Abstract
Objective
To characterize skeletal muscle fat (SMF), intermuscular adipose tissue (IMAT) and subcutaneous adipose tissue (SAT) in individuals with rheumatoid arthritis (RA), and assess the associations between these fat depots and physical function and physical activity.
Methods
Cross-sectional analysis from an RA cohort. SMF, IMAT and SAT were measured using computed tomography imaging of the mid-thigh cross-sectional region. Physical function was measured with the Health Assessment Questionnaire (HAQ) and a battery of performance-based tests that included quadriceps muscle strength, gait speed, repeated chair-stands, stair ascend, and single leg-stance. Physical activity was assessed using an activity monitor. Associations between SMF, IMAT and SAT, and physical function and activity were assessed by multiple linear regression models adjusted for potential confounders such as age, gender, body mass index, muscle area, and strength.
Results
Sixty subjects with RA (82% female, age 59 ± 10 years, BMI: 31.79 ± 7.16) were included. In the adjusted models, lower SMF was associated with greater gait speed, single leg stance, quadriceps strength, and physical activity, and less disability (R2Δ range .06−.25, p < .05); whereas IMAT did not associate with physical function or activity; and SAT was negatively associated with disability (HAQ) (R2Δ= .13 p<0.05) and weakly but positively associated with muscle strength. (R2Δ= .023, p<0.05).
Conclusions
Fat infiltration within the muscle seems to independently contribute to low physical function and activity in contrast to IMAT or SAT accumulation. Longitudinal studies are necessary to confirm the impact of SMF on disability and promoting health in persons with RA.
INTRODUCTION
Rheumatoid Arthritis (RA) affects around 1% of the population, and is an autoimmune disease driven by systemic inflammation that predominantly affects the synovial membrane of multiple joints. (1) The main clinical features of RA are poly-articular pain, swelling and stiffness, but, systemic inflammation in RA also promotes protein degradation, leading to loss of body cell mass (mostly lean mass) and concomitant increase in fat mass.(2, 3) This loss of lean mass and gain in fat mass is a well-known finding in persons with RA,(4) and could be accompanied by increase in fat content within or around the skeletal muscles. Increase in fat depots in and around the skeletal muscle may affect physical function and physical activity participation in those with RA. Investigating these fat depots may provide insight on alternate sources of disability and low physical activity levels (5) that persist in this population, despite relatively well-controlled disease and advanced medical management.(6)
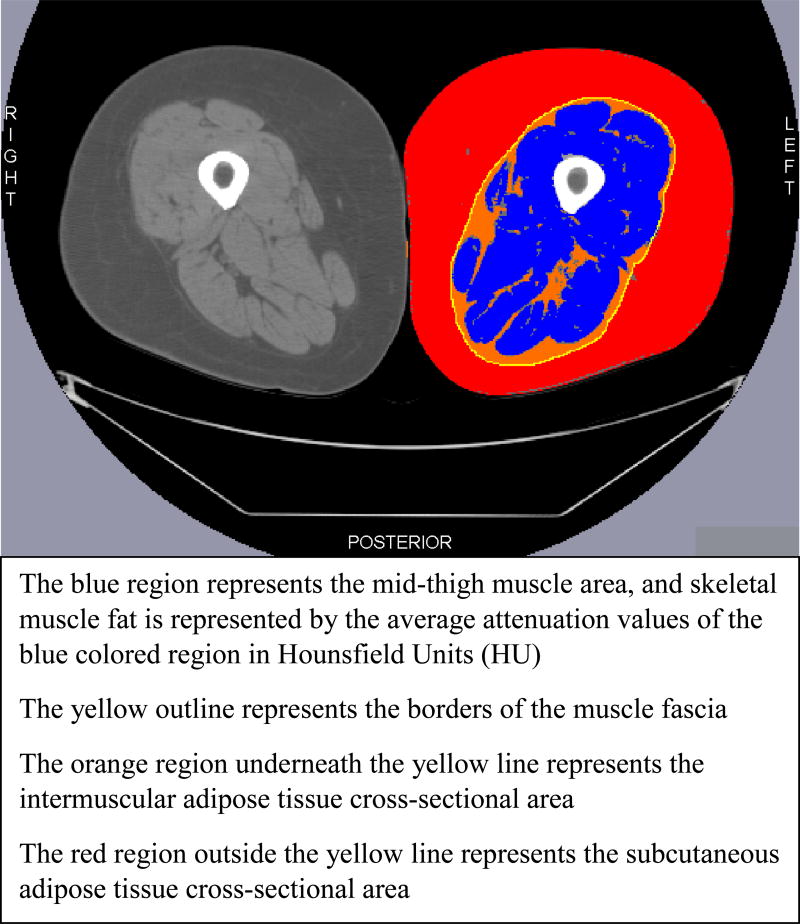
The mechanisms by which fat accumulation inside and around the muscle influence physical function are not fully established, but some studies have indicated that excessive fat infiltration may perpetrate chronic inflammatory pathways and produce toxic lipid by-products that could interfere with normal muscle metabolic and contractile functions.(7–9) These alterations in muscle physiological functions due to fat may consequently influence physical function. The fat depots inside and around the skeletal muscle that associate with physical function in non-RA populations are generally classified as skeletal muscle fat (SMF), intermuscular adipose tissue (IMAT), and subcutaneous adipose tissue (SAT). SMF is located within individual muscles and includes the fat inside the myocytes and around the muscle fibers. IMAT lies within the muscle fascia and is interspersed between groups of muscles. SAT lies outside the muscle fascia and directly underneath the skin.(10, 11) These fat depots can be assessed in the mid-thigh region using imaging techniques such as Computed Tomography (Figure 1).
