Abstract
Recent studies of signaling networks point out that an order of drugs to be administrated to the cancerous cells can be critical for optimal therapeutic outcomes of recalcitrant metastatic and drug-resistant cell types. In this study, a development of a polymeric nanoparticle system for sequential delivery is reported. The nanoparticle system can co-encapsulate and co-deliver a combination of therapeutic agents with different physicochemical properties [i.e. epidermal growth factor receptor (EGFR) inhibitor, erlotinib (Ei), and doxorubicin (Dox)]. Dox is hydrophilic and was complexed with anionic lipid, 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA), via ion pairing to form a hydrophobic entity. Then it was co-encapsulated with hydrophobic Ei in a poly(L-lactide)-b-polyethylene glycol (PLA-b-PEG) nanoparticle by nanoprecipitation. The complexation of Dox with DOPA greatly helps the encapsulation of Dox, and substantially reduces the release rate of Dox. This nanoparticle system was found to burst the release of Ei with a slow and sustained profile of Dox, which is an optimal course of administration for these two drugs as previously reported. The efficacy of this sequential delivery nanoparticle system was validated in vitro and its in vivo potential applicability was substantiated by fluorescent imaging of high tumor accumulation.
1 Introduction
Rational combination therapy has great potential in enhancing efficacy in treating cancer.(Choi et al., 2014; Guo et al., 2014; Miao et al., 2014; Xu et al., 2013) The idea is to apply multiple drugs with complementary and synergistic mechanisms to block multiple survival pathways in cancerous cells. Recent and continuously growing studies of cellular networking pathways indicate that a release order of drugs plays a critical role in optimal therapeutic outcomes.(He et al., 2016; Lee et al., 2012; Li et al., 2015; Pacardo et al., 2015; Qian et al., 2012; Ren et al., 2016; Zhang et al., 2011) It has also recently been validated that basal A type of triple negative breast cancer (TNBC) cells can remarkably be sensitized to the effects of DNA-damaging agents, such as doxorubicin (Dox), after EGFR signaling is suppressed.(Lee et al., 2012) The sensitization effect was derived from the rewiring of signaling networks induced by prolonged EGFR inhibition using an EGFR inhibitor, erlotinib (Ei). This rewiring showed the most effective DNA damaging efficacy for doxorubicin after a 24-hour time delay when compared with synergistic combination chemotherapy. Thus, there is a need for designing a drug delivery system that can not only co-deliver these two drugs with different physicochemical properties but also sequentially release them with a desired time delay.
Nanoparticles (NPs) are an emerging and versatile platform for drug delivery.(Blanco et al., 2015; Nel et al., 2009; Park et al., 2002; Peer et al., 2007) Through NPs, it is feasible to load multiple drugs with different physicochemical properties for combination therapy (Xiong and Lavasanifar, 2011; Xu et al., 2013) based on enhanced permeation and retention (EPR) effects (Hrkach et al., 2012; Maeda et al., 2000; Peer et al., 2007). In order to achieve such synergistically enhanced therapeutic effects for combination therapy, multiple drugs need to be co-encapsulated for co-delivery to the same cancer cells and be released with a desired order. To achieve sequential drug release, one approach is loading drugs into different layers inside the carrier. For instance, liposomes were used to achieve the sequential co-delivery of Ei and Dox by loading Dox inside the water core and Ei inside the lipid layer for different release profiles.(Morton et al., 2014) However, liposomes suffer from low stability, making it difficult to fine-tune the release.(Haluska et al., 2006; He et al., 2016; Lei and MacDonald, 2003; Zhang and Granick, 2006) Another sequential delivery system using near infrared radiation (NIR)-responsive hollow gold NP designed to deliver microRNA 21 inhibitor and Dox also greatly enhanced the anti-cancer efficacy against MDA-MB-231 and MCF-7 cell lines.(Ren et al., 2016)
In this study, a new polymeric NP design was used to demonstrate the sequential and controlled delivery concept using Ei and Dox as a pair of model drugs against basal A type of TNBC to achieve enhanced therapeutic outcomes. This polymeric NP design can release Ei followed by Dox in order to first sensitize TNBC cells for subsequent DNA damage using Dox. This NP design was validated in vitro through NP characterizations, stability evaluation, drug encapsulation efficiency, release kinetic profiles, and cytotoxicity evaluations against MDA-MB-468, a basal type A TNBC cell line. Finally, an in vivo bio-distribution study using a syngeneic model of breast cancer was also performed to evaluate the tumor accumulation capability of the NP system.
