Abstract
Stroke is one of the major causes of disability and mortality worldwide. It is well known that ischemic stroke can cause gray matter injury. However, stroke also elicits profound white matter injury, a risk factor for higher stroke incidence and poor neurological outcomes. The majority of damage caused by stroke is located in subcortical regions and, remarkably, white matter occupies nearly half of the average infarct volume. Indeed, white matter is exquisitely vulnerable to ischemia and is often injured more severely than gray matter. Clinical symptoms related to white matter injury include cognitive dysfunction, emotional disorders, sensorimotor impairments, as well as urinary incontinence and pain, all of which are closely associated with destruction and remodeling of white matter connectivity. White matter injury can be noninvasively detected by MRI, which provides a three-dimensional assessment of its morphology, metabolism, and function. There is an urgent need for novel white matter therapies, as currently available strategies are limited to preclinical animal studies. Optimal protection against ischemic stroke will need to encompass the fortification of both gray and white matter. In this review, we discuss white matter injury after ischemic stroke, focusing on clinical features and tools, such as imaging, manifestation, and potential treatments. We also briefly discuss the pathophysiology of WMI and future research directions.
Keywords: White matter injury, ischemic stroke, demyelination, oligodendrogenesis, axonal damage, therapy, MRI
1. Introduction
The mammalian neocortex is a sizeable structure with lamellar architecture and enlarges rapidly during early development. During primate evolution, the neocortex expanded greatly in size and this was paralleled by improvements in cognitive function. For example, the sum of neocortical gray matter (GM) and nearby white matter (WM) occupies only 10% to 20% of whole brain volume in insectivores, but accounts for 80% of whole brain volume in humans (Zhang and Sejnowski, 2000). According to neuroimaging studies, the volume of WM is 456 ± 48 cm3 in men and 392 ± 42 cm3 in women, which accounts for ~40% of total human brain volume (Pausova et al., 2007). The majority of WM tracts communicate across cortical areas and the rest join the cortex with subcortical structures. As the size of the brain enlarges during development, the WM immediately below the cortex expands disproportionally faster than cortical GM in order to unite distant cortical regions. Similar to GM, WM is critically dependent on a continuous supply of oxygen and glucose. However, WM receives less collateral circulation than GM and has a smaller blood supply, leading to extreme susceptibility to ischemia. Therefore, ischemic stroke rapidly and profoundly damages WM.
In the ischemic environment, glutamate and adenosine triphosphate (ATP), two major excitatory neurotransmitters, play pivotal roles in the pathophysiologic cascades of WMI after stroke. Glutamate and ATP lead to inflammation and oxidative stress (Matute and Ransom, 2012) and eventually induce oligodendrocyte death, axonal demyelination, WM structural damage, and neurobehavioral disorders (Lo et al., 2003). Hence, both GMI and WMI contribute significantly to neurological dysfunction in stroke. Preclinical and clinical studies of stroke have emphasized GMI over WMI, perhaps contributing to the failures of neuroprotectants designed to target neuronal death pathways (Ho et al., 2005; Wang and Shuaib, 2007). Thus, there is an urgent need for additional basic and clinical research on WMI, in the context of the entire brain as a sensitive organ system with highly heterogeneous cellular constituents.
2. Anatomy of WM
The principal components of GM include neuronal cell bodies, dendrites, and axons for local information processing, whereas WM mainly contains long extensions of myelinated and unmyelinated axons that are organized into tracts and surrounding glial cells and blood vessels. WM is classified into periventricular WM and deep WM based on anatomical location. Periventricular WM is found immediately adjacent to the ventricles (within ~1 cm) whereas deep WM is distinctly isolated from the ventricles and found beneath the cortex (Scheltens et al., 1992). WM tracts can be divided into three major categories according to their connectivity and functionality: (1) projection fibers—ascending and descending tracts connecting parts of the cerebral cortex and subcortical structures such as deep gray nuclei, the brainstem, cerebellum, and spinal cord—include pyramidal tracts, thalamic radiations and optic radiations, (2) association fibers—connections between various cortical subregions within the ipsilateral hemisphere—can be long or short and include subcortical U fibers, the cingulum, superior and inferior longitudinal fasciculi, the occipitofrontal fasciculus, and the uncinate fasciculus, (3) commissural fibers—the link connecting homologous areas of the bilateral hemispheres—include the corpus callosum and the anterior commissure (Wycoco et al., 2013).
Microscopically, WM consists of axons, oligodendrocytes, and astrocytes. Axons are the largest element of WM by volume and occupy about 87% of its space (Wang et al., 2008). Axons are tightly wrapped by vast myelin sheets whose integrity is critical for the accuracy and speed of nerve signal conduction (Susuki and Rasband, 2008). As a vital storage space for the neurotransmitter glutamate (Alix et al., 2008), axons act not only as cables that conduct action potentials, but also passageways for transporting biological mediators/nutrients between the cell soma and the synapse. Anterograde axonal transport provides nutrients such as glucose, proteins, and lipids to the distal synapse and to mitochondria for local energy production, whereas retrograde transport delivers a) misfolded and aggregated proteins from the axon terminal to the soma for clearance and b) trophic signals to the soma. Efficient conduction of electrical signals via WM demands a persistent supply of energy along the entire length of the axon. Thus, reduction of the local blood supply may destroy the electrophysiological properties of an entire axon.
Oligodendrocytes are myelin-producing cells responsible for axonal myelination under normal conditions and remyelination after axonal damage. Astrocytes—the most heterogeneous and abundant type of glial cell in the CNS—play important roles in maintaining homeostatic equilibrium and influence myelination (Lundgaard et al., 2014). Glutamate overload may cause excitotoxicity in oligodendrocytes by activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate or N-methyl-D-aspartate (NMDA) receptors. Astrocytes within WM express higher levels of glutamate transporters and exhibit greater capacity for glutamate metabolism compared to astrocytes within GM (Goursaud et al., 2009), supporting the view that glutamate clearance by astrocytes in WM is critical for the maintenance of healthy oligodendrocytes and robust myelination/remyelination. The architectural integrity and connectivity of interneuronal networks in the brain profoundly influences neural activity and function and is associated with behavioral performance and cognitive abilities (Paus et al., 2014). In a review of combined diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI) studies, Bennett described that the relationship between WM integrity and neural activity varies with aging and is influenced by the spatial proximity of the neural measures (Bennett and Rypma, 2013). The correlation between WM intactness and neural viability is positive in younger adults and in regions with direct, first-order connections, whereas there is an inverse correlation between these measures in older adults and in regions connected indirectly.
3. Pathophysiologic mechanisms underlying WMI in ischemia
3.1. Vulnerability of WM to cerebral ischemia
As mentioned earlier, WM is affected in most cases of human stroke, accounting for half of the lesion volume. However, WMI has largely been neglected in animal studies and in clinical trials, partially because 1) the oft-used rodent has much less WM relative to humans and 2) neurons are traditionally held to be more vulnerable to ischemia than oligodendrocytes. However, WM has lower blood flow than GM and there is little collateral circulation, especially in deep WM (Iadecola et al., 2009). Furthermore, oligodendrocytes, especially oligodendrocyte precursor cells (OPCs), are highly sensitive to ischemia-induced oxidative stress (Husain and Juurlink, 1995), excitotoxicity (McDonald et al., 1998; Karadottir et al., 2005; Salter and Fern, 2005; Micu et al., 2006; Domercq et al., 2007), and inflammation, which contribute to OPC death and WMI after ischemia. Importantly, WM becomes more susceptible to ischemia with age (Correa et al., 2011). In sum, WM is highly vulnerable to ischemia and is often injured more severely than GM.
3.2. Mechanisms
Ischemic stroke is a complex pathophysiological process influencing both GM and WM. The pathophysiologic mechanisms underlying ischemia have been mainly focused on animal studies and the potential mechanisms underlying human ischemic stroke remain poorly investigated and largely unknown. Nevertheless, animal studies indicate that excessive release of glutamate, activation of ATP receptors, oxidative stress, inflammation, and apoptosis all contribute to oligodendrocytic death and axonal damage after ischemic brain injury (Mifsud et al., 2014). Recent studies also report these mechanisms in human ischemic stroke. Below we summarize recent advances in mechanistic studies focusing on human ischemic stroke.
3.2.1 Excitotoxicity
The ubiquitous excitatory neurotransmitter glutamate is mostly located in intracellular vesicular stores in the brain. Abnormally high levels of glutamate in the extracellular fluid exert neurotoxic effects upon surrounding tissues under pathological conditions. Excessive glutamate in plasma and cerebrospinal fluid (CSF) contribute to the progressive neurological deterioration and infarct growth in stroke victims (Boyko et al., 2014). NMDA receptors are known to participate in glutamate-mediated brain injury, and are pivotal players in the neurotoxicity of brain ischemia. Dambinova and colleagues have reported that acute ischemic stroke patients exhibit higher levels of NMDA receptors in the bloodstream than healthy individuals, and that this characteristic has 97% sensitivity and 91% predictive value for diagnosing ischemic stroke within 72 hours after symptom onset (Dambinova et al., 2003). Furthermore, Campos et al demonstrated that high glutamate oxaloacetate transaminase concentrations in blood are associated with superior neurological outcomes in stroke patients (Campos et al., 2011).
3.2.2. Inflammation
Inflammatory reactions are common in stroke, even in the absence of infections, and induce activation of resident microglia and astrocyte, infiltration of peripheral immunocytes, and production of proinflammatory mediators, thereby leading to tissue necrosis and eventual brain damage (Jin et al., 2010). There is a positive association between elevated concentrations of inflammatory indicators in the peripheral circulation—including C-reactive protein (CRP), MMP-9 and interleukin-6 (IL-6)— and the initial severity as well as poor prognosis of ischemic stroke. In addition, these biomarkers are independent predictors of the first cerebral infarction and recurrent ischemic events (Nardi et al., 2012). Oh et al reported that multiple plasma inflammatory factors—such as MMP-9 and interleukin-18 (IL-18) receptor accessory protein—are modified in the acute stages of ischemic stroke, suggesting that immunity and inflammation participate in the development and progression of stroke (Oh et al., 2012). Zhang et al found that natural killer cell infiltrations peak between 2 and 5 days after ischemia in the ischemic hemispheres in humans, and induced necrosis and BBB damage through cytotoxic cytokines (Zhang et al., 2014d).
3.2.3. Oxidative stress
Oxidative stress is a major contributor to the pathogenesis of ischemic stroke. Increased production of free radicals elicts ischemia/reperfusion injury in the plasma membrane and intracellular components through the oxidative modification of lipids, protein and carbohydrates, resulting in necrosis or apoptosis (Nakka et al., 2008). Enhanced formation of advanced glycation end product, a marker of oxidative stress, was discovered in the skin of patients with chronic ischemic stroke or silent brain infarction (Ohnuki et al., 2009). Uric acid, which can alleviate glucose-driven oxidative stress, has been associated with a reduction in infarct volume and better prognosis in ischemic stroke patients (Amaro et al., 2015).
Cell apoptosis, endothelial dysfunction, and destruction of the integrity of the BBB, all play a role in the pathophysiologic processes underlying ischemia/reperfusion injury. These pathways do not work in isolation, but overlap and interact synergistically.
4. The relationship between WMI and ischemic stroke
Cerebral WMI is detected in more than half of normal elderly individuals (de Leeuw et al., 2001) and in 64-86% of stroke patients (Fu et al., 2005; Li et al., 2013). Furthermore, WMI progression is present in 8-28% of non-demented elderly patients (Enzinger et al., 2007) and 32% of ischemic stroke patients (Cho et al., 2015), implying that WMI has a dynamic course through late life and after injury.
4.1. WMI: a predictor of stroke
Cerebral WMI is probably caused by chronic hypoperfusion (Kurumatani et al., 1998; Debette et al., 2010) and is more frequent and severe in stroke patients than in healthy controls. It also is an important risk factor for stroke. The Framingham heart study demonstrated that the presence of severe WMI at baseline increases the risk of stroke death by two-fold and the risk of subsequent dementia four-fold (Debette et al., 2010). In the second ARTerial disease-Magnetic Resonance (SMART-MR) study, Conijn and colleagues reported that the presence of lacunar infarcts increases the risk of vascular and nonvascular mortality (Conijn et al., 2011). The severity of WMI is associated with an increased risk of vascular death and ischemic stroke, regardless of the co-existence of cerebrovascular disease. Lacunar infarcts and WMI are predictors of poor prognosis in patients with atherosclerotic diseases.