Figure 1.
Cross-sectional Bilateral Images of the Mid-thigh Region Illustrating Skeletal Muscle Fat, Intermuscular Adipose Tissue and Subcutaneous Adipose Tissue. The right mid-thigh cross-section shows the original image obtained by Computed Tomography, and the left mid-thigh cross-section represents the processed image with the fat depots.
The role of SMF, IMAT and SAT on physical function has been mostly studied in healthy elderly adults in large longitudinal studies; however, the overall evidence is not entirely consistent across studies despite robust study designs and an attempt to account for probable confounders. Higher SMF accumulation was associated with lower physical function in one longitudinal and two cross-sectional studies,(12–14) whereas no associations were reported in another longitudinal study.(15) Higher IMAT accumulation was significantly associated with decline in physical function in two longitudinal studies(16, 17), while no associations were found in another longitudinal study.(15) Higher SAT accumulation was associated with decline in physical function over time among women, but not in men,(16, 17) whereas one cross-sectional study reported no associations between SAT and physical function.(12) Three of the above studies also examined whether the associations of these fat depots on physical function decline were independent of muscle area and muscle strength; but conflicting results were reported.(13–15) As muscle area and strength are known to contribute to physical function, and also associate with SMF,(18) adjusting for these muscle characteristics would inform whether these fat depots influence physical function beyond what is already explained by muscle size and force generating capacity. Variations among the different study samples of healthy adults in terms of lifestyle, co-morbidities, degree of functional limitations or fat accumulation could drive these inconsistent findings.
Findings from healthy populations may not directly translate to populations with chronic conditions such as RA, who experience a greater degree of functional limitations, are less active, and have more loss of muscle mass and gain in fat mass compared to healthy populations. Currently, there is limited insight on how SMF, IMAT and SAT contribute to physical function in RA. While previous RA studies have investigated the role of overall body adiposity (19–22) in physical function, these studies used methods(19–21) (22) that do not quantify SMF or IMAT. To date, we are aware of only study in RA that used methods to distinguish these different fat depots and explored their associations with physical function.(23) In cross-sectional analyses, SMF and total fat outside the muscle (IMAT and SAT combined) were associated with lower physical function in RA,(23) but muscle strength was not taken into account when assessing the associations.
The current study sought to characterize SMF, IMAT and SAT separately, assess the associations of each fat depot with measures of physical function and physical activity, and determine whether the contributions of SMF, IMAT and SAT are unique and independent of commonly known factors that directly influence physical function such as age, gender, BMI, muscle area, and muscle strength. We hypothesized that after accounting for confounding SMF, IMAT and SAT would associate with physical function and physical activity.
METHODS
Design
The current study was an ancillary, cross-sectional analysis of baseline data from 60 individuals with RA who participated in a randomized clinical trial that compared two strengthening exercise programs to reverse muscle atrophy (Clinical Trials Registration NCT00924625). The study was conducted in the Department of Physical therapy at the University of Pittsburgh between December 2009 and September 2013. Computed tomography (CT) imaging was obtained at the University of Pittsburgh Radiology Department, and physical function measures were obtained in the Department of Physical Therapy.
Study Sample
Participants included those above 21 years of age, diagnosed with RA by a rheumatologist as per the 1987 American College of Rheumatology criteria, and able to ambulate independently. Participants were excluded if they had contra-indications that precluded safe participation in strength training of the lower extremity, such as cardiovascular disease, uncontrolled hypertension, neurological or muscular conditions affecting the lower limbs, or recent lower extremity surgery. All eligible participants signed an informed consent approved by the University of Pittsburgh Institutional Review Board prior to study enrollment.
Procedures and Measurements
SMF, IMAT and SAT were obtained from mid-thigh CT imaging using previously described methods.(24, 25) Briefly, we obtained a 10-mm thick axial image of the mid-thigh region at the femoral mid-point, which is the center of a line joining the lateral most part of the greater trochanter and the lateral femoral epicondyle. Scanning parameters were 120kVp and 200–250mA, and subjects were positioned in supine with both femurs in neutral position. Slice-O-Matic proprietary software was used to quantify SMF, IMAT and SAT from the CT images. The software differentiates between muscle, fat, and bone tissue based their physical density properties, which is measured on an interval scale in Hounsfeld Units (HU), with water as the reference at 0 HU. Muscle tissue was identified by values between 0 to 100 HU, while adipose tissue was identified by values from −190 to −30 HU. SMF accumulation was assessed using the average muscle density in HU, with lower average muscle density corresponding to higher amounts of SMF accumulation, and higher muscle density corresponding to low SMF accumulation. We obtained the average muscle density (HU) and muscle cross-sectional area (square centimeters) for both quadriceps muscle and total mid-thigh muscle area. SAT and IMAT were separated by tracing the muscle fascia, and their cross-sectional areas measured in square centimeters.(Figure 1) For analyses, all CT variables were averaged for both legs. This method to assess muscle area and fat content using CT imaging is reliable and valid with ICCs >0.98, and coefficients of variation less than 2%. (26)
Physical function was measured using the self-reported Health Assessment Questionnaire (HAQ) questionnaire and a battery of physical performance tests shown to be reliable, valid, and well tolerated in the RA population.(27–30) The HAQ assesses limitations during 20 activities of daily living, and is scored between 0 (no disability) to 3 (severe disability).(31) Maximum voluntary isometric strength of the quadriceps was measured using an isokinetic dynamometer (Biodex,Inc). Subjects sat on the dynamometer with 70 degrees of knee flexion and performed five trials of isometric knee extension per leg. The highest three trials for each leg were averaged, and further averaged for both limbs. Repeated chair-stand test consisted of the time taken to stand up from a chair five times. Stair climbing test recorded the time taken to ascend one flight of 12 stairs. Self-selected gait speed was calculated from the time taken to walk four meters. The single-leg stance test measured the time (up to 30 seconds) a subject could stand on one leg without losing balance. Each leg underwent three trials for single-leg stance, and the values from both limbs were averaged.
