Abstract
Introduction
Tranexamic acid (TXA) administration following trauma has not been proven to improve survival in the US. Trauma patients present to the hospital with a spectrum of fibrinolytic activity, in which a physiologic levels of fibrinolysis are associated with the lowest mortality. We hypothesize that trauma patients who present to the hospital with physiologic levels of fibrinolysis will have increased mortality if they receive TXA.
Material and Methods
Severely injured trauma patients followed prospectively from 2014 to 2016 we included in the analysis. The patient’s first thrombelastography (TEG) was used to stratify patients into fibrinolysis phenotypes which included fibrinolysis shutdown, physiologic fibrinolysis, and systemic hyperfibrinolysis. The primary outcome was in hospital mortality.
Results
232 patients were analyzed (11% received TXA) with an overall mortality rate of 20%. TXA administration was associated with a higher new injury severity score (NISS 49 vs 28 p=0.001) massive transfusion rate (69% vs 12% p<0.001) and mortality (52% vs 17% p<0.001). Hyperfibrinolysis and shutdown had higher mortality rates than physiologic (24% vs 30% vs 14% p=0.050). The effect of TXA within phenotypes, was not significant for shutdown (28% vs 38% p=0.604) but was significant in the physiologic group (11% vs 63% p<0.001) and systemic hyperfibrinolysis (19% vs 55% p=0.023). After adjusting for NISS, TXA remained a significant predictor of mortality for patients with physiologic fibrinolysis (p=0.018).
Conclusion
There was no clear benefit of receiving TXA in this study, and patients who present to the hospital with physiologic levels of fibrinolysis, who received TXA, had the highest mortality. The role of TXA in mature trauma systems remains unclear, and emerging data supports it may have adverse effects.
Keywords: Tranexamic acid, Fibrinolysis, Trauma, Trauma Induced Coagulopathy, Fibrinolysis Shutdown
Introduction
Trauma patients present to the hospital with a diverse range of injury patterns and degree of shock which results in a spectrum of fibrinolytic activity (1). A moderate level of fibrinolytic activity is associated with improved survival (2) while high levels of fibrinolysis (hyperfibrinolysis) are associated with increased mortality rates attributed to hemorrhagic death (3–5). Tranexamic acid is an anti-fibrinolytic medication that was associated with a reduction in mortality following trauma in the CRASH-2 trial(6). The protective role of TXA to reverse hyperfibrinolysis in trauma is suggested by a retrospective analysis of TXA use in severely injured patients in which patients in shock benefited from this medication (7), a risk factor for hyperfibrinolysis(1). TXA in elective cardiac (8) and orthopedic(9) surgery has also been associated with a reduction in blood product transfusion.
However, fibrinolysis inhibition (shutdown) after injury is associated with increased mortality(1, 2) and patients that remain in fibrinolysis shutdown for a week following injury have an even higher rate of mortality(10). The protective effect of fibrinolysis to maintain microvasculature patency has previously been demonstrated in animals in near lethal shock(11). Moreover, the potential dangers of blocking fibrinolysis with TXA were recently reported from the Miami group in which there was a two-fold increase in mortality when TXA was used non-selectively (12). It also remains perplexing that there is no benefit of goal-directed TXA use in trauma patients who are hyperfibrinolytic(13). The question remains which patients benefit from receiving this medications, and a selective use has been advocated (14).
The effects of TXA and impact of mortality when taking into consideration a patient’s fibrinolytic phenotype have not been assessed previously beyond hyperfibrinolysis (13). While our trauma center emphasizes thrombelastography (TEG) to guide the administration of TXA, clinicians often administer this medication before coagulation tests results are available. As fibrinolysis appears to have a protective effect following severe injury, we hypothesize that TXA in patients with a physiologic level of fibrinolysis will have an increase in mortality compared to other fibrinolytic phenotypes.
Methods
Patient population
Adult trauma patients (age> 18 years) meeting criteria for the highest level of activation at our level 1 trauma center (Denver Health Medical Center/University of Colorado) from 2014 to 2016 with a new injury severity score (NISS) >15 were included in this analysis. All patients had samples collected under protocols approved by the Colorado Multiple Institutional Review Board for prospective evaluation of coagulation in response to trauma. Patient demographics, injury mechanism, laboratory results, and transfusion requirements were recorded by professional research assistants (PRA) who provide onsite, continuous coverage of the emergency department (ED).
