunduz MK, Kurtenbach A, Volker M, Zrenner E, et al. A preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye (Lond) 2010;24:1149–1155. doi: 10.1038/eye.2009.328. [DOI] [PubMed] [Google Scholar]
- 44.Kur J, Burian MA, Newman EA. Light adaptation does not prevent early retinal abnormalities in diabetic rats. Sci Rep. 2016;6:21075. doi: 10.1038/srep21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarinci F, Jampol LM, Linsenmeier RA, Fawzi AA. Association of Diabetic Macular Nonperfusion With Outer Retinal Disruption on Optical Coherence Tomography. JAMA Ophthalmol. 2015;133:1036–1044. doi: 10.1001/jamaophthalmol.2015.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scarinci F, Nesper PL, Fawzi AA. Deep Retinal Capillary Non-perfusion is Associated with Photoreceptor Disruption in Diabetic Macular Ischemia. Am J Ophthalmol. 2016 doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arden GB, Wolf JE, Tsang Y. Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vision Res. 1998;38:1723–1729. doi: 10.1016/s0042-6989(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 48.Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. Br J Ophthalmol. 2005;89:764–769. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haugh LM, Linsenmeier RA, Goldstick TK. Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann Biomed Eng. 1990;18:19–36. doi: 10.1007/BF02368415. [DOI] [PubMed] [Google Scholar]
- 51.Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Birol G, Budzynski E, Flynn R, Linsenmeier RA. Metabolic responses to light in monkey photoreceptors. Curr Eye Res. 2010;35:510–518. doi: 10.3109/02713681003597255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 54.Xu Z, Wei Y, Gong J, Cho H, Park JK, et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57:204–213. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 56.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 57.Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R. Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest Ophthalmol Vis Sci. 2009;50:2351–2358. doi: 10.1167/iovs.08-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng L, Kern T. Role of nitric oxide, superoxide, peroxynitrite and poly(ADP-ribose) polymerase in diabetic retinopathy. Front Biosci. 2009;14:3974–3987. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 59.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013;110:16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SG, Lee CG, Yun IH, Hur DY, Yang JW, et al. Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin Experiment Ophthalmol. 2012;40:e47–57. doi: 10.1111/j.1442-9071.2011.02695.x. [DOI] [PubMed] [Google Scholar]
- 61.Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci. 2002;19:395–407. doi: 10.1017/s0952523802194028. [DOI] [PubMed] [Google Scholar]
- 62.Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, et al. TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia. 2014;57:1047–1056. doi: 10.1007/s00125-014-3194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos JM, Tewari S, Lin JY, Kowluru RA. Interrelationship between activation of matrix metalloproteinases and mitochondrial dysfunction in the development of diabetic retinopathy. Biochem Biophys Res Commun. 2013;438:760–764. doi: 10.1016/j.bbrc.2013.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tonade D, Liu H, Kern TS. Photoreceptor Cells Produce Inflammatory Mediators That Contribute to Endothelial Cell Death in Diabetes. Invest Ophthalmol Vis Sci. 2016;57:4264–4271. doi: 10.1167/iovs.16-19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med. 2016;22:439–445. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Eeden PE, Tee LB, Lukehurst S, Lai CM, Rakoczy EP, et al. Early vascular and neuronal changes in a VEGF transgenic mouse model of retinal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4638–4645. doi: 10.1167/iovs.06-0251. [DOI] [PubMed] [Google Scholar]
- 68.Berkowitz BA, Kern TS, Bissig D, Patel P, Bhatia A, et al. Systemic Retinaldehyde Treatment Corrects Retinal Oxidative Stress, Rod Dysfunction, and Impaired Visual Performance in Diabetic Mice. Invest Ophthalmol Vis Sci. 2015;56:6294–6303. doi: 10.1167/iovs.15-16990. [DOI] [PMC free article] [PubMed] [Google Scholar]