Physical activity was measured by the Sense Wear Armband (Bodymedia, Inc), which is a reliable and valid multi-sensor activity monitor.(32, 33) Subjects wore the monitor on the right upper arm for 8 days, up to 24 hours per day, except during water related activities. We calculated the daily time spent in activities of moderate or higher intensities. A minimum of 4 days with at least 10 hours of data was required to yield reliable estimates of physical activity.(34)
Demographics and biomedical characteristics included age, gender, race, education, body mass index (BMI), and disease duration and activity. BMI was calculated with weight and height measured on-site. Disease activity was measured by the disease activity score (DAS-28), a validated tool that involves the examination of 28 joints for tenderness and swelling, ESR, and patient reported global health on a scale of 0 to 100. Validated algorithms provide scores ranging from 0 to 9.4, with scores ≤ 3.2 indicating low disease activity, ≥3.2 ≤ 5.1 indicating moderate disease activity and scores > 5.1 indicating high disease activity.(35)
Statistical Analysis
The parent trial recruited sixty subjects who were all included in the current secondary analysis. With 60 subjects, the current study had 80% power to detect small to moderate associations (ρ = 0.35, α=0.05). The sample also achieved 80% power to detect an R-square of 0.16 with up to 4 covariates in a regression model, and an R-square increment of 0.11 with one predictor in the main set.
Data were described using means±standard deviations or medians(25th–75th percentiles) for continuous variables, and frequencies(percentages) for categorical variables. We calculated the 95% CI around the fat depots, and physical function and activity measures to provide estimates of precision. Univariate associations of average muscle density (proxy measure of SMF), and IMAT and SAT areas with physical function and physical activity were assessed using Pearson’s (r) or Spearman’s (rs) correlation coefficients, depending on data distribution. The strength of the correlations were interpreted based on the values provided by Cohen.(36)
We used multiple linear regression to assess the independent contribution of muscle density, IMAT and SAT (independent variables) towards physical function and physical activity (dependent variables) after accounting for potential confounders. We selected age, gender, BMI, muscle area and strength as potential confounders because of their known associations with physical function. Disease activity was not included as a potential confounder as we postulated that increase fat accumulation is a direct consequence of high disease activity (systemic inflammation). Since disease activity is now on the causal pathway for low physical function, it would not fulfil the criteria of a confounder. Separate models were created for each independent variable and its ability to explain the variance for each dependent variable. Regression modeling entailed two steps. First, we assessed the confounding effect of age, BMI, gender, and muscle area and strength by adding each potential confounder separately into the model with the independent variable. The potential confounder was included in the final adjusted model if it produced a change of 10% or higher in the effect size estimates of the independent variable (unstandardized beta-coefficients).(37) Given the relatively small sample size (N=60) we chose to only adjust for variables that met the definition of confounding to avoid saturating the models by merely adjusting for all potential confounders. Quadriceps strength was a dependent variable, but also selected as a potential confounder for the hierarchical models built to predict the other physical function and physical activity variables. This was done to assess whether muscle fat can explain alterations in physical function and PA beyond muscle force generating capacity. After entering the confounding variables in the regression models, we entered the independent variable to determine the magnitude of effect by observing the R2 change (R2 Δ) and the beta-coefficients. We ran regression diagnostics and performed data transformations (if needed) to ensure that the assumptions for linear regression (i.e., normality of the error distribution, linearity, homoscedasticity) were not violated. Statistical analyses were performed using the IBM SPSS software, version 21.(IBM Corporation)
For clarity of presentation, the results only contain data on muscle density and muscle cross-sectional area derived specifically from the quadriceps muscle. This was done for two reasons. First, the average density for the total mid-thigh muscle and quadriceps only were highly correlated (r >0.8). Second, because muscle strength was assessed only for the quadriceps muscles, it was appropriate to build regression models using quadriceps density and muscle area measures.
RESULTS
Subjects were on average 59 ± 10 years, mostly white (83%), female (82%), and obese (mean BMI: 31.79 ± 7.16), had median RA duration of 13.5 years, and moderate disease activity levels. (Table 1). Quadriceps density (SMF accumulation) was normally distributed with relatively lower variability and narrower confidence intervals compared to IMAT and SAT areas that were positively skewed with larger variability and wider confidence intervals. (Table 2)
Table 1.