Blood collection
Blood was collected in 3.5-mL tubes containing 3.2% citrate in the ambulance or upon arrival to the ED. Pre-hospital or emergency department (ED) healthcare workers drew study patient blood samples concurrently with the first set of blood samples used for in-hospital laboratory analysis. PRAs performed TEG assays within 2 hours of blood draw. TEGs were drawn for research purposes and not used for clinical management.
Outcomes
PRAs collected all clinical and demographic data on patients from the time they arrived to the ED until discharge or death. Blood transfusion requirements and TXA administration were recorded in the ED to 1, 1 to 2, 2 to 4, 5 to 6, 6 to 12, and 12 to 24 hour intervals from injury. Delayed use of TXA was categorized as TXA used from the 4–6 hour time interval or later. Massive transfusion was defined as >10 units RBC within 6 hours of injury. Mortality was determined during hospitalization, in addition to time from TXA administration to death, and cause of mortality.
Thrombelastography Assays
Viscoelastic assays were completed by a team of trained PRA with extensive experience in multiple types of TEG assays. Citrated blood samples were analyzed using the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics Corp., Niles, IL, USA). The following indices were obtained from the tracings of the TEG: reaction time (R-time min.), angle (°), maximum amplitude (MA [mm]), and lysis 30 min after MA (LY30 [%]). Fibrinolysis phenotypes were based on LY30 and previously reported ranges of phenotypes (1); shutdown LY30 <0.9%, physiologic 0.9–2.9%, and hyperfibrinolysis >2.9%.
Statistical Analysis
SPSS version 23 (IBM, Armonk, NY, USA) was used for statistical analysis. TEG measurements and clinical variables are presented as median and interquartile values. Patients who received TXA were contrasted to patients who did not receive TXA with Mann-Whitney U test for continuous variables and chi square or Fisher’s exact test for dichotomous variables. Fibrinolysis phenotypes characteristics were contrasted with a Kruskall-Wallis test for continuous variables or chi square/Fishers’s exact test for categorical. Alpha was set to 0.05 for all tests. Logistic regression analysis was used to adjust for injury severity between phenotypes. Additional variables to adjust for confounding were not feasible due to limited number of mortalities within phenotypes.
Results
Patient population
During the study time period 232 consecutive trauma activations with a NISS >15 were encountered. The patients were predominantly male (78%), young adults (median 33 years 26–49) with 33% sustaining penetrating injuries, and a median NISS of 33 (IQR 22–47). There were 43 (19%) patients who underwent a massive transfusion, and the overall mortality rate was 20%. TXA was administered in 11% (n=26) of these patients. A second drip of TXA was administered in 7 patients who received their first bolus of TXA without obtaining a second TEG before initiation of the TXA drip. Variables contrasting patients who received TXA versus no TXA are presented in Table 1. Those patients who received TXA were overall more severely injured (NISS 49 vs. 28 p=0.001) with a higher rate of massive transfusion (69% vs, 12% p<0.001) and mortality (50% vs. 17% p<0.001).
Table 1.
Patients Receiving TXA Compared to No TXA
| No TXA (n=206) | TXA (n=26) | P | |
|---|---|---|---|
| Age | 34(27–49) | 27 (24–54) | 0.214 |
| Male | 77% | 85% | 0.362 |
| Penetrating | 32% | 42% | 0.377 |
| NISS | 29 (22–43) | 48 (29–57) | 0.001 |
| INR | 1.2 (1.1–1.3) | 1.4 (1.2–1.8) | <0.001 |
| Plt Count | 255 (209–305) | 166 (133–237) | <0.001 |
| TEG- ACT | 121 (113–132) | 128 (121–136) | 0.010 |
| TEG- Angle | 71 (66–75) | 63 (60–72) | 0.003 |
| TEG- MA | 62 (57–67) | 54 (48–59) | >0.001 |
| TEG-LY30 | 1.9 (1.0–2.9) | 2.1 (0.6–9.4) | 0.324 |
| Massive Transfusion | 12% | 69% | <0.001 |
| Mortality | 17% | 50% | <0.001 |
Fibrinolysis Phenotype and TXA Administration
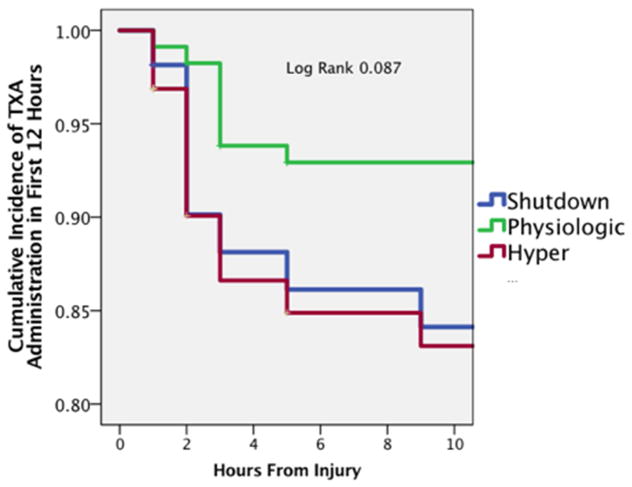
Physiologic fibrinolysis was the most common phenotype in this patient population (49%) followed by hyperfibrinolysis (28%) and shutdown (23%). TXA was more commonly administered in hyperfibrinolytic (16%) and shutdown patients (15%) than physiologic (6%), but this administration was not significantly different overall (p=0.143). Patients in shutdown and hyperfibrinolysis receiving TXA more frequently and earlier (Log rank p=0.087 Figure 1). However, in patients who received TXA, the delayed use of TXA was not significantly different between phenotypes (shutdown 25% physiologic 13% hyperfibrinolysis 20% p=0.999).