As argued above, WMI is a robust indicator of stroke (Leonards et al., 2012). There is a linear correlation between the National Institutes of Health Stroke Scale (NIHSS) and infarct volume (r=0.591; P<0.001), while this association falls with aggravated WMI burden from r=0.786 (P<0.001; absent WMI) to r=0.498 (P<0.001; severe WMI). Both WMI and infarct size are related to the NIHSS deficit. With increasing WMI load, similar infarct volumes cause more severe functional impairments. Helenius and Henninger demonstrated that pre-existing WMI is related to infarct volume and NIHSS (Helenius and Henninger, 2015). One study on a large single-center cohort (2485 intravenous thrombolysis-treated patients with ischemic stroke) showed that WMI on non-contrast CT scans upon admission is linked with a two-fold increase in symptomatic intracerebral hemorrhage (sICH) (Curtze et al., 2015a). It is also related to poor recovery 3 months after stroke. Not surprisingly, WMI load is an independent risk factor for adverse outcomes in IVT–treated patients with stroke (Curtze et al., 2015b). Furthermore, the severity of pre-existing WMI is an independent predictor of unfavorable long term functional outcomes in acute ischemic stroke patients undergoing endovascular therapy (Zhang et al., 2014b). Podigorska and colleagues report that the mortality at 1 year after stroke was 45.8% in patients with WMI, but 28% in those without WMI (Podgorska et al., 2002). WMI leads to a loss of WM bundle architecture and functional integrity (Lawrence et al., 2014), which may damage cerebral plasticity and impair compensatory mechanisms during post-stroke recovery (Ruber et al., 2012).
4.2. Stroke: a cause of WMI
As argued above, WMI can influence stroke risk. Conversely, ischemic stroke is an established cause of WMI. WM is profoundly injured by infarction in the supplying territory of small perforating vessels that link into the centrum semiovale and deeper fiber pathways. Occlusion of the large intracranial arteries, which supply both superficial GM and WM, offers an additional opportunity for WMI (Goldberg and Ransom, 2003). Several studies have demonstrated that the predominant lesions caused by stroke are located in subcortical regions, which comprise more than 85% of the total number of strokes (Wessels et al., 2006). Ho et al demonstrated that WM occupies a median of 49% of the infarct volume. About 95% of infarcts present with some involvement of WM bundles. As the connection between WMI and ischemic stroke is critically related to clinical outcomes, neuroprotection of WM should be more widely considered in designing therapeutic strategies (Ho et al., 2005).
In a prospective and longitudinal study, the cortical thickness and microstructural integrity of connecting fiber tracts were measured in 32 patients at both stroke onset and 6 months after the event (Duering et al., 2015). Duering and colleagues reported that focal cortical degenerative changes induced by acute infarcts were more significant in the areas with dense connections with the core region of injury, indicating that acute infarcts cause secondary neurodegeneration in remote brain regions via connecting fiber tracts. Cheng et al also reported a direct impact of focal subcortical ischemic lesions on remote cortical atrophy, secondary to retrograde or anterograde degeneration. These authors also discovered adaptive reconstruction in areas sharing connections with the ischemic infarct zone during stroke recovery (Cheng et al., 2015). Liu et al used diffusion tensor imaging (DTI) in 18 patients with acute subcortical infarcts at 1, 4 and 12 weeks after ischemia onset and found that the severities of motor deficits of patients at 4 and 12 weeks were greater than those at 1 week (Liu et al., 2015). Progressive degeneration of motor-related fiber pathways above and below the ischemic lesion occurred early after stroke and lasted for up to 3 months. Furthermore, progressive structural remodeling of contralateral WM tracts related to motor, cognitive, and sensory processing was positively associated with motor function recovery in the acute and sub-acute stages of stroke. The compensatory changes in fiber tracts in non-motor regions of the unaffected hemisphere after acute infarction reflect the significance of WM architectural integrity and plasticity, which enables the brain to mitigate the impact of the primary motor impairment.
5. Imaging analysis of WMI
5.1. Magnetic resonance imaging (MRI)
MRI is a noninvasive technique that can provide three-dimensional assessment of tissue morphology, metabolism, and function and has been widely applied to display the architecture and function of WM.
5.1.1. Routine MRI
Due to the differences in water content and myelination, T1, T2, and fluid-attenuated inversion recovery (FLAIR) sequences used in conventional MRI can accurately distinguish GM and WM (Forkel et al., 2014) and measure the volume of WM (Perrin et al., 2009). In theory, the volume of WM represents the quantity of axons, their diameter, the thickness of the medullary sheath, as well as their compactness (Paus et al., 2014). WMI presents as WM hyperintensities on T2-weighted or FLAIR sequences, and hypointensities on T1-weighted images relative to normal white and grey matter (Fig.1).
Figure 1.
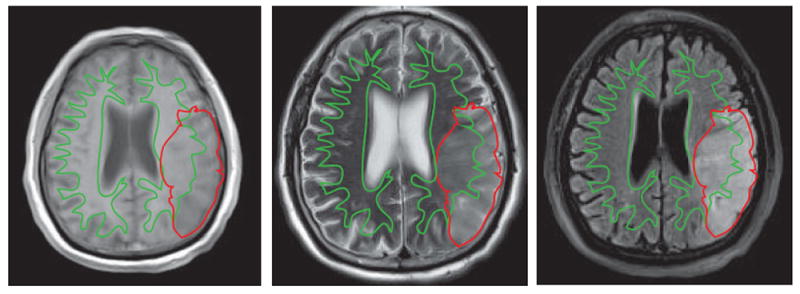
Transverse images in a 73-year-old man 48 hours after left middle cerebral artery (MCA) occlusion. T1 WI (a) shows mixed areas of abnormal signal intensity in the GM and WM of the temporal lobe. T2 WI (b) and FLAIR (c) images demonstrate high intensity signal in the infarct zone with subtle prominence of WM. WM is traced with a green line and the infarct region is traced with a red line. WM infarct regions are enclosed by both the red and green outlines, whereas GM infarct regions are defined as red regions outside the green outline.
5.1.2. Diffusion-weighted imaging (DWI)
DWI is sensitive to thermal movement of water molecules and can detect hyperacute infarction in the deep WM or in the brainstem (Oliveira-Filho et al., 2000). Swift attenuation of the apparent diffusion coefficient (ADC) in ischemic tissue produces hyperintensity on DWI that enables early diagnosis of ischemic stroke (Fig. 2). There is often a time-dependent change of ADC in WM fibers following ischemia. After a prompt decrease within one hour after stroke onset, the mean ADC in WM remains persistently low for 1 to 4 days, and then increases slowly. From one hour to a few days after stroke onset, there is a decline in ADC parallel with the WM fibers, while there is an increase in ADC perpendicular to the fiber bundles. This phenomenon might be attributed to the progression of the focal intracellular edema in glial cells and axons (Tamura et al., 2009).
Figure 2.
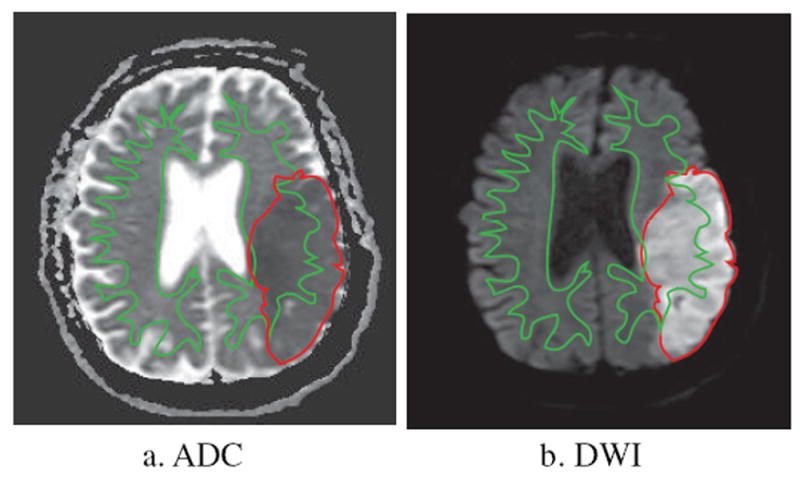
DWI image in the same patient as in Figure 1. a. ADC shows mixed areas of decreased signal intensity in the GM and WM of the temporal lobe. b. DWI demonstrates increased signal intensity in the infarct zone. WM infarct regions are enclosed by both the red and green outlines, whereas GM infarct regions are defined as red regions outside the green outline.
5.1.3. Diffusion tensor imaging (DTI)
DTI can detect the magnitude and directionality of water molecules in live tissue and is more sensitive than conventional MRI in detecting WMI (Fig. 3). As the movement of the water molecules in the GM is equivalent in every direction, the fractional anisotropy (FA) value of GM is very low. In contrast to GM, water in WM moves swiftly along the direction of the axon due to the axonal tract, while myelination inhibits water movement vertical to the fasciculation, presenting a high FA value (Beaulieu, 2002). By calculating the mean diffusivity (MD) and FA of water diffusion, DTI can assess the integrity and connectivity of WM tissue from a network perspective, and reconstruct the distribution of WM pathways in the brain three-dimensionally (Yap et al., 2013). Besides MD and FA, other diffusion metrics such as axial (AD) and radial (RD) diffusivity, which measure diffusivities parallel and perpendicular to the principal direction of the tensor respectively, can help determine the WM microstructural pathophysiology underlying the FA change (Burzynska et al., 2010). Attenuation in RD usually means ameliorative myelination, whereas an increase in AD reflects axon morphological damage (Tang et al., 2012). Using DTI in stroke patients, the RD enhancement is more general than the AD enhancement, which is interpreted as more severe and widespread demyelination than axonal degeneration (Song et al., 2003).
Figure 3.
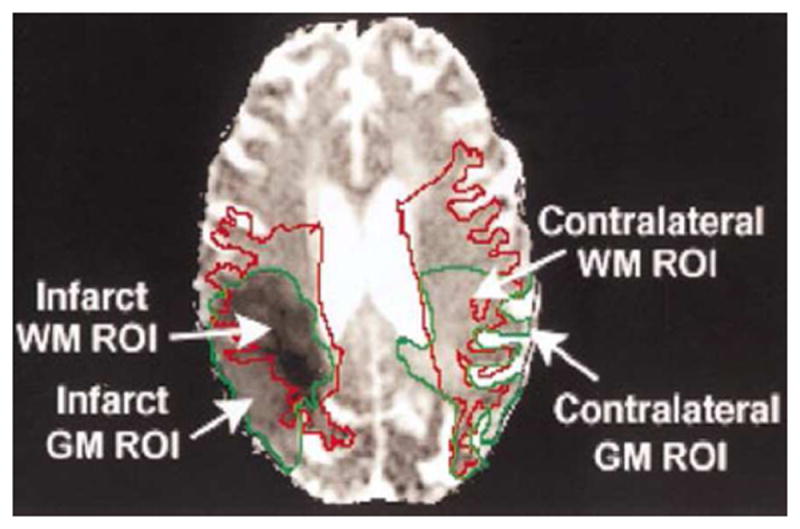
DTI image in an 87-year-old woman 3–4 days after middle cerebral artery occlusion. The region of interest (ROI) in WM was traced as a red line in both ipsilateral and contralateral hemispheres. The infarct regions were outlined with green lines. Infarct WM is enclosed by both the red and green outlines. The regions enclosed by the green outline but outside the red outline are infarct GM (adapted from Radiology 2000, 215:211-220 with permission).
5.1.4. Diffusion kurtosis imaging (DKI)
DKI is an improvement over DTI that captures the non-Gaussian quality of water diffusion in vivo (Umesh Rudrapatna et al., 2014) and exhibits enhanced sensitivity to the microstructural heterogeneity and complexity of brain tissue (Cheung et al., 2012). By supplying primary information, DKI compliments DTI well in demonstrating the structural tissue changes that reflect post-stroke tissue remodeling.
5.2. Magnetization transfer imaging (MTI)
MTI reveals the degree of myelination indirectly. Magnetization transfer ratio (MTR), or the exchange of magnetization between free water and combined water bound to macromolecules (McGowan, 1999), is positively associated with myelin amounts (Schmierer et al., 2008). Because macromolecules such as proteins and myelin lipids are the main source of this signal in WM (Kucharczyk et al., 1994), this parameter is also influenced by axonal cytoskeleton components that contain the same macromolecules (Nossin-Manor et al., 2013). A low MTR value means that the ability of macromolecules in the brain tissue to exchange magnetization with ambient water molecules decreases, indicating damage to myelin and axonal membrane (Patel and Markus, 2011).
5.3. Magnetic resonance spectroscopy (MRS)
MRS reveals the brain metabolism of critical metabolites such as N-acetylaspartate, total creatines, total cholines, and myoinositol, and exhibits higher sensitivity to underlying neuroaxonal injury or dysfunction than conventional imaging. N-acetylaspartate is an indicator of neuronal and axonal function and is often reduced in ischemia (Fig. 4). Creatines are found primarily in neurons, oligodendrocytes, and astrocytes and reflect the synthesis and degradation of phospholipid membranes. Myoinositol is a glial metabolite and often observed to be increased after stroke, which is interpreted as chronic gliosis. In addition, lactate, a by-product of anaerobic glycolysis, usually appears in ischemic regions (Fig. 4).
Figure 4.