Individual Characteristics of the Sample.
| Variables | Total Sample (N=60) |
|---|---|
|
| |
| Age in years, mean ± SD | 59.0 ± 9.8 |
| Number of Females, (%) | 49 (82) |
| Number of Caucasians, (%) | 50 (83) |
| Education Level, N (%) | |
| - High School | 16 (27) |
| - College Education | 44 (73) |
| Marital status, N (%) | |
| - Married | 32 (53) |
| - Single/never married | 12 (20) |
| - Other (divorced, widowed, etc.) | 16 (27) |
| Employment Status, N (%) | |
| - Regular Full Time or Part time | 20 (33) |
| - Retired (not due to health) | 20 (33) |
| - Retired or unable to work due to health | 11 (18) |
| - Other (modified or light duty, unemployed, homemaker) | 9 (14) |
| Height in meters, median (25th-75th percentile) | 1.63 (1.59–1.69) |
| Weight in kg, mean ± SD | 84.9 ± 21.1 |
| BMI in kg/m2 mean ± SD | 31.2 ± 7.2 |
| RA duration in years, median (25th–75th percentile) | 13.5 (6–22) |
| DAS-28 score, mean ± SD | 4.0 ± 1.3 |
| HAQ score, median (25th–75th percentile) | 0.88 (0.38–1.25) |
| Charleston Co-morbidity index, median (25th–75th percentile) | |
| Raw Score | 0 (0.0 – 1.0) |
| Age Adjusted Score | 2.0 (1.0 – 3.0) |
BMI: Body Mass Index, DAS: Disease Activity Score, HAQ: Health Status Questionnaire
Table 2.
Descriptive Statistics of the Dependent and Independent Variables along with Results of their Univariate Associations.
| Mean ± SD or Median (25th-75th Percentile) 95% Confidence Interval |
Univariate Correlation Coefficients (Pearson’s r or Spearman’s rho) |
|||
|---|---|---|---|---|
| Quadriceps Density, HU (Skeletal Muscle Fat)† |
Intermuscular Adipose Tissue, sq.cm |
Subcutaneous Adipose Tissue, sq.cm |
||
| Dependent Variables | ||||
| Quadriceps Strength, Newton-meters‡ | 141.6 (114.8 – 165.9) 134.3 – 158.7 | .454* | .072 | .075 |
| Single leg stance, seconds‡ | 12.7 (3.2–23.5) 11.0 −16.3 | .508* | −.211 | −.071 |
| Gait speed, meters/second | 1.06 ± 0.25 0.99 – 1.12 | .397* | −.389* | −.219 |
| 5-Chair-stand time, seconds | 12.5 (10.4–15.1) 12.0 −14.9 | −.244 | .270* | .128 |
| Stair ascend time, seconds | 6.3 (5.1 – 7.7) 6.1 −7.6 | −.576* | .372* | .289* |
| HAQ | 0.88 (0.38–1.25) 0.72 – 1.04 | −.418* | .424* | .190 |
| Physical Activity, minutes/day | 34.0 (16.0 −47.0) 31.1 −52.4 | .578** | −.388* | −.321* |
|
| ||||
| Independent Variables‡ | ||||
| Skeletal muscle fat (quadriceps density, HU) | 46.4 ± 4.7 45.1 – 47.6 | |||
| Intermuscular adipose tissue area (sq. cm) | 11.8 (7.9 – 17.3) 11.4 – 15.3 | |||
| Subcutaneous adipose tissue area (sq.cm) | 106.3 (69.4 – 153.4) 101.3 – 134.9 | |||
N = 60, except for HAQ (N = 59), Gait speed (N = 59), and Chair-stand (N=58) due to missing data, and Physical Activity (N=51) due to insufficient activity monitor data (<10 hours per day on 4 days).
significant at alpha level 0.05
Higher values of muscle density corresponds to lower amount of skeletal muscle fat
Values represent the averages of the right and left leg.
HAQ: Health Assessment Questionnaire, HU: Hounsfeld Units, sq.cm: square centimeter
Univariate associations indicated that greater muscle density (lower SMF) associated moderately with lesser time to ascend 12 steps, faster gait speed, longer single leg stance time, greater quadriceps strength, lower HAQ scores, and higher physical activity (p < .05). Larger IMAT area showed weak to moderate associations with slower gait speed, longer repeated chair-stand time, and longer time to ascend 12 steps, higher HAQ scores and lower physical activity levels (p < .05). Greater SAT area was weakly associated with longer time for to ascend 12 steps and lower physical activity levels (p < .05). (Table 2)
After identifying and controlling for confounders, greater muscle density (lower SMF) demonstrated moderate to large positive associations with gait speed, single leg stance time and physical activity, and negative associations with HAQ scores (R2Δ range .06−.25, p <.05). Greater muscle density (lower SMF) was weakly and positively associated with quadriceps strength (R2Δ = .023, p <.05), and not associated with stair ascent and repeated chair-stand time, (p >.05). (Table 3) IMAT did not associate with any measures of physical function or physical activity after controlling for confounding, (Table 4) while SAT showed moderate negative associations with HAQ scores (R2Δ = .13 p <.05), and weak positive associations with quadriceps strength (R2Δ = .023, p <.05). (Table 5)
Table 3.