Figure 1. Timing of TXA per Phenotype.
Figure 1 the temporal association of the administration of TXA after injury is shown stratified by fibrinolysis phenotype. The Y axis represents the cumulative incidence of patients that received TXA, while the X axis represents time from injury until the patient received this medication. No patient received TXA beyond 9 hours from injury.
Blood Product Utilization and TXA
When evaluating patients who required a massive transfusion (n=25 no TXA vs. 17 TXA), patients given TXA received more red blood cells (p=0.036), plasma (p=0.003), and cryoprecipitate (p=0.026) within 24 hours of injury (Table 2). Overall, TXA was not associated with improvement in mortality, when given to patients who required a massive transfusion (non-TXA 40% vs TXA 61% p=0.346) despite different, but not significant, NISS between groups [no TXA 38 (34–50) vs TXA 50 (40–57) p=0.104]. The median number of red blood cells transfused before or concurrent with TXA use in resuscitation was 9 units (4–16), with a median of 5 units of plasma (3–8) and the majority of patients had not received platelets or cryoprecipitate. Total blood products administered after TXA (until 24 hours after injury) was a median of 2 units of RBC (0–24), 6 units of plasma (0–15), and 1 of unit platelets (0–4), and the majority of patients did not receive cryoprecipitate. However, of the patients who received TXA, 26% went on to require another massive transfusion (10 or more units of RBC within 24 hours of injury after TXA) or were dead within 24 hours of injury. The percent of deaths associated with hemorrhage was higher in patients who received TXA (55% vs. 23% p=0.060) but no differences could be assessed within phenotypes due to a low overall number of deaths due to hemorrhage.
Table 2.
Blood Product use in Patients Undergoing MT
| No-TXA (n=25) | TXA (n=18) | P | |
|---|---|---|---|
| RBC | 19 (13–28) | 28 (17–51) | 0.036 |
| FFP | 8 (6–15) | 15 (14–28) | 0.003 |
| PLT | 2 (1–3) | 3 (2–9) | 0.124 |
| CRYO | 0 (0–2) | 2 (1–4) | 0.024 |
| NISS | 38 (34–50) | 50 (39–57) | 0.104 |
| Mortality | 40% | 61% | 0.346 |
TXA Impact on Outcomes
The overall mortality was different between phenotypes (p=0.050); hyperfibrinolysis (24%) and shutdown (30%) had higher mortality rates than physiologic (14%). When patients were stratified by TXA vs. non-TXA, non-TXA patients had improved survival (hyperfibrinolysis 19% vs. shutdown 28% vs. physiologic 11% p=0.023) compared to no difference in TXA patients (hyperfibrinolysis 56% vs. shutdown 38% vs. physiologic 63% p=0.585). The effect of TXA within phenotypes, was not significant for shutdown (28% vs. 38% p=0.604) but was significant in the physiologic group (11 vs. 63% p<0.001 Figure 2) and hyperfibrinolysis (19% vs. 55% p=0.023). After adjusting for injury severity (NISS) TXA remained a significant predictor of mortality for patients with physiologic levels of fibrinolysis (p=0.018) but was no longer significant for hyperfibrinolysis (p=0.116) and unchanged with shutdown (0.597). When evaluating patients who received at least one unit of blood (n=120), the same association of increased mortality in patients who received TXA in physiologic fibrinolysis (p=0.024 Figure 3) but not shutdown (p=0.999) or hyperfibrinolysis (p=0.245). Patients that received a second dose of TXA as a bolus had a morality rate of 43% which was not significantly different that the overall TXA cohort (p=0.0665). In the 13 patients who died after TXA use, 10 clinical TEGs was ordered before TXA was administered and available in the patient’s medical record. In this cohort there were only 3 patients with evidence of hyperfibrinolysis on their clinical TEG. In these remaining patients 7 patients TXA was given before completion of LY30 and was ordered most commonly by the trauma surgeon (n=5), 1 time by neurosurgery, and 1 time by anesthesia.