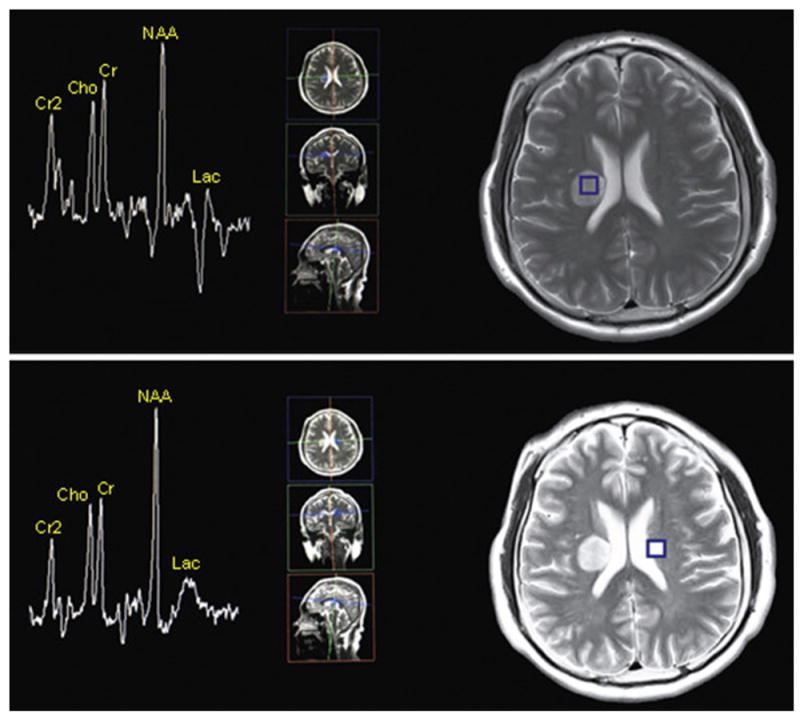
MRS images in a 43-year-old man 5 days after right MCA infarction. Compared with the MRS image in homologous parts of the contralateral hemisphere (b), the MRS image in the infarction area (figure a) shows decreases in N-acetylaspartate (NAA), total creatines (Cr) and total cholines (Cho), and an inversed peak of lactate (lac) appears in the infarction area. I: integral, A: amplitude
5.4. Blood oxygen-level dependent functional MRI (BOLD-fMRI)
BOLD-fMRI assesses cerebral blood flow by testing the magnetic susceptibility of deoxygenated versus oxygenated hemoglobin. BOLD-fMRI reflects the capacity of the networks in the brain and calculates the cerebral vascular reactivity reliably and reproducibly. Activated neurons increase regional blood flow and oxygenated hemoglobin, leading to a variation in BOLD signal. By mapping the fluctuations of BOLD signal related to an executive task, structural and functional information can be acquired simultaneously (Baird et al., 2005). Several studies have demonstrated that high WMI load is correlated with a dampening of BOLD signal (Patel et al., 2012), suggesting altered WM integrity in the neural network and neurological dysfunction. captures the non-Gaussian quality of water diffusion in vivo (Umesh Rudrapatna et al., 2014) and exhibits enhanced sensitivity to the microstructural heterogeneity and complexity of brain tissue (Cheung et al., 2012). By supplying primary information, DKI compliments DTI well in demonstrating the structural properties of the central nervous system.
Various imaging modalities for assessment of WMI are summarized in Table 1.
Table 1.
Imaging modalities for detection and quantitation of WMI
| Primary attribute | Imaging manifestations of WMI | |
|---|---|---|
| Routine MRI | Distinguishes GM and WM, measures the volume of WM | Hyperintensity on T2-weighted or FLAIR, hypointensity on T1-weighted images |
| DWI | Detects hyperacute infarction in deep WM or in the brainstem | Hyperintensity on DWI; time-dependent change of ADC in WM following ischemia |
| DTI | Assesses the integrity and connectivity of WM, reconstructs the distribution of WM pathways three-dimensionally. | Decreased FA values and increased MD values indicate structural breakdown of axons and disconnection/distortion of tracts. |
| DKI | Reflects post-stroke tissue remodeling, provides independent and complementary information relative to DTI | Increased MK, decreased FA, and MD value |
| MTI | Reveals the degree of myelination | Decreased MTR value |
| MRS | Measures the brain metabolism | Decreases in NAA, total Cr and total Cho |
5.5. CSF and blood biomarkers
Ischemic insults in the brain affect both GM and WM, as reflected in the biochemical composition of nearby CSF and blood. Hjalmarsson et al examined the content of glia-related inflammatory factors, such as YKL-40, glial fibrillary acidic protein (GFAP), monocyte chemotactic protein 1(MCP-1), and glycoprotein CD14, and neurodegeneration-related markers of myelin and cytoskeleton such as neurofilament light polypeptide (NFL), myelin basic protein (MBP), and T-Tau in CSF (Hjalmarsson et al., 2014). The authors reported that the CSF concentrations of NFL, T-tau, MBP, YKL-40 and GFAP were higher in patients with acute ischemic stroke than healthy subjects, and these markers were positively correlated with clinical stroke severity. Furthermore, the myelinated axon marker NFLcorrelated with the degree of WMI. Elevated CSF/serum albumin ratios indicate BBB dysfunction and are correlated with WMI in subcortical ischemic vascular disease patients with dementia (Wallin et al., 2012). Other biomarkers such as plasma CRP, IL-6, homocysteine, urine albumin/creatinine ratio, and CSF MMP-9—all which are involved in inflammation and endothelial dysfunction—have been associated with WMI in patients with subcortical ischemic vascular disease (Vilar-Bergua et al., 2015).
In sum, the available information about candidate biomarkers in blood and CSF highlights multiple interconnected pathways in the pathogenesis of WMI caused by ischemia. However, data on human CSF biochemical parameters related to stroke are very limited, and the sensitivity and specificity of these biomarkers in blood and CSF are still unknown. Future studies on specific biomarkers in blood and CSF after WMI may shed more light on underlying mechanisms, thereby advancing diagnostic strategies and improve our ability to monitor the efficacy of treatments.
5.6. Electrophysiological examination
Transcranial magnetic stimulation (TMS), a non-invasive tool to map electrophysiological changes in cortical excitability, has revealed new insights into the physiological mechanisms behind motor disturbances and neuroplasticity after ischemic stroke (Dimyan and Cohen, 2010). Lyseniuk et al have demonstrated that the absence of motor evoked potentials (MEP) in the affected hemispheres is associated with poor functional recovery, while recordable MEP (even if reduced in amplitude and prolonged in latency) is associated with better prognosis in patients with ischemic stroke (Lyseniuk et al., 2014). Furthermore, increases in central motor conduction time (CMCT), which indicates axonal or demyelinating damage of the corticospinal tract, has been reported in the affected hemisphere (Prashantha et al., 2013). MEP, when combined with CMCT, evaluates the functional integrity of corticospinal pathways, and reveals neurophysiological changes in these tracts in ischemic stroke (Dimyan and Cohen, 2010). Using TMS, Prashantha et al showed that the resting motor threshold (rMT), which is linked with neuronal membrane excitability and white matter microstructure, was reduced over time in the unaffected side in stroke patients, suggesting a progressive increase in cortical excitability of the contralateral hemisphere that can be attributed to damaged transcallosal inhibition and intracortical inhibition in the contralateral hemisphere (Prashantha et al., 2013). Jiang et al concluded that BMSC transplantation can reduce cerebral infarct volume and improve functional outcomes in rat, perhaps through electrophysiological effects on the motor cortex and associated pathways (Jang et al., 2011).
Brainstem auditory evoked potentials (BAEP), which are generated from auditory nerves and the brainstem, and somatosensory evoked potentials (SEP), which are generated from the spinal cord and the dorsal column-lemniscal system, can be used to assess the integrity of two different afferent pathways in patients (Burghaus et al., 2013). In several clinical studies, BAEP and SEP have been used as predictors of stroke prognosis. Zhang et al demonstrated that the bilateral absence of short-latency SEP N20 and BAEP wave V had 100% specificity and positive predictive value for poor outcomes in patients with massive hemispheric infarction (Zhang et al., 2011b). Joo et al discovered that constraint-induced movement treatment improved motor recovery in rats with ischemic stroke and coincided with beneficial changes in SEP suggesting that the reestablishment of somatosensory pathways likely contribute to functional recovery (Joo et al., 2012).
Electroencephalography (EEG) records the electrical activity created by the brain with a high temporal resolution and provides a dynamic view of rapid variations in functional activity (Duru et al., 2015). EEG functional connectivity is determined to a great extent by WM axons, especially cortico-cortical axons (Wan et al., 2011). The velocity of impulse transmission is highly plastic and mainly dependent on myelination. The synchrony of electrical activities between different cortical areas is essential for optimal neuropsychological performance. By relying on multiple spatiotemporal dynamic scalp patterns, such as frequency, phase spectra, covariance, and coherence, EEG can provide explicit multi-scale assessments of functional continuity in the brain, and WMI breaks this normal EEG coherence (Nunez et al., 2015). It has been demonstrated that resting state theta rhythm power detected by EEG is linked with default mode network activity in a set of brain regions that are active when an individual is at wakeful rest (Scheeringa et al., 2008).
In sum, electrophysiological examinations reflect the network connectivity and functional continuity in the brain, and can reveal robust neurophysiological changes after stroke and during rehabilitation. Establishment of electrophysiological criteria for the diagnosis of WMI in ischemic stroke may help us quantitatively evaluate the severity of WMI and the effectiveness of treatment interventions.
6. Clinical manifestations
WMI may cause cognitive dysfunction, emotional or affective disorders, sensorimotor impairments, gait disturbances, disequilibrium, as well as urinary incontinence and pain. These clinical manifestations are mainly related to the subcortical/WM topography of stroke and damage to WM tracts, in particular junctional or nodal areas with massive fiber convergence. The destruction of WM integrity is therefore thought to play an important role in neurological impairments (Corbetta et al., 2015).
6.1. Cognitive dysfunction
Cognitive dysfunction exists in about 64% of patients with stroke, of which, up to one third develop dementia (Nichols and Holmes, 2002). Decreased WM integrity induced either locally or remotely by ischemia has been reported to contribute to cognitive deficits in stroke (Molko et al., 2002). Cognitive dysfunction depends on volume and strategic location of brain infarction, site and range of cerebral WM, bilaterality and number of stroke lesions, and other co-existent pathologies (Grysiewicz and Gorelick, 2012). Some studies have shown that cognitive dysfunction is correlated with WMI in the frontal lobes, basal ganglia, and thalamus of stroke patients—in whom fronto-striato-thalamic pathways are impaired—and subsequent involvement of the dorsolateral prefrontal cortex and anterior cingulate, regions known to support attention and executive function (Cumming et al., 2013).
In a community-based sample of older adults free of clinical stroke, Dong et al found a negative relationship between WMI burden and cognitive performance, irrespective of brain atrophy (Dong et al., 2015). Chaudhari et al demonstrated that, in addition to poor educational background, location of infarct, and baseline stroke severity, WMI load is also an independent predictor for post-stroke cognitive dysfunction (Chaudhari et al., 2014). In the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study, MRI scans were performed on 142 mild to moderate stroke or transient ischemic attack patients within seven days of stroke onset (Kliper et al., 2014). Patients were cognitively assessed again one year after the event. Patients with preexisting WMI were more susceptible to cognitive dysfunction, independent of the volume and location of ischemic lesions. The integrity of the normal appearance of WM may be a marker for “brain reserve properties” and an indicator of higher cognitive abilities. In a single-center study, 426 subjects with cerebral small vessel disease without dementia (between 50 and 85 years old) were recruited and underwent MRI scanning (Tuladhar et al., 2015). There was an inverse relationship between WMI burden and cortical thickness in frontotemporal regions, representing a region-specific association. WMI load was positively correlated with cortical thickness in paracentral areas, which may be a compensatory reaction to increased WMI. Furthermore, heavy WMI burden was linked with decreased global efficiency and increased distance of pathways, indicating that network breakdown contributes to the development of cognitive disturbances.
Lacunar infarcts account for more than 20% of stroke and commonly involve WM (Fern et al., 2014). Compared with healthy individuals, the general mental status, memory, attention, executive function, and language abilities are lower in patients with silent lacunar infarcts in the basal ganglia. Fern et al demonstrated that tract-specific damage of WM and region-specific lesions and network disruption accompanied cognitive declines and eventually led to dementia. DTI of patients with lacunar infarcts reveal decreased FA and increased MD across several WM regions either close to or relatively far from lesions, implying that WM damage not only influences nearby areas, but also extends across considerable distances (Chen et al., 2015b). Lunven et al directed a prospective, longitudinal study that measured microstructural markers of neglect restoration after ischemia in the MCA territory (Lunven et al., 2015). They found that disruption of WM fronto-parietal circuits (intrahemispheric disconnection) is a critical element of spatial neglect. In addition, dynamic changes in WM of the splenium of the corpus callosum (interhemispheric disconnection) contributes to the persistence of long-term neglect. These two findings underscore the clinical significance of network integrity and continuity, both within and across the hemispheres. Dacosta-Aguayo et al examined the relationship between changes in cerebral WM integrity and cognitive recovery in patients 3 months after ischemic stroke (Dacosta-Aguayo et al., 2014). In comparison to healthy individuals, WM integrity was affected in both hemispheres in stroke patients. Patients with poor cognitive recovery who performed worse in attention, motor, executive and processing speed functions experienced greater WMI in the non-injured hemisphere.
In addition to affecting lesion volume and localization, WM changes are also instrumental in the manifestation and severity of neurological impairments and predict recovery or secondary cognitive dysfunction after stroke.