Adjusted Regression Models of Associations between Quadriceps Density, HU (Skeletal Muscle Fat) † and Physical Function and Physical Activity.
| Dependent Variable |
Step | Confounder variable |
Independent variable |
Beta- coefficient |
R2 | R2Δ | R2 Adjusted |
F- change |
P- value |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Quad Strength | 1 | Age | .104 | .771 | .758 | 62.727 | <.001 | ||
| BMI | −.143 | ||||||||
| Quad Area | .896* | ||||||||
| 2 | Quad Density | .212* | .794 | .023 | .779 | 6.118 | .017 | ||
|
| |||||||||
| Stair Climb (Inverse) | 1 | Age | −.444* | .627 | .607 | 31.426 | <.001 | ||
| BMI | −.541* | ||||||||
| Strength | .410* | ||||||||
| 2 | Quad Density | .006 | .627 | .000 | .600 | .002 | .961 | ||
|
| |||||||||
| Gait Speed | 1 | BMI | −.276* | .142 | .127 | 9.438 | .003 | ||
| 2 | Quad Density | .306* | .226 | .084 | .198 | 6.051 | .017 | ||
|
| |||||||||
| Single Leg Stance | 1 | Age | −.213 | .172 | .157 | 12.027 | .001 | ||
| 2 | Quad Density | .416* | .304 | .132 | .279 | 10.801 | .002 | ||
|
| |||||||||
| Chair Rise Time (Inverse) | 1 | Age | −.298 | .195 | .166 | 6.660 | .003 | ||
| 2 | BMI | Quad Density | −.383* | ||||||
| −.004 | .195 | .000 | .150 | .001 | .979 | ||||
|
| |||||||||
| HAQ | 1 | Age | −.200 | .230 | .188 | 5.471 | .002 | ||
| BMI | .240 | ||||||||
| Strength | −.211 | ||||||||
| 2 | Quad Density | −.345* | .286 | .056 | .233 | 4.237 | .044 | ||
|
| |||||||||
| Physical Activity (Sqrt) | 1 | BMI | −.296 | .167 | .133 | 4.818 | .012 | ||
| Strength | −.547* | ||||||||
| Quad Area | .395 | ||||||||
| 2 | Quad Density | .530* | .415 | .248 | .377 | 19.881 | <.001 | ||
significant at alpha level 0.05
Higher values of muscle density corresponds to lower amount of skeletal muscle fat
BMI: Body Mass Index, Quad: Quadriceps muscle, HAQ: Health Assessment Questionnaire, Sqrt: Square root transformed
Table 4.
Adjusted Regression Models of the Associations between Intermuscular Adipose Tissue (area in square cm), and Measures of Physical Function, and Physical Activity
| Dependent Variable |
Step | Confounder Variable |
Independent Variable |
Beta Coefficient |
R2 | R2 Δ | R2 Adjusted |
F- change |
p- value |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Strength | 1 | Age | .099 | .788 | .772 | 51.025 | <.001 | ||
| BMI | .176 | ||||||||
| Gender | −.294* | ||||||||
| Quad Area | 1.111* | ||||||||
| 2 | IMAT | −.037 | .788 | .001 | .769 | .150 | .700 | ||
|
| |||||||||
| Stair Climb (Inverse) | 1 | BMI | −.701* | .438 | .419 | 22.242 | <.001 | ||
| Quad Area | .593* | ||||||||
| 2 | IMAT | .046 | .439 | .001 | .409 | .100 | .753 | ||
|
| |||||||||
| Gait Speed | 1 | Age | −.262* | .212 | .184 | 7.539 | .001 | ||
| 2 | BMI | −.353* | |||||||
| IMAT | −.093 | .216 | .004 | .174 | .294 | .590 | |||
|
| |||||||||
| Single Leg Stance (log) | 1 | BMI | −.215 | .164 | .135 | 5.602 | .006 | ||
| Quad Area | .362* | ||||||||
| 2 | IMAT | −.216 | .186 | .022 | .143 | 1.516 | .223 | ||
|
| |||||||||
| Chair Rise Time (Inverse) | 1 | BMI | −.275 | .179 | .149 | 6.008 | .004 | ||
| Gender | .255* | ||||||||
| 2 | IMAT | −.101 | .184 | .005 | .139 | .323 | .572 | ||
|
| |||||||||
| HAQ | 1 | BMI | .168 | .125 | .110 | 8.133 | .006 | ||
| 2 | IMAT | .258 | .157 | .032 | .127 | 2.141 | .149 | ||
|
| |||||||||
| Physical Activity (Sqrt) | 1 | BMI | −.198 | .162 | .145 | 9.495 | .003 | ||
| 2 | IMAT | −.285 | .202 | .039 | .168 | 2.369 | .130 | ||
IMAT: Intermuscular Adipose Tissue, BMI: Body Mass Index, Quad: Quadriceps muscle, HAQ: Health Assessment Questionnaire, Sqrt: Square root transformed
significant at alpha level 0.05
Table 5.
Adjusted Regression Models of the Associations between Subcutaneous Adipose Tissue (area in square cm), and Measures of Physical Function, and Physical Activity.