2 Materials and Methods
2.1 Materials
N,N-Diisopropylethylamine (DIEA), N-hydroxysuccinimide (NHS), tetrahydrofuran (THF), dichloromethane (DCM), dimethyl sulfoxide (DMSO), acetonitrile (ACN), chloroform and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC·HCl) were purchased from Sigma-Aldrich (St. Louis, MO). Poly(L-lactide) (MW 20,000) with terminal carboxylate groups was purchased from Akina (West Lafayette, IN). Methoxy-poly(ethylene-glycol)-amine (mPEG-NH2 (MW 5,000) was purchased from Laysan Bio Inc. (Arab, AL). 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Doxorubicin hydrochloride (Dox) and erlotinib (Ei) were purchased from LC Laboratories (Woburn, MA). Dialysis membrane tubing (MWCO 100,000 Da) was purchased from Spectrum (Rancho Dominguez, CA). Amicon® Ultra-4 centrifugal filter (MWCO 100,000 Da) was purchased from Millipore (Billerica, MA). Acrodisc® syringe filters (0.45 µm and 0.2 µm) were purchased from Pall Corp. (Port Washington, NY). Cyanine 5.5 (Cy 5.5) NHS ester was purchased from Lumiprobe Corp. (Hallandale Beach, FL).
2.2 Synthesis and characterization of sequential delivery NP
To validate the formation of a hydrophobic DOPA/Dox complex, 0.5 ml of water containing Dox (200 µg/ml) was mixed with 0.5 ml of chloroform containing different amounts of DOPA. The hydrophobicity of DOPA/Dox complex was assessed by shake-flask method. The mixture was intensely vortexed for 5 min, and was allowed to reach the phase separation overnight at 4 °C. Then the two immiscible phases were separated by pipette drawing. The amount of Dox included in each phase was measured using fluorescence plate reader (485 nm/600 nm). Proton nuclear magnetic resonance (1H NMR) spectroscopy was used to examine the complexation of DOPA and Dox. DOPA, Dox, and DOPA/Dox were dissolved in CDCl3, DMSO-d6, and CDCl3/DMSO-d6 (1:1 by volume), respectively. To encapsulate a hydrophobic DOPA/Dox complex in NPs, Dox dissolved in DMSO was mixed in THF with DOPA dissolved in THF at a predetermined molar ratio.
The nano-precipitation method was used for preparing NPs.(Fessi et al., 1989; Legrand et al., 2007; Quintanar-Guerrero et al., 1998; Zhou et al., 2015) A di-block copolymer, PLA-b-mPEG, was made by conjugating PLA-COOH with H2N-mPEG through the carbodiimide-mediated coupling reaction.(Cheng et al., 2007; Kamaly et al., 2013) Then the PLA-b-mPEG polymer, DOPA/Dox complex, and Ei were co-dissolved in THF to reach a polymer concentration of 5 mg/ml. Then this solution was dropwise added to stirring water (water:THF = 5:1 by volume). This mixture was stirred for 2 hours to remove THF through evaporation at room temperature. Then, NPs were washed by ultrafiltration with water (3,000 g, 15 min, MWCO 100 kDa) to remove residual solvent, un-encapsulated Dox, and Ei. The NPs were then resuspended in phosphate-buffered saline (PBS) at pH 7.4. In order to determine the amount of Ei loaded, the NPs were dissolved in acetonitrile, and the drug was quantified using HPLC (Agilent 1100 series). Dox was quantified with a fluorescence plate reader (485 nm/600 nm). The encapsulation efficiency (EE) was determined by dividing the amount of drug loaded by the amount of drug initially added during the NP formation. Dynamic light scattering (DLS) was employed to measure the size of NPs (0.2 mg/ml).