Figure 2. TXA Effect on Mortality between Phenotypes.
*=P<0.05; Hyper = hyperfibrinolysis
Figure 2 The non-adjusted association between phenotypes and mortality contrasting the effects of tranexamic acid is shown. The Y axis represents the percent mortality and X axis are fibrinolysis phenotypes. In the shutdown phenotype there were 54 patients (8 received TXA), in the physiologic phenotype there were 114 patients (8 received TXA), and in the hyperfibrinolytic phenotype there were 64 patients (10 received TXA).
Figure 3. TXA Effect on Mortality between Phenotypes in Patients Requiring Blood Product Resuscitation.
*=P<0.05; Hyper = hyperfibrinolysis
Figure 3 The association between fibrinolytic phenotypes and mortality contrasting the effects of tranexamic acid on patients required blood products during resuscitation is illustrated. The Y axis represents the percent mortality and X axis are fibrinolysis phenotypes. In the shutdown phenotype there were 33 patients (8 received TXA), in the physiologic phenotype there were 48 patients (8 received TXA), and in the hyperfibrinolytic phenotype there were 39 patients (10 received TXA).
Discussion
Despite our institution’s protocol of LY30 to guide TXA administration, there were similar percentages of patients with their first TEG reflective of hyperfibrinolysis or shutdown who received this medication, with the lowest number of patients with physiologic levels of fibrinolysis. This administration of TXA without TEG data is likely due to the obligatory time to obtain the LY30 read out, which is nearly an hour after blood is assayed in the Haemonetics 5000 device. In patients who did not receive TXA, physiologic fibrinolysis was associated with the lowest mortality rate. However, TXA was associated with increased mortality in this group, with no effect on shutdown patients, consistent with the hypothesis that a moderate level of fibrinolysis is protective after injury. After adjusting for injury severity, TXA was associated with increased mortality in trauma patients who presented to the hospital in a physiologic state of fibrinolysis, which also persisted when only including patients who received a blood transfusion within 24 hours of injury. TXA was not associated with an overall improvement in mortality in patients whom underwent a massive transfusion. Within the patients who received TXA, bleeding remained the most common cause of death.
Our findings are consistent with the report from the Houston group (13) that TXA did not improve survival in patients who presented with hyperfibrinolysis, and have perhaps identified a population of patients who are harmed by this medications, as described by the Miami group (12). Our results are concerning in that the use of this medication does not appear to be reflective of the patient’s fibrinolytic status, rather their physiologic status. While the temptation to inhibit fibrinolysis in an actively bleeding patient seems rational, it may have lethal consequences. The protective role of fibrinolysis has been proven in animal models in the 1960’s. Hardaway demonstrated that irreversible shock was associated with impaired fibrinolysis (15), a process that could be reversed by increasing fibrinolysis (16).
TXA use during massive transfusion was associated with higher overall blood product use compared to those patients who did not receive TXA, but received a MT, and a 26% rate of needing an additional 10 or more units of blood after the medication was given or death within 24 hours of injury. While TXA can reduce plasma fibrinolytic activity within 30 minutes, specific organs can paradoxically have increased fibrinolytic activity early after the medication is given, with some organs having sustained inhibition after plasma levels have appeared to normalize within 90 minutes after administration (17). Thus, administration of this medication may paradoxically increase organ specific bleeding, while causing fibrinolysis shutdown in others. Given that systemic inhibition of fibrinolysis appears to have adverse effects, a role of topical TXA, which in orthopedic surgery has the same efficacy of reducing blood products (18), would be worthy of investigation in future studies.