| Dependent Variable |
Step | Confounder Variable |
Independent Variable |
Beta Coefficient |
R2 | R2 Δ | R2 Adjusted |
F- change |
p- value |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Strength (sqrt) | 1 | Age | .112 | .788 | .772 | 51.025 | <.001 | ||
| BMI | −.567* | ||||||||
| Gender | .084 | ||||||||
| Area | 1.143* | ||||||||
| 2 | SAT | .305* | .811 | .023 | .793 | 6.497 | .014 | ||
|
| |||||||||
| Stair Climb (Inverse) | 1 | Age | −.303* | .619 | .591 | 22.349 | .000 | ||
| BMI | −.809* | ||||||||
| Gender | .221 | ||||||||
| Area | SAT | .615* | |||||||
| 2 | .086 | .621 | .002 | .586 | .256 | .615 | |||
|
| |||||||||
| Gait Speed | 1 | Age | −.262* | .213 | .170 | 4.973 | .004 | ||
| BMI | −.605* | ||||||||
| 2 | Gender | −.054 | |||||||
| SAT | .239 | .227 | .014 | .170 | .985 | .325 | |||
|
| |||||||||
| Single Leg Stance | 1 | Age | −.333* | .288 | .236 | 5.557 | .001 | ||
| BMI | −.595* | ||||||||
| Gender | .029 | ||||||||
| Area | .303 | ||||||||
| 2 | SAT | .237 | .302 | .014 | .237 | 1.060 | .308 | ||
|
| |||||||||
| Chair Rise Time (Inverse) | 1 | Age | −.254* | .240 | .198 | 5.685 | .002 | ||
| BMI | −.356 | ||||||||
| 2 | Gender | .232 | |||||||
| SAT | −.041 | .240 | .000 | .183 | .030 | .864 | |||
|
| |||||||||
| HAQ | 1 | BMI | .894* | .133 | .102 | 4.311 | .018 | ||
| 2 | Gender | .361* | |||||||
| SAT | −.718* | .260 | .127 | .220 | 9.433 | .003 | |||
|
| |||||||||
| Physical Activity (Sqrt) | 1 | Age | −.333* | .324 | .280 | 7.497 | .000 | ||
| BMI | −.657* | ||||||||
| 2 | Gender | −.408* | |||||||
| SAT | .286 | .344 | .020 | .287 | 1.408 | .241 | |||
significant at alpha level 0.05
SAT: Subcutaneous Adipose Tissue, BMI: Body Mass Index, Quad: Quadriceps muscle, HAQ: Health Assessment Questionnaire, Sqrt: Square root transformed
DISCUSSION
This is the first study to investigate the individual roles of SMF, IMAT, and SAT on objectively measured physical function and physical activity outcomes in RA. Study findings demonstrate that muscle density (SMF) significantly associated with most physical function measures (4 of 6) and to physical activity in individuals with RA, even after accounting for confounders such as muscle area and strength, BMI, age, and gender. In contrast, associations of IMAT and SAT with physical function and physical activity significantly attenuated after accounting for muscle area, strength, BMI, age or gender. These findings are unique and suggest that physical function and physical activity may not be solely influenced by the amount of muscle or its ability to generate force, but, also by the amount of fat within the muscle.
We are only aware of one previous study in RA that investigated SMF with respect to physical function.(23) Kramer et al reported that SMF significantly associated with lower physical function in regression models simultaneously adjusted for appropriate confounders such as subject demographics, RA disease characteristics, muscle area and thigh fat area (SAT +IMAT combined). However, the unique variability explained by SMF, SAT and IMAT on physical function was not ascertained, and the role of muscle strength was not assessed. To our knowledge, our study is the first to demonstrate that SMF independently explains a significant amount of variability in physical function (4 of 6 measures) and physical activity independent of muscle strength. These findings suggest that there might be underlying mechanisms not related to muscle force-generating capacity that can also affect an individual’s physical function or activity participation. Although not directly studied, it has been postulated that fat encroachment within the skeletal muscle can affect the contractile, neuromuscular, and metabolic functions by altering the muscle’s extracellular matrix and connective tissue properties,(9) and through release of toxic lipid by-products.(8) Few small RA studies, conducted over 40 years ago, explored the histological properties of muscle spindles in RA and found accumulation of fluid, thickening of the muscle spindle capsule, and fibrotic changes in the intrafusal muscle fibers. (38–40) There is a possibility that fat encroachment around the muscle spindles may affect the intrafusal muscle fibers in a similar fashion, however, this investigation would require studies that directly assess muscle ultrastructure and intramyocellular lipid content in RA.
The role of SMF on physical function may also depend on the type of activity carried out by the contracting muscle and the energy source utilized. We observed that SMF had greater associations with activities that challenged neuromuscular control (single leg stance) and muscle endurance (walking) compared to those that replicated a quick strong burst in muscle activity (e.g. isometric contraction or climbing up a flight of stairs). The reason that we observed only a small association between SMF and quadriceps strength and no associations with stair ascend or chair stand time could be that the source of energy used during these activities is largely glycogen and not intramyocellular lipid, which is represented by SMF (25).
In contrast to SMF, IMAT did not explain additional variability in physical function or activity, and the associations significantly attenuated after accounting for confounding, with BMI being the most consistent confounder. The role of IMAT on physical function has not been previously reported in RA, but there seems to be conflicting results between non-RA studies that also adjusted for the effect of BMI and other confounders.(15, 17) Murphy et al reported that IMAT was associated with a small but significant risk of developing mobility limitations over time (Hazard Ratio range: 1.00–1.47, (95% CI 1.00–2.02)),(17) whereas Reinders et al observed no associations between IMAT and worsening of physical function measures over time (Odds ratio range: 1.00–1.14, (95% CI: 0.82–1.54)).(15) Based on the current RA and previous non-RA findings, it is likely that the magnitude of the independent associations between IMAT and physical function or physical activity is small after accounting for overall body adiposity such as BMI. Although IMAT was not a significant predictor of physical function or activity, it has been associated with metabolic complications, such as poor glucose metabolism and chronic systemic inflammation in the elderly, obese and diabetes populations.(10, 41–45) Recently, a cross-sectional study in RA reported that IMAT accumulation was associated with greater insulin resistance.(46)
SAT accumulation did not associate with most physical function variables or physical activity, but, was positively associated with strength and negatively with HAQ scores (R2Δ = .023 and .127, respectively). Similar to IMAT, most associations between SAT and physical function or activity significantly attenuated after accounting for BMI, as well as age, gender, and muscle area. Although previous studies in RA have not specifically studied SAT, prior RA studies reported positive associations between regional adiposity and disability, which contradict the current study results.(19, 23) Giles et al and Kramer et al observed a detrimental effect of overall body and appendicular fat mass, and total thigh fat area (SAT and IMAT combined), respectively, on HAQ scores in adjusted models.(19, 23) In healthy elderly populations, the associations between SAT and physical function is inconsistent among studies. Among studies that accounted for BMI and demographics, Therkelson et al reported no associations,(12) whereas Murphy et al, and Beavers et al reported significant associations between SAT and worsening of physical function over time in women, but not men. (16, 17) There does exist some literature in healthy elderly and obese populations suggesting that subcutaneous fat mass, particularly in the lower body region, is not associated with adverse metabolic complications, and may be attributed to favorable metabolic profile (43, 47, 48). In RA, being overweight and obese was associated with lower mortality, (49, 50) but, the RA studies only used BMI as a proxy measure of body fat, and did not assess SAT specifically. Therefore, SAT may not be a fat depot that adversely affects physical or metabolic health. It is also possible that the protective associations between SAT and disability, and strength could be due to a strong negative confounding effect of BMI. Further investigation in RA is necessary to confirm these findings, and tease out the role of BMI as a confounder or effect modifier of these relationships.