6.2. Depression
Depression is a frequent and severe emotional disorder that emerges in approximately 33% of stroke survivors (Esparrago Llorca et al., 2015) and is closely linked with additional disability, cognitive deficits, and even death (Whyte and Mulsant, 2002). Several studies have shown that WMI in the frontal and temporal lobes are related to depressive symptoms. In addition, WMI involvement in specific WM fiber tracts, such as the cingulum bundle, uncinate fasciculus, and superior longitudinal fasciculus may be an underlying cause of depression (Taylor et al., 2013). In comparison to healthy controls, a reduction in FA was found in the bilateral anterior limb of the internal capsule in stroke patients, although no patient exhibited frank lesions at this site (Yasuno et al., 2014). There was a negative correlation between the increase in FA values and a reduction in depression scores 6 months after stroke. Yasuno et at also argued that the decrease in FA is linked with an attenuation of dementia risk, indicating that microstructural breakdown in WM is the result of axonal damage, which may be induced by Wallerian degeneration secondary to neuronal death (Thomalla et al., 2004). Consistent with these views, the anterior limb of the internal capsule—an intercept point in the frontal-subcortical loops (Axer and Keyserlingk, 2000)—plays an essential role in behavior and emotional control (Duran et al., 2009). As a result, the destruction of WM within frontal-subcortical circuits may help trigger the onset of depressive symptoms (Budde et al., 2007).
6.3. Motor deficits
Motor circuits execute motor movements in a synergistic fashion and their integrity is a predictor of motor function following stroke (Gauthier et al., 2014). Wu et al reported that acute stroke lesions in WM are linked with more severe neurological deficits at admission and long-term disabilities (Wu et al., 2015). Zhang et al showed that WM structural complexity, which is negatively correlated with motor disability changes in the stroke-affected hemisphere, is lower than in the unaffected hemisphere (Zhang et al., 2008). Riley et al also found that damaged motor tracts, specifically those descending from the primary motor and dorsal premotor cortices, can benefit from behavioral therapy in individuals with chronic stroke (Riley et al., 2011). Furthermore, tract-specific lesions are more significant than infarct size or baseline behavioral status at predicting prognosis, which can be used to identify those subjects who might benefit the most from treatment. By reinforcing ‘fungible’ trajectories, neuroplasticity can help restore brain function after stroke (Zaaimi et al., 2012). This success of neuroplasticity after stroke depends on the viability and integrity of the remaining tissue and the extent of functional reconstruction that they are able to reach (Lindenberg et al., 2012).
7. Management and treatment
WM is intimately involved in the relay of motor and sensory information to and from the cerebral cortex, and determines the degree of cognitive function. If only neuronal somas but not axons were protected after stroke, the axonal demyelination and damage would still interfere with neuronal signal transduction and function. Thus, interventions targeted at both GM and WM are expected to improve post-stroke quality of life. Thus far, however, all treatments targeting WMI are still limited to preclinical animal studies and there is no specific treatment for human WMI in ischemic stroke. Potential treatments targeting WMI include risk factor management, thrombolytic therapy, prevention of WMI, stimulation of oligodendrogenesis and axonal plasticity, and cell therapy.
7.1. Risk factor control
Studies have confirmed that hypertension plays a role in the loss of BBB integrity (Choi et al., 2015) and is a strong predictor of WMI. Adequate control of hypertension might alleviate the progression of WMI. Godin et al (Godin et al., 2011) prospectively compared subjects exhibiting high systolic blood pressure (SBP) (≥160 mmHg) at baseline with or without antihypertensive medication. They found that subjects with antihypertensive treatment had smaller increases in WMI volume at a 4-year follow-up than those without hypertensive treatment (0.24 cm3 vs. 1.60 cm3, P=0.008). Individuals with diabetes mellitus are more likely to suffer severe and early WMI (J et al., 2009). Consistent with these findings, Chen et al found that hyperglycemia aggravates WMI in the ischemic area and worsens functional prognosis in diabetic mice (Chen et al., 2011). The underlying mechanism may be that hyperglycemia inhibits the proliferation and survival of OPCs by promoting oxidative stress and stimulating MMP-9 activity (Yatomi et al., 2015).
Homocysteine is a risk factor for WMI in stroke subjects (Tseng et al., 2009). Cloonan et al demonstrated a positive correlation between WMI load and homocysteine (β=0.11, p=0.003) as well as hemoglobin A1c levels (β=0.1, p=0.008), both of which are associated with endothelial dysfunction via oxidative stress (Cloonan et al., 2015). Obviously, controls of other traditional cardiovascular risk factors such as diabetes mellitus, hyperlipidemia, and chronic renal failure may also ameliorate the destruction of WM.
7.2. Thrombolytic therapy
To date, recombinant tissue-type plasminogen activator (r-tPA) is the only effective approach for the treatment of acute ischemic stroke, both for GMI and WMI. Pantoni et al demonstrated that patients with acute lacunar infarcts have a good clinical response to thrombolysis (Pantoni et al., 2014). In addition to thrombolytic function, tPA can inhibit oligodendrocyte apoptosis and maintain WM integrity after ischemic stroke through an epidermal growth factor-like effect (Correa et al., 2011). However, less than 5% of ischemic stroke patients qualify for tPA treatment. Thus, other approaches that can either prevent WMI or stimulate oligodendrogenesis and axonal plasticity are urgently needed for stroke recovery.
7.3. Pharmacological neuroprotectants
Neuroprotection from ischemic injury includes the goal of quelling harmful molecular and cellular pathways that lead to ischemic cell death and is defined as a correction of pathophysiological manifestations of ischemic brain damage. As a supplementary or adjunct approach to thrombolysis, a number of pharmacological neuroprotectants, such as free radical scavengers, anti-inflammatory drugs, and excitatory amino acid antagonists, have shown efficacy and safety in animal stroke models (Table 2).
Table 2.
Pharmacological neuroprotectants for WMI under investigation
| Class | Representative drugs | Potential mechanisms |
|---|---|---|
| Antioxidant | Ebselen, Arundic acid, Citicoline | Scavenger of free radicals and ROS; decrease of NO production and S100 calcium-binding protein expression |
| Anti-inflammatory | Minocycline | Inhibition of MMP-9 and microglia activation, attenuation of apoptosis |
| Anti-excitotoxicity | Magnesium | Blocker of glutamate transport and efflux, reverser of Na+-Ca2+ exchange |
| Growth factors | BDNF, NAMPT, Erythropoietin | Stimulation of oligodendrogenesis, inhibition of OPC apoptosis |
7.3.1. Prevention of WMI
Antioxidants and anti-inflammatory agents have been shown to attenuate WMI (Shereen et al., 2011). The antioxidant ebselen can reduce oligodendrocyte and axonal damage and enhance neurological outcomes when administered 2 hours after the onset of stroke (Imai et al., 2001). Arundic acid downregulates inducible nitric oxide synthase in astrocytes. With decreased nitric oxide production, there is less toxicity in neighboring cells such as oligodendrocytes. Furthermore, arundic acid may reduce the expression of S100 calcium-binding protein, which is primarily expressed in astrocytes and is correlated with the lesions (Aurell et al., 1991). Citicoline is an essential component of membrane phospholipids, which are degraded into fatty acids and free radicals in cerebral ischemia. Citicoline has been extensively tested in clinical trials (Clark et al., 1997; Clark et al., 1999; Davalos et al., 2002; Alix et al., 2008; Davalos et al., 2012). Although largely negative results were reported, thus far citicoline is the only neuroprotective drug with consistent neuroprotective benefits against stroke, especially in less severely injured elderly patients. In addition to its neuroprotective effects, citicoline can also prevent WMI (Lee et al., 2009).
Minocycline is a potent anti-inflammatory agent and has been shown to be beneficial in patients with acute ischemic stroke in several clinical trials (Lampl et al., 2007; Amiri-Nikpour et al., 2015). It is highly likely that minocycline exerts its neuroprotective effects by targeting both gray matter injury (GMI) and WMI. In line with this view, minocycline was reported to be protective against WMI in ischemic stroke by targeting multiple mechanisms underlying WMI. For example, minocycline inhibits the activation of MMP-9, thereby alleviating WMI induced by hypoxia in spontaneously hypertensive/stroke prone rats (Jalal et al., 2015). As mentioned above, minocycline also has potent anti-inflammatory effects and may prevent WMI by inhibiting microglial activation in neonatal models of hypoxia-ischemia (Carty et al., 2008).
Treatments targeting excitotoxicity have been quite promising in preclinical studies. Blockers of axonal Na+ ion channels reverse Na+-Ca2+ exchange and decrease Na+-dependent glutamate transport, thereby inhibiting excitotoxic damage to oligodendrocytes. Glutamate transport inhibitors are thought to block the glutamate efflux triggered by the collapse of ionic gradients, especially the transmembrane Na+ gradient (Li et al., 1999; Baltan et al., 2008; Alix and Fern, 2009).
Finally, inhibition of histone deacetylase may also reduce stroke injury, rescue neurons from apoptosis, and sustain WM function (Baltan et al., 2013).
7.3.2. Stimulation of oligodendrogenesis and axonal plasticity
Oligodendrogenesis and axonal plasticity play important roles in maintaining the structure and function of axons. Therefore, interventions targeting oligodendrogenesis and axonal plasticity might improve behavioral outcomes. Ischemic stroke induces endogenous oligodendrogenesis and there are large numbers of newly generated OPCs in the subventricular zone after ischemia. However, only a small amount of OPCs are able to survive, migrate to, and differentiate into mature myelin producing oligodendrocyte in the ischemic region. Therefore pharmacological drug/growth factors that can stimulate and protect endogenous oligodendrogenesis are critical for WM reorganization. Aspirin, a standard therapy for the prevention and treatment of ischemic stroke, possesses a variety of pharmacological activities and neuroprotective effects. Chen et al reported that aspirin can accelerate the proliferation and differentiation of OPCs as well as oligodendrocyte myelination after WMI (Chen et al., 2014c).
Brain-derived neurotrophic factor (BDNF) stimulates oligodendrogenesis, resulting in enhanced remyelination and fiber connectivity and superior functional outcomes in rats subjected to subcortical damage in ischemic stroke (Ramos-Cejudo et al., 2015). Nicotinamide phosphoribosyltransferase (NAMPT) has also been implicated in neuroprotection against ischemic brain injury. NAMPT is secreted into extracellular space after ischemia, where it binds to and protects WM against ischemic injury, suggesting a novel role for secreted NAMPT in WM protection after ischemic injury (Jing et al., 2014). Erythropoietin inhibits OPC apoptosis, stimulates oligodendrogenesis, and improves neurological outcomes after neonatal hypoxic ischemic injury (Iwai et al., 2010). Erythropoietin has also been reported to enhance WM reorganization, restore local cerebral blood flow, and expedite functional recovery (Li et al., 2009).
Despite the successes in animal models, almost all the pharmacological agents that show potential in pre-clinical studies of ischemic injury have failed to translate into positive outcomes in clinical trials (Hoyte et al., 2004).
7.4. Cell therapy
Cell-based therapies can increase neuroplasticity and improve the architecture and physiology of cerebral tissue, which promote neurological recovery after ischemic stroke (Chen et al., 2014b). The underlying mechanisms contributing to these therapeutic effects include cell replacement, nutritional support, immunoregulation, and activation of endogenous pro-survival processes such as neurogenesis, vasculogenesis, and oligodendrogenesis (Liu et al., 2014).
Adipose-derived mesenchymal stem cell (ADMSC) administration can facilitate the reconstruction of WM fiber tracts and alleviate functional deficits in an experimental model of subcortical stroke with WMI. Through paracrine effects, immunomodulatory actions, and trophic factor production, ADMSC therapy can reduce lesion volume, inhibit cell death, stimulate axonal growth, and increase oligodendrocyte proliferation and myelin formation (Otero-Ortega et al., 2015). Human umbilical cord blood cells (HUSBC) transplantation attenuates inflammation, enhances vascular remodeling and promotes white matter axonal recovery (Yan et al., 2015). Bone marrow stromal cells (BMSC) are the most popular cell type for stroke cell therapy because of an abundant supply, simple isolation procedure, and convenient intravenous route of administration. Intravenously delivered BMSCs can cross the BBB and migrate to peri-infarct regions, where they accelerate axonal remodeling by upregulating the secretion of axonal regeneration markers and down-regulating the release of axonal extension inhibitory molecules in reactive astrocytes (Shen et al., 2008). In combination with Niaspan, BMSCs can increase the numbers of OPCs and oligodendrocytes, the density of myelin and axons, and the expression of synaptic proteins, which is related to WM reconstruction (Ye et al., 2013). Grafted neural progenitor cells selectively migrate towards the ischemic boundary regions and enhance WM reorganization after stroke (Jiang et al., 2006). Transplanted OPCs have shown potent WM protective effects and enhance spatial learning and memory after hypoxic ischemic brain injury. OPCs prevent WMI by multiple mechanisms; OPCs generate a myelin sheath, stimulate BDNF expression, enhance the proliferation of neural stem cells, and inhibit hypoxia-induced neuronal apoptosis (Chen et al., 2015a). As a large amount of OPCs can be produced in vitro, OPC therapy is a very promising avenue for WMI therapy.