Alternatives resuscitation strategies can attenuate fibrinolysis in a more physiologic manner, such as plasma first resuscitation. Plasma is an effective buffer of fibrinolytic activity (19) and effectively reverses hyperfibrinolysis and reduces mortality in hyperfibrinolytic rodents (20). This effective colloid also has been shown to enhance metabolic recovery (21) from hemorrhagic shock, restore the endothelial glycoalyx (22), reduce pulmonary injury (23) and reduce gastrointestinal damage (24). Additional blood products that impact fibrinolysis include cryoprecipitate, which has been found to have a similar survival advantage as TXA (25).
There is no current study from the United States that has demonstrated a survival advantage with TXA. The CRASH-2 study (6) was predominantly conducted in developing countries, that did not have the same blood bank capacity as in the United States. Difference in transfusion strategies may also explain why a benefit of TXA is apparent in Europe (7, 26) but not the United States (12, 13). European resuscitation strategies rely on recombinant fibrinogen and prothrombin complex during resuscitation (27). These products do not contain the numerous antifibrinolytic proteins found in plasma (19). In a sense, this European strategy of resuscitation needs an antifibrinolytic therapy during resuscitation, as it may dilute fibrinolytic regulators. The effect of plasma dilution of these regulatory proteins with increased fibrinolysis is evident in vitro (19) and clinically (4). Therefore, in the absence of early plasma, TXA may have a role, but is not without some inherent risks to the patient. Furthermore, the majority of severely injured patients may develop a second wave of fibrinolysis resistance after resuscitation, and the time that these patients remain in shutdown is a risk for postinjury complications (28) and mortality (10).
This analysis of trauma patients with the selective use of TXA is inherently biased to treating patients in whom bleeding is not controlled. Despite our institutions algorithm to treat patients based on their TEG LY30 values, a similar number of patients in shutdown received TXA as patients who were hyperfibrinolytic. TXA appeared to be used in dire situations the majority of the time, and may have been used to treat patients who were bleeding because they were dying, and not dying from bleeding. This question has been investigated previously, finding the majority of trauma deaths from hemorrhage are not attributed to TIC (29). TXA does not inhibit major bleeding, as was documented in the current data because 26% of these patients were dead or underwent a massive transfusion after TXA was delivered.
While studies in the past have suggested a modest improvement in survival when using this medication, the benefit may not be applicable to mature urban trauma centers (6, 25). More importantly, trauma patients present to the hospital with a spectrum of coagulation abnormalities (30, 31). Individualized coagulation resuscitation guided by viscoelastic assays reduces mortality by 50% in patients undergoing massive transfusion (32). Employing a goal-based resuscitation strategy can improve mortality as opposed to resuscitation with fixed ratios of red blood cells, plasma and platelets which does not improve overall survival (33). Finally, trauma patients do not share the same pathophysiology as elective surgery. TXA has been shown to reduce blood loss in elective surgery (34), however, in the setting of massive bleeding from injury where profound tissue ischemia exists there does not appear to be the same beneficial effect. This study is also limited to 26 patients whom received TXA and has limited power to control for multiple potential confounding variables. As previously discussed, the increase in mortality in patients who received TXA could be related to a last ditch effort to salvage patients, rather than a direct cause of increased mortality.
While this study represents a single center experience, the results are consistent with previously published literature in the United States that TXA use does not benefit all trauma patients. Several multicenter randomized control trials with TXA in trauma are currently being conducted in the United States in a variety of clinical settings, which further support that the indications for using this medication remain unclear. Until the results of these studies are available, empiric use of TXA in trauma patients in this country remains a questionably beneficial practice.
Acknowledgments
This study was supported in part by National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, in addition to the National Heart Lung and Blood Institute UM1-HL120877. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional research support was provided by Haemonetics Corporation with shared intellectual property.