The morphology of the NPs was investigated by FEI CM20 transmission electron microscopy (TEM). A 10-µl solution of 0.1 mg/ml freshly made NPs in PBS was dropped on carbon-coated copper grids. Then an excess amount of the liquid was removed by filter paper. Next the grid was immersed in a staining solution (1%(w/v) uranyl acetate) for 2 min and finally dried under the fume hood.(Booth et al., 2011)
The release kinetics of the NPs was studied by an indirect method using dialysis. The NP solution was transferred into the dialysis membrane tubing (MWCO 100 kDa). Then the dialysis tubing was immersed in 500 ml of PBS solution at 37 °C. At predetermined times (1, 4, 8, and 24 hours), a small fraction of the sample inside the dialysis tubing was collected. The individual amounts of Ei and Dox retained inside the NPs at each sampling time were determined using HPLC and fluorescence, respectively, as described above.
2.2 In vitro cell tests for cytotoxicity
The Dulbecco's Modified Eagle Medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) and 1% penicillin-streptomycin (Invitrogen) was used as a cell culture medium for the TNBC cell line MDA-MB-468 (ATCC). Cells were cultivated in a humidified environment at 37 °C with 5% CO2.
MTS assay was used to evaluate the cytotoxicity of the NPs. Cells were plated in a 96-well plate with ~5,000 cells per well and then cells were treated with different NP formulations. The cytotoxicity of NPs was examined by the MTS assay (Promega, Madison WI) following the protocol provided by the vendor. The cell viability was obtained using the following equation:
| (eq. 1) |
The positive control was the absorbance obtained at 470 nm from the cells without treatment and the negative control was the absorbance obtained at 470 nm from a blank well containing the cell culture medium and MTS reagents.
2.4 In vivo non-invasive imaging
The R7 murine breast cancer cell line was derived from a mammary tumor from a transgenic MMTV-Ron mouse.(Zinser et al., 2006) For our in vivo experiments, 150,000 R7 cells were orthotopically injected into the inguinal mammary fat pads of syngeneic FVB female mice following protocols previously described.(Wagh et al., 2011) Tumor formation was examined by manual palpation after 10-day post-injection. Alfalfa-free diet was fed to the mice upon identification of palpable tumors. To label PLA with Cy5.5, Cy5.5-NH2 was conjugated to PLA-COOH through the carbodiimide-mediated coupling reaction. Near infrared fluorescence (NIFR) NPs were prepared by blending 10 wt% of PLA-Cy5.5 and 90 wt% PLA-PEG before nanoprecipitation. NPs were then injected through the tail vein, and mice were imaged 1 hour, 4 hours, and 24 hours after the injection using a fluorescent imaging system (Bruker In-Vivo MS FX PRO). All animal treatments and surgical procedures followed approved protocols and were performed in accordance with the Institutional Animal Care and Use Committee of the University of Cincinnati.