At present, the field of cell-based therapy in stroke is still in its infancy. Several small clinical trials of cell transplantation have been completed but have not yielded satisfactory evidence favoring substantial clinical improvements. In addition, multiple issues, such as target populations, type and source of cells, cell dosage, optimal timing and administration routes must be given more attention (Leak et al., 2014). In the meantime, safety considerations, such as the potential for malignant transformation and side effects such as epilepsy, injection site injury, and immune allergic reactions remain important concerns (Ding et al., 2013).
7.5. Non-pharmaceutical therapies for WHI
7.5.1. Physical exercise
Through adjustment of blood pressure, weight control, improvement of metabolic disorders, and clearance of abnormal rheological properties of blood, regular physical exercise can protect endothelial function and prevent degenerative changes (Zhang et al., 2011a). In addition to well-established musculoskeletal and cardiorespiratory effects, physical activity contributes to a reduced risk and initial severity of stroke and amelioratives neurobehavioral outcome. Furthermore, early and more frequent exercise within the first 6 months after stroke is associated with improved mobility, greater independence, and reduced disability (Egan et al., 2014). Physical exercise improves stroke outcomes via multiple mechanisms, including mitigation of inflammatory responses, attenuation of apoptosis, enhancement of angiogenesis, neurogenesis and even synaptogenesis, improvements in cerebral blood circulation, inhibition of glutamate over-activation, and strengthening of brain microvascular integrity (Zhang et al., 2014c).
In a rat ischemic model subjected to transient MCA occlusion, Ding et al demonstrated that exercise pre-conditioning significantly decreased infarct size and cerebral edema in the frontoparietal cortex and the dorsolateral striatum, with significant improvements in neurological outcomes. In contrast to pre-exercised rats, ischemic rats without exercise exhibited ultrastructural alterations with reduced luminal area, swollen astrocyte end-feet, parenchymal edema, and abnormally thin basal lamina were (Ding et al., 2006). Epidemiologic studies have shown that physical exercise is related to a reduced risk and severity of stroke in humans (Ding et al., 2004). Using T1 WI and DTI, an increase in GM volume in the prefrontal cortex and microstructural changes of FA in corresponding WM regions were observed in healthy humans after intensive exercise (Taubert et al., 2010).
Zheng et al found that post-stroke exercise effectively facilitates axonal regeneration of newborn projection neurons and promotes the rehabilitation of new neuronal networks within the basal ganglia after the ischemia insult, all of which is pivotal for the recovery of motor behavioral function (Zhang et al., 2013). Zhang et al demonstrated that exercise training facilitates the recovery of motor performance and contralesional pyramidal tract regeneration after cerebral infarction (Zhang et al., 2014a). In addition, Seo et al found that early motor balance and coordination training with treadmill training after stroke enhanced the production of synaptophysin, which is thought to reflect axonal sprouting and synaptogenesis in subcortical regions of the ischemic hemisphere, along with improvements in sensorimotor abilities and brain plasticity (Seo et al., 2010). Clinical evidence suggests that physical activity after stroke is associated with behavioral improvement and structural alterations in the brain (Kim et al., 2005). Wan et al. reported that intensive rehabilitation programs for patients with nonfluent aphasia led to structural changes in the right hemisphere, and this was correlated with improvements in speech, indicating that intensive therapy might induce contralateral WM changes in chronic stroke patients with Broca’s aphasia (Wan et al., 2014).
Physical exercise triggers endogenous neuroprotection and is an effective strategy to alleviate ischemia/reperfusion brain damage from stroke. However, the potential mechanisms underlying this therapy are complex and manifold, and the protection of WMI is still undefined. On the other hand, some studies suggest that excessively intense exercise exacerbates brain damage, perhaps due to stress-induced increase of corticosterone levels (Ploughman et al., 2005). As a result, the most effective timing, intensity and duration of physical activity needs to be clearly defined.
7.5.2. Acupuncture
Acupuncture is an important modality of traditional Chinese medicine and has been applied to the treatment of various neurological disorders. A number of clinical studies and evidence-based literature analyses have demonstrated that acupuncture has a positive effect on post-stroke rehabilitation (Huang et al., 2012). However, the underlying mechanisms are still unclear. Clinical trials suggest that acupuncture can modulate the balance between neurochemicals, regulate cerebral microcirculation, and influence the metabolism and activity of neurons. Preclinical studies have shown that acupuncture exerts its protective effect on stroke through mitigation of inflammatory reactions and promotion of neurogenesis and angiogenesis (Li and Wang, 2013).
Using DTI, Shen et al found that the FA values in the infarct areas and cerebral peduncles were higher in stroke patients with acupuncture than control subjects (Shen et al., 2012). These results suggest that acupuncture may postpone Wallerian degeneration and facilitate structural reorganization in and beyond ischemic lesion areas, which is associated with functional recovery after stroke. A positron emission tomography study showed that electroacupuncture in stroke patients can bilaterally excite cerebral structures related to motor function, demonstrating that acupuncture influences the neural network related to brain plasticity, thereby contributing to the recovery of motor performance (Fang et al., 2012). Furthermore, Li et al speculated that acupuncture has a preconditioning effect that can induce cerebral ischemic resistance via antioxidant and anti-apoptotic pathways (Li and Wang, 2013).
Before clinical application of acupuncture treatment, acupuncturists require intensive training and practice, and the evidence of the efficacy of acupuncture for stroke is not sufficiently strong due to a dearth of strict standards and randomized controlled trials. These weaknesses obstruct the clinical promotion of acupuncture in the treatment for stroke.
7.5.3. Brain stimulation
Rehabilitation is crucial for stroke patients. Repetitive transcranial magnetic stimulation (rTMS) is a new non-invasive brain stimulation technique. Applying rTMS over the primary sensory cortex might alleviate WMI. Brodie et al. showed that WM volume in the cortex underlying the TMS coil may be a novel predictor for the behavioral response to the application of 5-Hz rTMS over the ipsilateral primary somatosensory, followed by motor practice (Brodie et al., 2014). Zheng et al found that a combination of transcranial direct current stimulation and physical therapy can facilitate the microstructural restoration of WM fibers in descending motor tracts, which are related to positive clinical outcomes after stroke (Zheng and Schlaug, 2015).
7.6. Hypothermia
Therapeutic hypothermia may be a potent neuroprotective treatment for patients with acute brain injury, as hyperthermia contributes to poor prognosis (Baldwin et al., 2010). Hypothermia mitigates ischemic brain injury by retarding metabolism and energy depletion, suppressing the release of excitatory amino acids, diminishing oxygen free radical production, attenuating inflammatory response, regulating gene expression, preventing BBB destruction, and balancing cell survival and death pathways (Han et al., 2015). A meta-analysis of 101 publications reporting the efficacy of hypothermia in animal models with ischemic stroke showed that induced hypothermia resulted in 44% average reduction in infarct volume and 46% average improvement in functional scores (van der Worp et al., 2007). An in vitro OPC culture study demonstrated that mild hypothermia regulates the balance between apoptosis/differentiation and proliferation, leading to an increase in cell number (Imada et al., 2010). Several experimental studies indicate that hypothermia has beneficial effects on neurogenesis, angiogenesis, and synapse organization after stroke, all of which are associated with neural network reconstruction and functional recovery (Han et al., 2015). In humans, therapeutic hypothermia has been shown to be effective and safe in patients with global cerebral ischemia induced by cardiac arrest or neonatal hypoxic-ischemic encephalopathy (Hemmen and Lyden, 2009). For example, the “cooling for acute ischemic brain damage” study reported that therapeutic hypothermia is feasible and safe in patients with acute ischemic stroke (Krieger et al., 2001). The growth of the lesion size on DWI is less in hypothermic patients than in control subjects (De Georgia et al., 2004). Five observational case series including 112 patients with severe MCA infarction demonstrated that hypothermia reduces mortality, improves prognosis in survivors, and exerts an anti-edema effect after malignant MCA infarction (Bardutzky and Schwab, 2007). However, the “intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke” study showed that there were no differences in sICH rates, clinical recovery level, and long term death rates between the hypothermia and normothermia groups, and that more patients developed pneumonia after hypothermia (Hemmen et al., 2010). Furthermore, a meta-analysis including seven parallel-controlled clinical trials demonstrated that hypothermia did not affect stroke severity and mortality. The researchers suggested that this result might be subject to the high heterogeneity and limited sample size of included studies and that large randomized controlled clinical trials are still needed (Lakhan and Pamplona, 2012).
To date, induced hypothermia is considered an ideal modality for stroke therapy in animal studies, but clinical trials have yet to yield conclusive evidence to confirm efficacy, safety, and feasibility. Furthermore, issues such as the depth and duration of cooling, therapeutic window, methods for hypothermia delivery, rewarming rates, and adverse effects still need to be resolved before clinical application of therapeutic hypothermia (Faridar et al., 2011).
Berger et al found that a combination of hypothermia and BDNF therapy reduce infarct volumes through a synergistic reduction in striatal glutamate (Berger et al., 2004). Goossens et al reviewed all neuroprotective strategies that have been combined with therapeutic hypothermia in experimental models of ischemic stroke, and found that hypothermia is a promising therapy to combine with other neuroprotective compounds (Goossens and Hachimi-Idrissi, 2014). However, only rt-PA, caffeinol, and decompressive hemicraniectomy have been combined with hypothermia in the clinic.
7.7. Ischemic conditioning
Ischemic conditioning or ischemic tolerance of the brain is a naturally protective but transient mechanism induced by exposure to subtoxic insults. In this phenomenon, subtoxic insults promote the recovery of cells or tissues from a subsequent, more severe ischemic and hypoxic episode (N et al., 2015). The mechanisms underlying the induction and maintenance of ischemic/hypoxic resistance in the brain are complex and intertwined, and include the reinforcement of the neurovascular network, improvements in cerebral circulation, regulation of the expression of neurotrophin, reductions in inflammation, and modulation of survival/ apoptotic signaling pathways (Wang et al., 2015). A large number of preclinical studies have shown ischemic preconditioning as well as postconditioning reduce infarct volume and facilitate brain recovery after stroke in animal models (Prasad et al., 2012). Transient ischemic attacks without apparent structural damage as identified by imaging may protect the brain against a subsequent “stroke,” as manifested by superior clinical presentation and outcome, and reductions in infarct size (Stetler et al., 2009). According to Selim et al, ischemic conditioning should be applied in the primary and secondary prevention of stroke, as an additive intervention for acute ischemic stroke, and as a prophylactic therapy against delayed ischemic injury in subarachnoid hemorrhage (Selim and Wang, 2015).
Large adequately powered clinical trials are still needed to standardize optimal protocols for the ischemic conditioning procedure and to further assess the potential benefits of ischemic conditioning in human brain ischemia (Wang et al., 2015).
8. The future of WMI research and therapies
The majority of mechanisms and hypotheses of ischemic WMI have been studied and verified in animal stroke models, but remain poorly investigated in human ischemic stroke. There are major differences between human and animal models and the human condition is far more complex than rodent injury models. Furthermore, we only have limited access to central nervous system in humans. The establishment of animal models with a more complex brain that is not so different from the human brain would be a welcome trend for future WMI research. Furthermore, more mechanistic studies need to be performed in human ischemic stroke.
Although ischemic GMI and WMI share some common characteristics, WMI exhibits unique properties. Furthermore, axons, oligodendrocytes, and astrocytes, along with surrounding microglia, progenitor cells, and vasculature constitute a highly complex framework for WM-targeting strategies. Multiple cell types and intercellular signaling cascades contribute to the maintenance of WM integrity and connectivity (Hayakawa and Lo, 2015). Thus, further investigations of WMI should emphasize cellular networks rather than solely focusing on oligodendrocytes and axons.
WM is involved in the relay of sensorimotor information and is intimately linked with cognitive function. Although there is a natural increase in oligodendrogenesis under ischemic conditions, the scale of this response is not sufficient for full neurorecovery. Therefore, therapeutic approaches that promote oligodendrogenesis are urgently needed for WM repair. Given that stem cells can differentiate into multiple neural cell types and that the brain has only limited and transient capacity for endogenous neurogenesis and oligodendrogenesis after ischemia, stem cells may be the most promising approach for WM and GM recovery.
Accumulating evidence suggests that non-pharmacological approaches, such as therapeutic hypothermia and ischemic/hypoxic conditioning might salvage brain tissue in both GM and WM and provide new opportunities for stroke (Chen et al., 2014a).
Ischemic WMI is a complex process involving multiple intertwined mechanisms. One of the many lessons we have learned from the failure of clinical neuroprotectant trials is that treatments of ischemic stroke need to target multiple underlying mechanisms over time, perhaps with a “cocktail” approach. Therefore, a combination of pharmacological and non-pharmacological strategies, such as hypothermia combined with neuroprotective reagents, stem cell therapy with brain stimulation, ischemic conditioning with revascularization may yield more positive results in the future.