This study also characterized fat content in those with RA. We were able to compare our ranges of SMF, SAT and IMAT to those from the Healthy Aging and Body Composition Study as they used similar methods to measure and report fat values. SMF (quadriceps attenuation) in our RA sample was slightly higher than the healthy ABC study (41.1 ± 6.9 HU), SAT was greater compared to the healthy older adults (77.9 ± 47.1 cm2) and IMAT was similar to that reported in older adults (10.2 ± 6.6 cm2).(18)
The current study is not without limitations. The study sample consisted mainly of Caucasian women. However, this sample is representative of the RA populations examined in most western developed societies in terms of gender distribution, BMI, and disease characteristics.(23, 46) The cross-sectional design also precludes temporal or causal inferences. The current study investigated fat depots as the primary pathway for reduced physical function and physical activity, however, it can also be argued that physical inactivity may lead to fat accumulation, and consequently to muscle weakness and impaired function. These relationships between fat depots, physical function and physical activity are likely circular and self-perpetuating in nature, hence future longitudinal studies in RA are necessary to investigate the temporal relationship between fat depots and physical function and activity behavior.
In summary, this study demonstrates that fat infiltration within the muscle (SMF) has a unique association with physical function and physical activity in individuals with RA, that is independent of body size, muscle area or muscle strength. These findings suggest that fat encroachment may drive some metabolic and physical changes within the muscle that may consequently contribute to worse physical function and low physical activity levels in RA. In contrast, fat infiltration outside the muscle (IMAT and SAT), does not seem to explain much variability in physical function or physical activity beyond what is explained by overall body size (BMI), demographics, or muscle area. These findings are clinically relevant because they suggest that fat within the muscle may be an alternate source of disability in this population. Future longitudinal studies assessing the effect of fat infiltration within muscle on physical function and activity will be necessary to confirm these findings, and to examine whether targeting these fat depots may be beneficial in promoting the health of those with RA.
Supplementary Material
Significance and Innovation.
Shift in body composition towards higher overall body fat mass and lower lean body mass is a known finding in individuals with RA, however, there is limited information on whether accumulation of fat in specific depots within the muscle (skeletal muscle fat) and around the muscles (intermuscular adipose tissue and subcutaneous adipose tissue) contributes to functional limitations in this population.
To address the gaps in knowledge this study investigated associations between skeletal muscle fat, intermuscular adipose tissue and subcutaneous adipose tissue, and physical function and physical activity in RA after accounting for factors that are likely to influence physical function and physical activity. Findings demonstrated that accumulation of skeletal muscle fat, but not intermuscular or subcutaneous adipose tissue was independently associated with both lower physical function and activity in RA after accounting for confounding.
Fat encroachment within the muscle may be a more important contributor to worse physical function and low physical activity levels in RA compared to fat accumulated outside the muscle. Future longitudinal studies would be necessary to assess whether fat in the muscle needs to be directly targeted to promote health in those with RA.