3 Results and discussion
3.1 Synthesis and Characterization of Sequential Co-Delivery Nanoparticles
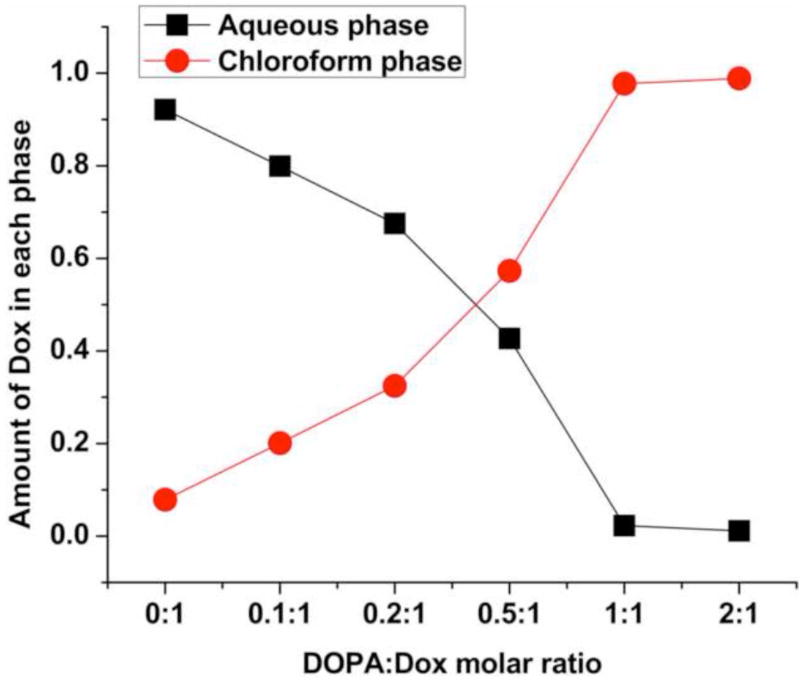
As previously reported, the sensitization of basal A type of TNBC to Dox derived by pretreating the MDA-MB-468 cells with Ei for at least 8 hours before Dox was applied.(Lee et al., 2012; Li et al., 2015) It was confirmed from our free dose-response test (Fig. S1) that the simultaneous loading of free Ei and Dox did not enhance the cytotoxic efficacy of Dox. It was also confirmed that the sequential free loading of Ei followed by Dox with a 24-hour time delay could result in the reported enhanced cytotoxic efficacy. In order to realize a NP design for the sequential delivery of Ei followed by Dox with the desired time delay, the release kinetics of Dox was suppressed using DOPA as a counter-ion. Doxorubicin hydrochloride is highly water soluble and could form a hydrophobic complex with DOPA. The conventional shake-flask method was used to examine the hydrophobicity of the complex formed. As shown in Fig. 1, more than 90% of Dox remained in the aqueous phase in the absence of DOPA. However, as the amount of DOPA increased, Dox moved from the aqueous phase to the chloroform phase. When a molar ratio of DOPA to Dox reached 1:1 and above, almost 100% of Dox was transferred to the chloroform phase, suggesting a complete complexation of Dox and DOPA and high lipophilicity of the DOPA/Dox complex (Fig. S2).
Figure 1.
Distribution of Dox between the aqueous and chloroform phases (water:chloroform = 1:1 by volume) in terms of DOPA-to-Dox molar ratios.
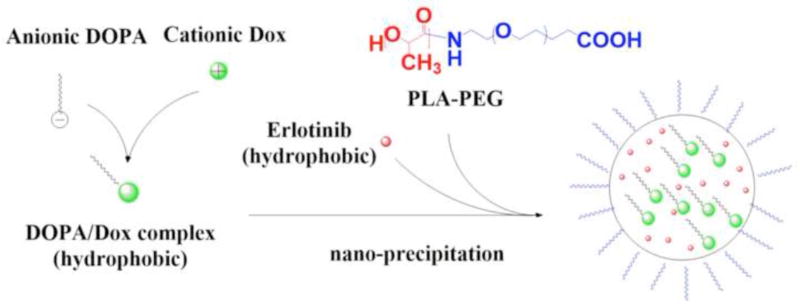
In addition, the complexation of DOPA/Dox was studied using proton nuclear magnetic resonance (1H NMR) spectroscopy (Fig. S3).(Song et al., 2016) The peak assigned to the primary amine of Dox at 2.1 ppm was greatly reduced in the DOPA/Dox complex, indicating an electron density change resulting from the complexation (Fig. S4). Chloroform was used to demonstrate the migration of Dox as a result of the DOPA/Dox complexation. However, when the hydrophobic DOPA/Dox complex was co-encapsulated with Ei in the NP, water-miscible THF was used as a solvent for nanoprecipitation. A schematic for this NP synthesis route is shown in Fig. 2.
Figure 2.
Synthesis route of nanoparticle (NP(Ei→Dox)) for sequential delivery.