9. Conclusion
WM is involved in the relay of motor and sensory information to and from the cerebral cortex and subcortical structures. As a consequence, WMI results in profound cognitive dysfunction, emotional disorders, and impairments in motor function. WM is exquisitely vulnerable to ischemic brain injury; thus, stroke generally involves both GM and WM. Most studies have emphasized GM and overlooked the critical role of WM in neurorecovery after ischemic stroke. However, true and long-lasting neuroprotection cannot be attained without parallel protection of WM. Protecting the integrity and connectivity of WM is expected to facilitate the rehabilitation of axonal networks and improve stroke prognosis. Therefore future stroke therapies need to be designed to be inclusive of both GM and WM.
Table 3.
Neurorepair based on cell therapy for WMI under investigation
| Source of stem cells | Potential mechanisms |
|---|---|
| ADMSC | Immunomodulation, anti-apoptotic, promotion of trophic factor production, stimulation of axonal growth and oligodendrogenesis, |
| HUSBC | Anti-inflammatory, enhancement of vascular remodeling |
| BMSC | Stimulation of axonal growth and oligodendrogenesis |
| OPCs | Stimulation of BDNF expression, enhancement of neurogenesis, anti-apoptotic and remyelinated the damaged axon |
Acknowledgments
This project was supported by National Institutes of Health/NINDS grants NS079345 (to G. C.), VA Merit Review grants BX002346 (to G. C.), and Chinese 12th Five science and technology support program 2013BAI07B01 (to X. J).
Abbreviations
- AD
axial diffusivity
- ADC
apparent diffusion coefficient
- ADMSC
adipose-derived mesenchymal stem cell
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ATP
adenosine triphosphate
- BAEP
Brainstem auditory evoked potential
- BBB
blood brain barrier
- BMSC
bone marrow stromal cells
- BOLD-fMRI
blood oxygen-level dependent functional MRI
- Ca2+
calcium ion
- CMCT
central motor conduction time
- CNS
central nervous system
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- CT
computer tomography
- DKI
diffusion kurtosis imaging
- DTI
diffusion tensor imaging
- DW-MRI
diffusion-weighted magnetic resonance imaging
- DWI
diffusion-weighted imaging
- EEG
electroencephalography
- FA
fractional anisotropy
- FLAIR
fluid-attenuated inversion recovery
- fMRI
functional magnetic resonance imaging
- GFAP
glial fibrillary acidic protein
- GluRs
glutamate receptors
- GM
gray matter
- GMI
gray matter injury
- HUSBC
human umbilical cord blood cells
- IL-6
interleukin-6
- IL-18
interleukin-18
- MBP
myelin basic protein
- MCA
middle cerebral artery
- MCP-1
monocyte chemotactic protein 1
- MD
mean diffusivity
- MEP
motor evoked potential
- MMP-9
matrix metalloproteinase 9
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MT
magnetization transfer
- MTR
magnetization transfer ratio
- NFL
neuroinflament light polypeptide
- NIHSS
National Institutes of Health Stroke Scale
- NMDA
N-methyl-D-aspartate
- OPCs
oligodendrocyte progenitor cells
- RD
radial diffusivity
- rMT
resting motor threshold
- ROS
reactive oxygen species
- rTMS
repetitive transcranial magnetic stimulation
- r-tPA
recombinant tissue-type plasminogen activator
- SEP
somatosensory evoked potential
- SBP
systolic blood pressure
- sICH
symptomatic intracerebral hemorrhage
- SMART-MR
Second Manifestations of ARTerial disease-Magnetic Resonance
- TABASCO
Tel Aviv Brain Acute Stroke Cohort
- TMS
transcranial magnetic stimulation
- WM
white matter
- WMI
white matter injury
- Zn2+
zinc ion
Footnotes
Conflict of interest
All authors have no actual or potential conflicts of interest, including any financial, personal or other relationships with other people or organizations within three years of beginning of the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alix JJ, Dolphin AC, Fern R. Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. The Journal of physiology. 2008;586:4069–4089. doi: 10.1113/jphysiol.2008.155077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Annals of neurology. 2009;66:682–693. doi: 10.1002/ana.21767. [DOI] [PubMed] [Google Scholar]
- Amaro S, Llull L, Renu A, Laredo C, Perez B, Vila E, Torres F, Planas AM, Chamorro A. Uric acid improves glucose-driven oxidative stress in human ischemic stroke. Annals of neurology. 2015;77:775–783. doi: 10.1002/ana.24378. [DOI] [PubMed] [Google Scholar]
- Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, Rezaei Y. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta neurologica Scandinavica. 2015;131:45–50. doi: 10.1111/ane.12296. [DOI] [PubMed] [Google Scholar]
- Axer H, Keyserlingk DG. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. Journal of neuroscience methods. 2000;94:165–175. doi: 10.1016/s0165-0270(99)00132-6. [DOI] [PubMed] [Google Scholar]
- Baird AA, Colvin MK, Vanhorn JD, Inati S, Gazzaniga MS. Functional connectivity: integrating behavioral, diffusion tensor imaging, and functional magnetic resonance imaging data sets. Journal of cognitive neuroscience. 2005;17:687–693. doi: 10.1162/0898929053467569. [DOI] [PubMed] [Google Scholar]
- Baldwin K, Orr S, Briand M, Piazza C, Veydt A, McCoy S. Acute ischemic stroke update. Pharmacotherapy. 2010;30:493–514. doi: 10.1592/phco.30.5.493. [DOI] [PubMed] [Google Scholar]
- Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Morrison RS, Murphy SP. Novel protective effects of histone deacetylase inhibition on stroke and white matter ischemic injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10:798–807. doi: 10.1007/s13311-013-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke; a journal of cerebral circulation. 2007;38:3084–3094. doi: 10.1161/STROKEAHA.107.490193. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Rypma B. Advances in functional neuroanatomy: a review of combined DTI and fMRI studies in healthy younger and older adults. Neuroscience and biobehavioral reviews. 2013;37:1201–1210. doi: 10.1016/j.neubiorev.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Schabitz WR, Wolf M, Mueller H, Sommer C, Schwab S. Hypothermia and brain-derived neurotrophic factor reduce glutamate synergistically in acute stroke. Experimental neurology. 2004;185:305–312. doi: 10.1016/j.expneurol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Boyko M, Gruenbaum SE, Gruenbaum BF, Shapira Y, Zlotnik A. Brain to blood glutamate scavenging as a novel therapeutic modality: a review. Journal of neural transmission (Vienna, Austria : 1996) 2014;121:971–979. doi: 10.1007/s00702-014-1181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie SM, Borich MR, Boyd LA. Impact of 5-Hz rTMS over the primary sensory cortex is related to white matter volume in individuals with chronic stroke. The European journal of neuroscience. 2014;40:3405–3412. doi: 10.1111/ejn.12717. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnetic resonance in medicine. 2007;57:688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Liu WC, Dohmen C, Haupt WF, Fink GR, Eggers C. Prognostic value of electroencephalography and evoked potentials in the early course of malignant middle cerebral artery infarction. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34:671–678. doi: 10.1007/s10072-012-1102-1. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. NeuroImage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Campos F, Sobrino T, Ramos-Cabrer P, Castellanos M, Blanco M, Rodriguez-Yanez M, Serena J, Leira R, Castillo J. High blood glutamate oxaloacetate transaminase levels are associated with good functional outcome in acute ischemic stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1387–1393. doi: 10.1038/jcbfm.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26:477–485. doi: 10.1016/j.ijdevneu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Chaudhari TS, Verma R, Garg RK, Singh MK, Malhotra HS, Sharma PK. Clinico-radiological predictors of vascular cognitive impairment (VCI) in patients with stroke: a prospective observational study. Journal of the neurological sciences. 2014;340:150–158. doi: 10.1016/j.jns.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Chen F, Qi Z, Luo Y, Hinchliffe T, Ding G, Xia Y, Ji X. Non-pharmaceutical therapies for stroke: mechanisms and clinical implications. Progress in neurobiology. 2014a;115:246–269. doi: 10.1016/j.pneurobio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke; a journal of cerebral circulation. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Frontiers in human neuroscience. 2014b;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zuo S, Wang J, Huang J, Zhang X, Liu Y, Zhang Y, Zhao J, Han J, Xiong L, Shi M, Liu Z. Aspirin promotes oligodendrocyte precursor cell proliferation and differentiation after white matter lesion. Frontiers in aging neuroscience. 2014c;6:7. doi: 10.3389/fnagi.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LX, Ma SM, Zhang P, Fan ZC, Xiong M, Cheng GQ, Yang Y, Qiu ZL, Zhou WH, Li J. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PloS one. 2015a;10:e0115997. doi: 10.1371/journal.pone.0115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang A, Tang J, Wei D, Li P, Chen K, Wang Y, Zhang Z. Association of white matter integrity and cognitive functions in patients with subcortical silent lacunar infarcts. Stroke; a journal of cerebral circulation. 2015b;46:1123–1126. doi: 10.1161/STROKEAHA.115.008998. [DOI] [PubMed] [Google Scholar]
- Cheng B, Schulz R, Bonstrup M, Hummel FC, Sedlacik J, Fiehler J, Gerloff C, Thomalla G. Structural plasticity of remote cortical brain regions is determined by connectivity to the primary lesion in subcortical stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:1507–1514. doi: 10.1038/jcbfm.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JS, Wang E, Lo EH, Sun PZ. Stratification of heterogeneous diffusion MRI ischemic lesion with kurtosis imaging: evaluation of mean diffusion and kurtosis MRI mismatch in an animal model of transient focal ischemia. Stroke; a journal of cerebral circulation. 2012;43:2252–2254. doi: 10.1161/STROKEAHA.112.661926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AH, Kim HR, Kim W, Yang DW. White matter hyperintensity in ischemic stroke patients: it may regress over time. Journal of stroke. 2015;17:60–66. doi: 10.5853/jos.2015.17.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Cui Y, Kim BG. Interaction between hypertension and cerebral hypoperfusion in the development of cognitive dysfunction and white matter pathology in rats. Neuroscience. 2015;303:115–125. doi: 10.1016/j.neuroscience.2015.06.056. [DOI] [PubMed] [Google Scholar]
- Clark WM, Warach SJ, Pettigrew LC, Gammans RE, Sabounjian LA. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Citicoline Stroke Study Group. Neurology. 1997;49:671–678. doi: 10.1212/wnl.49.3.671. [DOI] [PubMed] [Google Scholar]
- Clark WM, Williams BJ, Selzer KA, Zweifler RM, Sabounjian LA, Gammans RE. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke; a journal of cerebral circulation. 1999;30:2592–2597. doi: 10.1161/01.str.30.12.2592. [DOI] [PubMed] [Google Scholar]
- Cloonan L, Fitzpatrick KM, Kanakis AS, Furie KL, Rosand J, Rost NS. Metabolic determinants of white matter hyperintensity burden in patients with ischemic stroke. Atherosclerosis. 2015;240:149–153. doi: 10.1016/j.atherosclerosis.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conijn MM, Kloppenborg RP, Algra A, Mali WP, Kappelle LJ, Vincken KL, van der Graaf Y, Geerlings MI. Cerebral small vessel disease and risk of death, ischemic stroke, and cardiac complications in patients with atherosclerotic disease: the Second Manifestations of ARTerial disease-Magnetic Resonance (SMART-MR) study. Stroke; a journal of cerebral circulation. 2011;42:3105–3109. doi: 10.1161/STROKEAHA.110.594853. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, Astafiev SV, Rengachary J, Zinn K, Lang CE, Connor LT, Fucetola R, Strube M, Carter AR, Shulman GL. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85:927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, Hernangomez M, Montagne A, Liot G, Guaza C, Maubert E, Ali C, Vivien D, Docagne F. Tissue plasminogen activator prevents white matter damage following stroke. The Journal of experimental medicine. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. International journal of stroke : official journal of the International Stroke Society. 2013;8:38–45. doi: 10.1111/j.1747-4949.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- Curtze S, Haapaniemi E, Melkas S, Mustanoja S, Putaala J, Sairanen T, Sibolt G, Tiainen M, Tatlisumak T, Strbian D. White Matter Lesions Double the Risk of Post-Thrombolytic Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2015a;46:2149–2155. doi: 10.1161/STROKEAHA.115.009318. [DOI] [PubMed] [Google Scholar]
- Curtze S, Melkas S, Sibolt G, Haapaniemi E, Mustanoja S, Putaala J, Sairanen T, Tiainen M, Tatlisumak T, Strbian D. Cerebral computed tomography-graded white matter lesions are associated with worse outcome after thrombolysis in patients with stroke. Stroke; a journal of cerebral circulation. 2015b;46:1554–1560. doi: 10.1161/STROKEAHA.115.008941. [DOI] [PubMed] [Google Scholar]
- Dacosta-Aguayo R, Grana M, Fernandez-Andujar M, Lopez-Cancio E, Caceres C, Bargallo N, Barrios M, Clemente I, Monserrat PT, Sas MA, Davalos A, Auer T, Mataro M. Structural integrity of the contralesional hemisphere predicts cognitive impairment in ischemic stroke at three months. PloS one. 2014;9:e86119. doi: 10.1371/journal.pone.0086119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clinical chemistry. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Davalos A, Alvarez-Sabin J, Castillo J, Diez-Tejedor E, Ferro J, Martinez-Vila E, Serena J, Segura T, Cruz VT, Masjuan J, Cobo E, Secades JJ International Citicoline Trial on acUte Stroke trial, i. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- Davalos A, Castillo J, Alvarez-Sabin J, Secades JJ, Mercadal J, Lopez S, Cobo E, Warach S, Sherman D, Clark WM, Lozano R. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke; a journal of cerebral circulation. 2002;33:2850–2857. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study Journal of neurology, neurosurgery, and psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke; a journal of cerebral circulation. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabilitation and neural repair. 2010;24:125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DC, Lin CH, Shyu WC, Lin SZ. Neural stem cells and stroke. Cell transplantation. 2013;22:619–630. doi: 10.3727/096368912X655091. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li J, Luan X, Ding YH, Lai Q, Rafols JA, Phillis JW, Clark JC, Diaz FG. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124:583–591. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurological research. 2006;28:184–189. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- Domercq M, Sanchez-Gomez MV, Sherwin C, Etxebarria E, Fern R, Matute C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. Journal of immunology (Baltimore, Md : 1950) 2007;178:6549–6556. doi: 10.4049/jimmunol.178.10.6549. [DOI] [PubMed] [Google Scholar]
- Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, DeCarli C, Sacco RL, Stern Y, Wright CB. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85:441–449. doi: 10.1212/WNL.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology. 2015;84:1685–1692. doi: 10.1212/WNL.0000000000001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran FL, Hoexter MQ, Valente AA, Jr, Miguel EC, Busatto GF. Association between symptom severity and internal capsule volume in obsessive-compulsive disorder. Neuroscience letters. 2009;452:68–71. doi: 10.1016/j.neulet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Duru AD, Duru DG, Yumerhodzha S, Bebek N. Analysis of correlation between white matter changes and functional responses in thalamic stroke: a DTI & EEG study. Brain imaging and behavior. 2015 doi: 10.1007/s11682-015-9397-1. [DOI] [PubMed] [Google Scholar]
- Egan KJ, Janssen H, Sena ES, Longley L, Speare S, Howells DW, Spratt NJ, Macleod MR, Mead GE, Bernhardt J. Exercise reduces infarct volume and facilitates neurobehavioral recovery: results from a systematic review and meta-analysis of exercise in experimental models of focal ischemia. Neurorehabilitation and neural repair. 2014;28:800–812. doi: 10.1177/1545968314521694. [DOI] [PubMed] [Google Scholar]
- Enzinger C, Fazekas F, Ropele S, Schmidt R. Progression of cerebral white matter lesions -- clinical and radiological considerations. Journal of the neurological sciences. 2007;257:5–10. doi: 10.1016/j.jns.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Esparrago Llorca G, Castilla-Guerra L, Fernandez Moreno MC, Ruiz Doblado S, Jimenez Hernandez MD. Post-stroke depression: an update. Neurologia (Barcelona, Spain) 2015;30:23–31. doi: 10.1016/j.nrl.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Fang Z, Ning J, Xiong C, Shulin Y. Effects of Electroacupuncture at Head Points on the Function of Cerebral Motor Areas in Stroke Patients: A PET Study. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:902413. doi: 10.1155/2012/902413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridar A, Bershad EM, Emiru T, Iaizzo PA, Suarez JI, Divani AA. Therapeutic hypothermia in stroke and traumatic brain injury. Frontiers in neurology. 2011;2:80. doi: 10.3389/fneur.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern RF, Matute C, Stys PK. White matter injury: Ischemic and nonischemic. Glia. 2014;62:1780–1789. doi: 10.1002/glia.22722. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, Kalra L, Murphy DG, Williams SC, Catani M. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain : a journal of neurology. 2014;137:2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2005;76:793–796. doi: 10.1136/jnnp.2003.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LV, Mark VW, Taub E, McCullars A, Barghi A, Rickards T, Hicks J, Uswatte G. Motor recovery from constraint induced movement therapy is not constrained by extent of tissue damage following stroke. Restorative neurology and neuroscience. 2014;32:755–765. doi: 10.3233/RNN-130366. [DOI] [PubMed] [Google Scholar]
- Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123:266–273. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Ransom BR. New light on white matter. Stroke; a journal of cerebral circulation. 2003;34:330–332. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- Goossens J, Hachimi-Idrissi S. Combination of therapeutic hypothermia and other neuroprotective strategies after an ischemic cerebral insult. Current neuropharmacology. 2014;12:399–412. doi: 10.2174/1570159X12666140424233036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud S, Kozlova EN, Maloteaux JM, Hermans E. Cultured astrocytes derived from corpus callosum or cortical grey matter show distinct glutamate handling properties. Journal of neurochemistry. 2009;108:1442–1452. doi: 10.1111/j.1471-4159.2009.05889.x. [DOI] [PubMed] [Google Scholar]
- Grysiewicz R, Gorelick PB. Key neuroanatomical structures for post-stroke cognitive impairment. Current neurology and neuroscience reports. 2012;12:703–708. doi: 10.1007/s11910-012-0315-2. [DOI] [PubMed] [Google Scholar]
- Han Z, Liu X, Luo Y, Ji X. Therapeutic hypothermia for stroke: Where to go? Experimental neurology. 2015;272:67–77. doi: 10.1016/j.expneurol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Lo EH. Brain-peripheral cell crosstalk in white matter damage and repair. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbadis.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius J, Henninger N. Leukoaraiosis Burden Significantly Modulates the Association Between Infarct Volume and National Institutes of Health Stroke Scale in Ischemic Stroke. Stroke; a journal of cerebral circulation. 2015;46:1857–1863. doi: 10.1161/STROKEAHA.115.009258. [DOI] [PubMed] [Google Scholar]
- Hemmen TM, Lyden PD. Hypothermia after acute ischemic stroke. Journal of neurotrauma. 2009;26:387–391. doi: 10.1089/neu.2008.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, Wijman CA, Rapp KS, Grotta JC, Lyden PD. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke; a journal of cerebral circulation. 2010;41:2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson C, Bjerke M, Andersson B, Blennow K, Zetterberg H, Aberg ND, Olsson B, Eckerstrom C, Bokemark L, Wallin A. Neuronal and glia-related biomarkers in cerebrospinal fluid of patients with acute ischemic stroke. Journal of central nervous system disease. 2014;6:51–58. doi: 10.4137/JCNSD.S13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, Young D, Donnan GA. Is white matter involved in patients entered into typical trials of neuroprotection? Stroke; a journal of cerebral circulation. 2005;36:2742–2744. doi: 10.1161/01.STR.0000189748.52500.a7. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Kaur J, Buchan AM. Lost in translation: taking neuroprotection from animal models to clinical trials. Experimental neurology. 2004;188:200–204. doi: 10.1016/j.expneurol.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tang C, Wang S, Lu Y, Shen W, Yang J, Chen J, Lin R, Cui S, Xiao H, Qu S, Lai X, Shan B. Acupuncture regulates the glucose metabolism in cerebral functional regions in chronic stage ischemic stroke patients--a PET-CT cerebral functional imaging study. BMC neuroscience. 2012;13:75. doi: 10.1186/1471-2202-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain J, Juurlink BH. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain research. 1995;698:86–94. doi: 10.1016/0006-8993(95)00832-b. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke; a journal of cerebral circulation. 2009;40:S40–44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada S, Yamamoto M, Tanaka K, Seiwa C, Watanabe K, Kamei Y, Kozuma S, Taketani Y, Asou H. Hypothermia-induced increase of oligodendrocyte precursor cells: Possible involvement of plasmalemmal voltage-dependent anion channel 1. Journal of neuroscience research. 2010;88:3457–3466. doi: 10.1002/jnr.22520. [DOI] [PubMed] [Google Scholar]
- Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke; a journal of cerebral circulation. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J SRF, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriguti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochimica et biophysica acta. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:1145–1153. doi: 10.1038/jcbfm.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DK, Park SI, Han YM, Jang KS, Park MS, Chung YA, Kim MW, Maeng LS, Huh PW, Yoo DS, Jung SW. Motor-evoked potential confirmation of functional improvement by transplanted bone marrow mesenchymal stem cell in the ischemic rat brain. Journal of biomedicine & biotechnology. 2011;2011:238409. doi: 10.1155/2011/238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of leukocyte biology. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z, Xing J, Chen X, Stetler RA, Weng Z, Gan Y, Zhang F, Gao Y, Chen J, Leak RK, Cao G. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1613–1621. doi: 10.1038/jcbfm.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HW, Hyun JK, Kim TU, Chae SH, Lee YI, Lee SJ. Influence of constraint-induced movement therapy upon evoked potentials in rats with cerebral infarction. The European journal of neuroscience. 2012;36:3691–3697. doi: 10.1111/ejn.12014. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain research. 2005;1052:16–21. doi: 10.1016/j.brainres.2005.05.070. [DOI] [PubMed] [Google Scholar]
- Kliper E, Ben Assayag E, Tarrasch R, Artzi M, Korczyn AD, Shenhar-Tsarfaty S, Aizenstein O, Hallevi H, Mike A, Shopin L, Bornstein NM, Ben Bashat D. Cognitive state following stroke: the predominant role of preexisting white matter lesions. PloS one. 2014;9:e105461. doi: 10.1371/journal.pone.0105461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, Mayberg MR, Furlan AJ. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2001;32:1847–1854. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- Kucharczyk W, Macdonald PM, Stanisz GJ, Henkelman RM. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology. 1994;192:521–529. doi: 10.1148/radiology.192.2.8029426. [DOI] [PubMed] [Google Scholar]
- Kurumatani T, Kudo T, Ikura Y, Takeda M. White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke; a journal of cerebral circulation. 1998;29:1058–1062. doi: 10.1161/01.str.29.5.1058. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Pamplona F. Application of mild therapeutic hypothermia on stroke: a systematic review and meta-analysis. Stroke research and treatment. 2012;2012:295906. doi: 10.1155/2012/295906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83:304–311. doi: 10.1212/WNL.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Zheng P, Ji X, Zhang JH, Chen J. From apoplexy to stroke: historical perspectives and new research frontiers. Progress in neurobiology. 2014;115:1–5. doi: 10.1016/j.pneurobio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kang JS, Kim YI. Citicoline protects against cognitive impairment in a rat model of chronic cerebral hypoperfusion. Journal of clinical neurology (Seoul, Korea) 2009;5:33–38. doi: 10.3988/jcn.2009.5.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonards CO, Ipsen N, Malzahn U, Fiebach JB, Endres M, Ebinger M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke; a journal of cerebral circulation. 2012;43:3046–3051. doi: 10.1161/STROKEAHA.111.646554. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, Panda S, Kapke A, Lu M, Ewing JR, Chopp M. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke; a journal of cerebral circulation. 2009;40:936–941. doi: 10.1161/STROKEAHA.108.527713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Simoni M, Kuker W, Schulz UG, Christie S, Wilcock GK, Rothwell PM. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke; a journal of cerebral circulation. 2013;44:3063–3070. doi: 10.1161/STROKEAHA.113.002775. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Q. Acupuncture therapy for stroke patients. International review of neurobiology. 2013;111:159–179. doi: 10.1016/B978-0-12-411545-3.00008-0. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Human brain mapping. 2012;33:1040–1051. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Dang C, Chen X, Xing S, Dani K, Xie C, Peng K, Zhang J, Li J, Zhang J, Chen L, Pei Z, Zeng J. Structural remodeling of white matter in the contralesional hemisphere is correlated with early motor recovery in patients with subcortical infarction. Restorative neurology and neuroscience. 2015;33:309–319. doi: 10.3233/RNN-140442. [DOI] [PubMed] [Google Scholar]
- Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J. Cell based therapies for ischemic stroke: from basic science to bedside. Progress in neurobiology. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature reviews. Neuroscience. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunven M, Thiebaut De Schotten M, Bourlon C, Duret C, Migliaccio R, Rode G, Bartolomeo P. White matter lesional predictors of chronic visual neglect: a longitudinal study. Brain : a journal of neurology. 2015;138:746–760. doi: 10.1093/brain/awu389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyseniuk VP, Balitskii AP, Samosiuk NI. The application of transcranial magnetic stimulation for the functional diagnostics of motor disturbances in the patients presenting with ischemic stroke. Voprosy kurortologii, fizioterapii, i lechebnoi fizicheskoi kultury. 2014:9–14. [PubMed] [Google Scholar]
- Matute C, Ransom BR. Roles of white matter in central nervous system pathophysiologies. ASN neuro. 2012;4 doi: 10.1042/AN20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- McGowan JC. The physical basis of magnetization transfer imaging. Neurology. 1999;53:S3–7. [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS neuroscience & therapeutics. 2014;20:603–612. doi: 10.1111/cns.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N, Pappata S, Mangin JF, Poupon F, LeBihan D, Bousser MG, Chabriat H. Monitoring disease progression in CADASIL with diffusion magnetic resonance imaging: a study with whole brain histogram analysis. Stroke; a journal of cerebral circulation. 2002;33:2902–2908. doi: 10.1161/01.str.0000041681.25514.22. [DOI] [PubMed] [Google Scholar]