Acknowledgments
Funding Source: Supported by the Mentored Research Scientist Development Award, National Center for Medical Rehabilitation Research, NIH (K01 HD058035), and the Research Development Fund, School of Health and Rehabilitation Science, University of Pittsburgh
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. Journal of Clinical Investigation. 1994;93(6):2379–86. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. Journal of Rheumatology. 1992;19(10):1505–10. [PubMed] [Google Scholar]
- 4.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. International Journal of Cardiology. 2002;85(1):89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 5.Sokka T, Hakkinen A, Kautiainen H, Maillefert JF, Toloza S, Mork Hansen T, et al. Physical inactivity in patients with rheumatoid arthritis: data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59(1):42–50. doi: 10.1002/art.23255. [DOI] [PubMed] [Google Scholar]
- 6.Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36(5):685–95. doi: 10.1007/s00296-015-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. International journal of endocrinology. 2014;2014:309570. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;23(8):391–8. doi: 10.1016/j.tem.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–31. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297(5):E987–98. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH. Measuring body fat distribution and content in humans. Curr Opin Clin Nutr Metab Care. 2002;5(5):481–7. doi: 10.1097/00075197-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Therkelsen KE, Pedley A, Hoffmann U, Fox CS, Murabito JM. Intramuscular fat and physical performance at the Framingham Heart Study. Age (Dordrecht, Netherlands) 2016;38(2):31. doi: 10.1007/s11357-016-9893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 15.Reinders I, Murphy RA, Koster A, Brouwer IA, Visser M, Garcia ME, et al. Muscle Quality and Muscle Fat Infiltration in Relation to Incident Mobility Disability and Gait Speed Decline: the Age, Gene/Environment Susceptibility-Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2015;70(8):1030–6. doi: 10.1093/gerona/glv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97(3):552–60. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy RA, Reinders I, Register TC, Ayonayon HN, Newman AB, Satterfield S, et al. Associations of BMI and adipose tissue area and density with incident mobility limitation and poor performance in older adults. Am J Clin Nutr. 2014;99(5):1059–65. doi: 10.3945/ajcn.113.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 19.Giles JT, Bartlett SJ, Andersen RE, Fontaine KR, Bathon JM. Association of body composition with disability in rheumatoid arthritis: impact of appendicular fat and lean tissue mass. Arthritis Rheum. 2008;59(10):1407–15. doi: 10.1002/art.24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dao H-H, Do Q-T, Sakamoto J. Abnormal body composition phenotypes in Vietnamese women with early rheumatoid arthritis. Rheumatology. 2011;50(7):1250–8. doi: 10.1093/rheumatology/ker004. [DOI] [PubMed] [Google Scholar]
- 21.Book C, Karlsson MK, Akesson K, Jacobsson LTH. Early rheumatoid arthritis and body composition. Rheumatology. 2009;48(9):1128–32. doi: 10.1093/rheumatology/kep165. [DOI] [PubMed] [Google Scholar]
- 22.Chen YM, Chen HH, Hsieh CW, Hsieh TY, Lan JL, Chen DY. A close association of body cell mass loss with disease activity and disability in Chinese patients with rheumatoid arthritis. Clinics (Sao Paulo) 2011;66(7):1217–22. doi: 10.1590/S1807-59322011000700016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer HR, Fontaine KR, Bathon JM, Giles JT. Muscle density in rheumatoid arthritis: Associations with disease features and functional outcomes. Arthritis Rheum. 2012 doi: 10.1002/art.34464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Strandberg S, Wretling ML, Wredmark T, Shalabi A. Reliability of computed tomography measurements in assessment of thigh muscle cross-sectional area and attenuation. BMC Med Imaging. 2010;10:18. doi: 10.1186/1471-2342-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88(5):604–9. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Piva SR, Almeida GJ, Wasko MC. Association of physical function and physical activity in women with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(8):1144–51. doi: 10.1002/acr.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy R, Cooper R, Shah I, Harridge S, Guralnik J, Kuh D. Is chair rise performance a useful measure of leg power? Aging Clin Exp Res. 2010;22(5–6):412–8. doi: 10.1007/bf03324942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54(5):737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 31.Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ) Clin Exp Rheumatol. 2005;23(5 Suppl 39):S14–8. [PubMed] [Google Scholar]
- 32.Almeida GJ, Irrgang JJ, Fitzgerald GK, Jakicic JM, Piva SR. Reliability of Physical Activity Measures During Free-Living Activities in People After Total Knee Arthroplasty. Phys Ther. 2015 doi: 10.2522/ptj.20150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida GJ, Wert DM, Brower KS, Piva SR. Validity of physical activity measures in individuals after total knee arthroplasty. Arch Phys Med Rehabil. 2015;96(3):524–31. doi: 10.1016/j.apmr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida GJ, Wasko MC, Jeong K, Moore CG, Piva SR. Physical activity measured by the SenseWear Armband in women with rheumatoid arthritis. Phys Ther. 2011;91(9):1367–76. doi: 10.2522/ptj.20100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954–60. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.J. C. Statistical power analysis for the behavioral sciences. Second. Hillsdale: NJ1988; [Google Scholar]
- 37.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 38.Magyar E, Talerman A, de Bruijn WC, Mohacsy J, Wouters HW. Muscle spindles in rheumatoid arthritis. An ultrastructural study. Virchows Arch A Pathol Anat Histol. 1979;382(2):191–200. doi: 10.1007/BF01102874. [DOI] [PubMed] [Google Scholar]
- 39.Magyar E, Talerman A, Mohacsy J, Wouters HW, de Bruijn WC. Muscle changes in rheumatoid arthritis. A review of the literature with a study of 100 cases. Virchows Arch A Pathol Anat Histol. 1977;373(3):267–78. doi: 10.1007/BF00432241. [DOI] [PubMed] [Google Scholar]
- 40.Magyar E, Talerman A, Wouters HW. Histological abnormalities in the muscle spindles in rheumatoid arthritis. Ann Rheum Dis. 1973;32(2):143–50. doi: 10.1136/ard.32.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–5. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 42.Franco C, Veldhuis JD, Iranmanesh A, Brandberg J, Lonn L, Andersson B, et al. Thigh intermuscular fat is inversely associated with spontaneous GH release in post-menopausal women with abdominal obesity. Eur J Endocrinol. 2006;155(2):261–8. doi: 10.1530/eje.1.02211. [DOI] [PubMed] [Google Scholar]
- 43.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65(3):295–9. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 46.AbouAssi H, Tune KN, Gilmore B, Bateman LA, McDaniel G, Muehlbauer M, et al. Adipose depots, not disease-related factors, account for skeletal muscle insulin sensitivity in established and treated rheumatoid arthritis. J Rheumatol. 2014;41(10):1974–9. doi: 10.3899/jrheum.140224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48(2):301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 48.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34(6):949–59. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 49.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Archives of internal medicine. 2005;165(14):1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(10):1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.