Footnotes
Presented at the Academic Surgical Congress Las Vegas NV, February 2017
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. The journal of trauma and acute care surgery. 2014;77:811–817. doi: 10.1097/TA.0000000000000341. discussion 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, et al. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. Journal of the American College of Surgeons. 2016;222:347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. The journal of trauma and acute care surgery. 2013;75:961–967. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. The journal of trauma and acute care surgery. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. discussion 370. [DOI] [PubMed] [Google Scholar]
- 5.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. The Journal of trauma. 2009;67:125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 6.collaborators C-t. Shakur H, Roberts I, Bautista R, Caballero J, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 7.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Annals of surgery. 2015;261:390–394. doi: 10.1097/SLA.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 8.Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, et al. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. The New England journal of medicine. 2017;376:136–148. doi: 10.1056/NEJMoa1606424. [DOI] [PubMed] [Google Scholar]
- 9.Barrachina B, Lopez-Picado A, Remon M, Fondarella A, Iriarte I, et al. Tranexamic Acid Compared with Placebo for Reducing Total Blood Loss in Hip Replacement Surgery: A Randomized Clinical Trial. Anesthesia and analgesia. 2016;122:986–995. doi: 10.1213/ANE.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 10.Meizoso JP, Karcutskie CAt, Ray JJ, Namias N, Schulman CI, et al. Persistent Fibrinolysis Shutdown Associated with Increased Mortality in Severely Injured Trauma Patients. Journal of the American College of Surgeons. 2016 doi: 10.1016/j.jamcollsurg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Hardaway RM, Drake DC. Prevention of “irreversible” hemorrhagic shock with fibrinolysin. Annals of surgery. 1963;157:39–47. doi: 10.1097/00000658-196301000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, et al. Do all trauma patients benefit from tranexamic acid? The journal of trauma and acute care surgery. 2014;76:1373–1378. doi: 10.1097/TA.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 13.Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, et al. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. The journal of trauma and acute care surgery. 2015;78:905–911. doi: 10.1097/TA.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 14.Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, et al. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016;56(Suppl 2):S110–114. doi: 10.1111/trf.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardaway RM, Brune WH, Geever EF, Burns JW, Mock HP. Studies on the role of intravascular coagulation in irreversible hemorrhagic shock. Annals of surgery. 1962;155:241–250. doi: 10.1097/00000658-196200000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardaway RM, Burns JW. Mechanism of action of fibrinolysin in the prevention of irreversible hemorrhagic shock. Annals of surgery. 1963;157:305–309. doi: 10.1097/00000658-196302000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reust DL, Reeves ST, Abernathy JH, 3rd, Dixon JA, Gaillard WF, 2nd, et al. Temporally and regionally disparate differences in plasmin activity by tranexamic acid. Anesthesia and analgesia. 2010;110:694–701. doi: 10.1213/ANE.0b013e3181c7eb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Chen Z, Cui S, Li Z, Yuan Z. Topical versus systemic tranexamic acid after total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e4656. doi: 10.1097/MD.0000000000004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, et al. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. Journal of the American College of Surgeons. 2015;220:872–879. doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore HBME, Morton AP, Gonzalez E, Fragoso M, Chapman MP, Dzieciatkowska M, Hansen KC, Banerjee A, Sauaia A, Silliman CC. Shock Induced Systemic Hyperfibrinolysis is Attenuated by Plasma First Resuscitation. Journal of Trauma and Acute Care Surgery. 2015 doi: 10.1097/TA.0000000000000792. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Moore HB, Moore EE, Wither MJ, Nemkov T, et al. Plasma First Resuscitation Reduces Lactate Acidosis, Enhances Redox Homeostasis, Amino Acid and Purine Catabolism in a Rat Model of Profound Hemorrhagic Shock. Shock. 2016;46:173–182. doi: 10.1097/SHK.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesthesia and analgesia. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40:195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ban K, Peng Z, Pati S, Witkov RB, Park PW, et al. Plasma-Mediated Gut Protection After Hemorrhagic Shock is Lessened in Syndecan-1−/− Mice. Shock. 2015;44:452–457. doi: 10.1097/SHK.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Archives of surgery. 2012;147:113–119. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 26.Wafaisade A, Lefering R, Bouillon B, Bohmer AB, Gassler M, et al. Prehospital administration of tranexamic acid in trauma patients. Critical care. 2016;20:143. doi: 10.1186/s13054-016-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical care. 2016;20:100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore HBME, Gonzalez E, Huebner BJ, Sheppard F, Banerjee A, Sauaia A, Silliman CC. Reperfusion Shutdown: Delayed Onset of Fibrinolysis Resistance after Resuscitation from Hemorrhagic Shock Is Associated with Increased Circulating Levels of Plasminogen Activator Inhibitor-1 and Postinjury Complications. Blood. 2016;128:206. [Google Scholar]
- 29.Morton AP, Moore EE, Wohlauer MV, Lo K, Silliman CC, et al. Revisiting early postinjury mortality: are they bleeding because they are dying or dying because they are bleeding? The Journal of surgical research. 2013;179:5–9. doi: 10.1016/j.jss.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. The journal of trauma and acute care surgery. 2013;74:1223–1229. doi: 10.1097/TA.0b013e31828b7fa1. discussion 1229–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, et al. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156:570–577. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Annals of surgery. 2016;263:1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA: the journal of the American Medical Association. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. The Cochrane database of systematic reviews. 2011:CD001886. doi: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]