- N TV, Sangwan A, Sharma B, Majid A, Gk R. Cerebral Ischemic Preconditioning: the Road So Far. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9278-z. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Molecular neurobiology. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Nardi K, Milia P, Eusebi P, Paciaroni M, Caso V, Agnelli G. Admission leukocytosis in acute cerebral ischemia: influence on early outcome. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2012;21:819–824. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human brain mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossin-Manor R, Card D, Morris D, Noormohamed S, Shroff MM, Whyte HE, Taylor MJ, Sled JG. Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T(1) imaging. NeuroImage. 2013;64:505–516. doi: 10.1016/j.neuroimage.2012.08.086. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R, Fields RD. EEG functional connectivity, axon delays and white matter disease. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2015;126:110–120. doi: 10.1016/j.clinph.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Kim OJ, Shin DA, Song J, Yoo H, Kim YK, Kim JK. Alteration of immunologic responses on peripheral blood in the acute phase of ischemic stroke: blood genomic profiling study. Journal of neuroimmunology. 2012;249:60–65. doi: 10.1016/j.jneuroim.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T. Advanced glycation end products in patients with cerebral infarction. Internal medicine (Tokyo, Japan) 2009;48:587–591. doi: 10.2169/internalmedicine.48.1390. [DOI] [PubMed] [Google Scholar]
- Oliveira-Filho J, Ay H, Schaefer PW, Buonanno FS, Chang Y, Gonzalez RG, Koroshetz WJ. Diffusion-weighted magnetic resonance imaging identifies the “clinically relevant” small-penetrator infarcts. Archives of neurology. 2000;57:1009–1014. doi: 10.1001/archneur.57.7.1009. [DOI] [PubMed] [Google Scholar]
- Otero-Ortega L, Gutierrez-Fernandez M, Ramos-Cejudo J, Rodriguez-Frutos B, Fuentes B, Sobrino T, Hernanz TN, Campos F, Lopez JA, Cerdan S, Vazquez J, Diez-Tejedor E. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Stem cell research & therapy. 2015;6:121. doi: 10.1186/s13287-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Fierini F, Poggesi A. Thrombolysis in acute stroke patients with cerebral small vessel disease. Cerebrovascular diseases (Basel, Switzerland) 2014;37:5–13. doi: 10.1159/000356796. [DOI] [PubMed] [Google Scholar]
- Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. International journal of stroke : official journal of the International Stroke Society. 2011;6:47–59. doi: 10.1111/j.1747-4949.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- Patel MJ, Boada FE, Price JC, Sheu LK, Tudorascu DL, Reynolds CF, Iii, Aizenstein HJ. Association of small vessel ischemic white matter changes with BOLD fMRI imaging in the elderly. Psychiatry research. 2012;204:117–122. doi: 10.1016/j.pscychresns.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Pesaresi M, French L. White matter as a transport system. Neuroscience. 2014;276:117–125. doi: 10.1016/j.neuroscience.2014.01.055. [DOI] [PubMed] [Google Scholar]
- Pausova Z, Paus T, Abrahamowicz M, Almerigi J, Arbour N, Bernard M, Gaudet D, Hanzalek P, Hamet P, Evans AC, Kramer M, Laberge L, Leal SM, Leonard G, Lerner J, Lerner RM, Mathieu J, Perron M, Pike B, Pitiot A, Richer L, Seguin JR, Syme C, Toro R, Tremblay RE, Veillette S, Watkins K. Genes, maternal smoking, and the offspring brain and body during adolescence: design of the Saguenay Youth Study. Human brain mapping. 2007;28:502–518. doi: 10.1002/hbm.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. NeuroImage. 2009;45:1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Podgorska A, Hier DB, Pytlewski A, Czlonkowska A. Leukoaraiosis and stroke outcome. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2002;11:336–340. doi: 10.1053/jscd.2002.130123. [DOI] [PubMed] [Google Scholar]
- Prasad SS, Russell M, Nowakowska M, Williams A, Yauk C. Gene expression analysis to identify molecular correlates of pre- and post-conditioning derived neuroprotection. Journal of molecular neuroscience : MN. 2012;47:322–339. doi: 10.1007/s12031-012-9751-3. [DOI] [PubMed] [Google Scholar]
- Prashantha DK, Sriranjini SJ, Sathyaprabha TN, Nagaraja D, Pal PK. Evaluation of the motor cortical excitability changes after ischemic stroke. Annals of Indian Academy of Neurology. 2013;16:394–397. doi: 10.4103/0972-2327.116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L, Rodriguez-Frutos B, Fuentes B, Vallejo-Cremades MT, Hernanz TN, Cerdan S, Diez-Tejedor E. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke; a journal of cerebral circulation. 2015;46:221–228. doi: 10.1161/STROKEAHA.114.006692. [DOI] [PubMed] [Google Scholar]
- Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, Cramer SC. Anatomy of stroke injury predicts gains from therapy. Stroke; a journal of cerebral circulation. 2011;42:421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79:515–522. doi: 10.1212/WNL.0b013e31826356e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Valk J, Algra PR, van der Hoop RG, Nauta J, Wolters EC. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity. Brain : a journal of neurology. 1992;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magnetic resonance in medicine. 2008;59:268–277. doi: 10.1002/mrm.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M, Wang M. Ischemic Preconditioning: The Long-Awaited Savior of Neuroprotection. Has It Arrived? Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2015;12:655–656. doi: 10.1007/s13311-015-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HG, Kim DY, Park HW, Lee SU, Park SH. Early motor balance and coordination training increased synaptophysin in subcortical regions of the ischemic rat brain. Journal of Korean medical science. 2010;25:1638–1645. doi: 10.3346/jkms.2010.25.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–1754. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Li M, Wei R, Lou M. Effect of acupuncture therapy for postponing Wallerian degeneration of cerebral infarction as shown by diffusion tensor imaging. Journal of alternative and complementary medicine (New York, N Y) 2012;18:1154–1160. doi: 10.1089/acm.2011.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen A, Nemkul N, Yang D, Adhami F, Dunn RS, Hazen ML, Nakafuku M, Ning G, Lindquist DM, Kuan CY. Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia-ischemia-induced thrombotic stroke. J Cereb Blood Flow Metab. 2011;31:1155–1169. doi: 10.1038/jcbfm.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Zhang F, Liu C, Chen J. Ischemic tolerance as an active and intrinsic neuroprotective mechanism. Handbook of clinical neurology. 2009;92:171–195. doi: 10.1016/S0072-9752(08)01909-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Current opinion in cell biology. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Kurihara N, Machida Y, Nishino A, Shimosegawa E. How does water diffusion in human white matter change following ischemic stroke? Magnetic resonance in medical sciences : MRMS : an official journal of Japan Society of Magnetic Resonance in Medicine. 2009;8:121–134. doi: 10.2463/mrms.8.121. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Tseng YL, Chang YY, Liu JS, Su CS, Lai SL, Lan MY. Association of plasma homocysteine concentration with cerebral white matter hyperintensity on magnetic resonance images in stroke patients. Journal of the neurological sciences. 2009;284:36–39. doi: 10.1016/j.jns.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, Reid AT, Shumskaya E, de Laat KF, van Norden AG, van Dijk EJ, Norris DG, de Leeuw FE. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke; a journal of cerebral circulation. 2015;46:425–432. doi: 10.1161/STROKEAHA.114.007146. [DOI] [PubMed] [Google Scholar]
- Umesh Rudrapatna S, Wieloch T, Beirup K, Ruscher K, Mol W, Yanev P, Leemans A, van der Toorn A, Dijkhuizen RM. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology NeuroImage. 2014;97:363–373. doi: 10.1016/j.neuroimage.2014.04.013. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain : a journal of neurology. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- Vilar-Bergua A, Riba-Llena I, Nafria C, Bustamante A, Llombart V, Delgado P, Montaner J. Blood and CSF biomarkers in brain subcortical ischemic vascular disease: involved pathways and clinical applicability. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1038/jcbfm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin A, Ohrfelt A, Bjerke M. Characteristic clinical presentation and CSF biomarker pattern in cerebral small vessel disease. Journal of the neurological sciences. 2012;322:192–196. doi: 10.1016/j.jns.2012.07.068. [DOI] [PubMed] [Google Scholar]
- Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain and language. 2014;136:1–7. doi: 10.1016/j.bandl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q, Kerr C, Pritchett D, Hamalainen M, Moore C, Jones S. Dynamics of dynamics within a single data acquisition session: variation in neocortical alpha oscillations in human MEG. PloS one. 2011;6:e24941. doi: 10.1371/journal.pone.0024941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs & aging. 2007;24:537–546. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- Wang SS, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:4047–4056. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Reis C, Applegate R, 2nd, Stier G, Martin R, Zhang JH. Ischemic conditioning-induced endogenous brain protection: Applications pre-, per- or post-stroke. Experimental neurology. 2015;272:26–40. doi: 10.1016/j.expneurol.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels T, Wessels C, Ellsiepen A, Reuter I, Trittmacher S, Stolz E, Jauss M. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR American journal of neuroradiology. 2006;27:35–39. [PMC free article] [PubMed] [Google Scholar]
- Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biological psychiatry. 2002;52:253–264. doi: 10.1016/s0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- Wu O, Cloonan L, Mocking SJ, Bouts MJ, Copen WA, Cougo-Pinto PT, Fitzpatrick K, Kanakis A, Schaefer PW, Rosand J, Furie KL, Rost NS. Role of Acute Lesion Topography in Initial Ischemic Stroke Severity and Long-Term Functional Outcomes. Stroke; a journal of cerebral circulation. 2015;46:2438–2444. doi: 10.1161/STROKEAHA.115.009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycoco V, Shroff M, Sudhakar S, Lee W. White matter anatomy: what the radiologist needs to know. Neuroimaging clinics of North America. 2013;23:197–216. doi: 10.1016/j.nic.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. Neurorestorative Therapy of Stroke in Type 2 Diabetes Mellitus Rats Treated With Human Umbilical Cord Blood Cells. Stroke; a journal of cerebral circulation. 2015;46:2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. Journal of neural transmission (Vienna, Austria : 1996) 2013;120:1369–1395. doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Taguchi A, Yamamoto A, Kajimoto K, Kazui H, Kudo T, Kikuchi-Taura A, Sekiyama A, Kishimoto T, Iida H, Nagatsuka K. Microstructural abnormality in white matter, regulatory T lymphocytes, and depressive symptoms after stroke. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society. 2014;14:213–221. doi: 10.1111/psyg.12084. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Tanaka R, Shimada Y, Yamashiro K, Liu M, Mitome-Mishima Y, Miyamoto N, Ueno Y, Urabe T, Hattori N. Type 2 diabetes reduces the proliferation and survival of oligodendrocyte progenitor cells in ishchemic white matter lesions. Neuroscience. 2015;289:214–223. doi: 10.1016/j.neuroscience.2014.12.054. [DOI] [PubMed] [Google Scholar]
- Ye X, Yan T, Chopp M, Zacharek A, Ning R, Venkat P, Roberts C, Chen J. Combination BMSC and Niaspan treatment of stroke enhances white matter remodeling and synaptic protein expression in diabetic rats. International journal of molecular sciences. 2013;14:22221–22232. doi: 10.3390/ijms141122221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain : a journal of neurology. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wen H, Hu X, Li C, Zeng J. Effects of physical training on pyramidal tract regeneration in hypertensive rats with focal cerebral infarction. Zhonghua yi xue za zhi. 2014a;94:1488–1493. [PubMed] [Google Scholar]
- Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011a;177:170–176. doi: 10.1016/j.neuroscience.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Zhang J, Puri AS, Khan MA, Goddeau RP, Jr, Henninger N. Leukoaraiosis predicts a poor 90-day outcome after endovascular stroke therapy. AJNR American journal of neuroradiology. 2014b;35:2070–2075. doi: 10.3174/ajnr.A4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Butler AJ, Sun CK, Sahgal V, Wittenberg GF, Yue GH. Fractal dimension assessment of brain white matter structural complexity post stroke in relation to upper-extremity motor function. Brain research. 2008;1228:229–240. doi: 10.1016/j.brainres.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang L, Yang X, Wan Y, Jia J. The effects of exercise preconditioning on cerebral blood flow change and endothelin-1 expression after cerebral ischemia in rats. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014c;23:1696–1702. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Zhang QW, Deng XX, Sun X, Xu JX, Sun FY. Exercise promotes axon regeneration of newborn striatonigral and corticonigral projection neurons in rats after ischemic stroke. PloS one. 2013;8:e80139. doi: 10.1371/journal.pone.0080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao Z, Wang D, Zhang T, Sun B, Mu L, Wang J, Liu Y, Kong Q, Liu X, Zhang Y, Zhang H, He J, Li H, Wang G. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP-10. Journal of neuroinflammation. 2014d;11:79. doi: 10.1186/1742-2094-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su YY, Haupt WF, Zhao JW, Xiao SY, Li HL, Pang Y, Yang QL. Application of electrophysiologic techniques in poor outcome prediction among patients with severe focal and diffuse ischemic brain injury. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2011b;28:497–503. doi: 10.1097/WNP.0b013e318231c852. [DOI] [PubMed] [Google Scholar]
- Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Frontiers in human neuroscience. 2015;9:229. doi: 10.3389/fnhum.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]