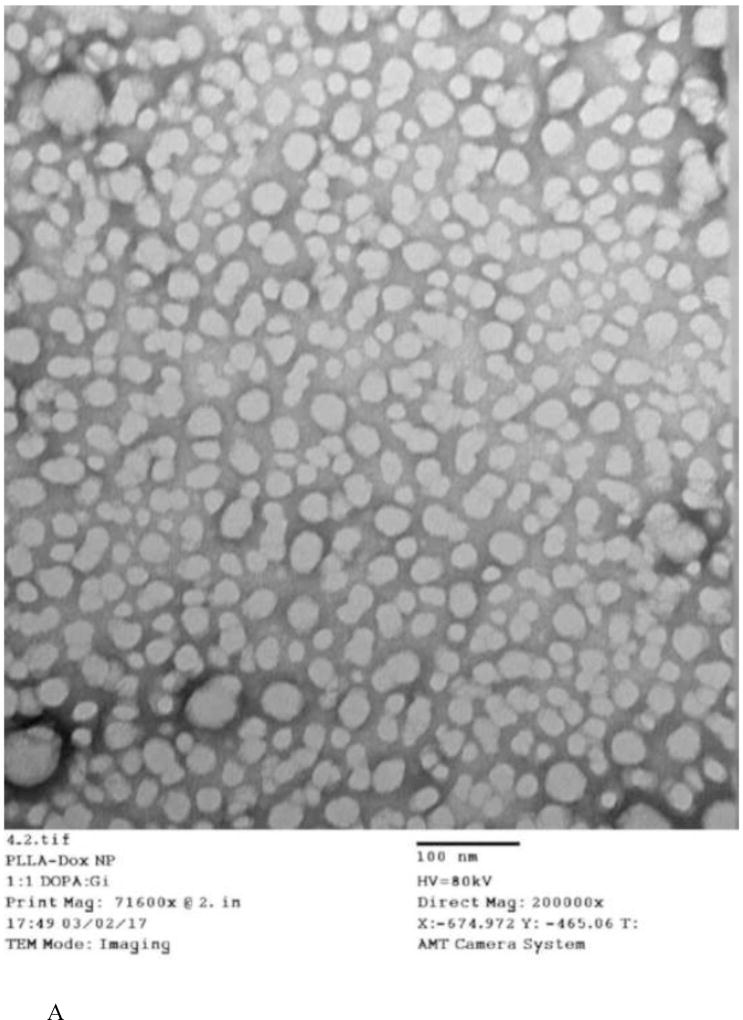
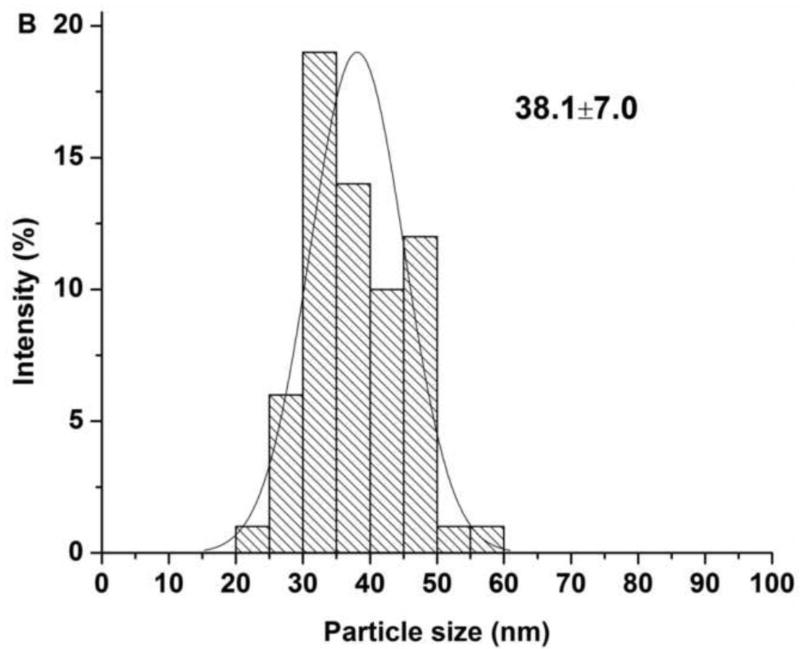
The characterization results for sequential delivery nanoparticle, NP(Ei→Dox), and single drug encapsulating nanoparticles, NP(Dox) and NP(Ei), are summarized in Table. 1. The morphology of the individual NP designs was assessed through TEM (Fig. 3A). The NPs for the sequential delivery (NP(Ei→Dox)) exhibited a uniform particle size distribution from DLS measurements, ranging from ~70 to ~80 nm. However, the sizes of the NPs observed through the TEM images were smaller than the diameters measured by DLS (Fig. 3B). This could be attributed to the shrinkage of the NPs as TEM assessed the sample in a dried form as compared to in a wet form when DLS was used. Similar observations were previously reported.(Liu et al., 2007; Tang et al., 2006; Zhang et al., 2004)
Table 1.
Properties of sequential-delivery nanoparticles (NP(Ei→Dox)) and single drug encapsulating nanoparticles (NP(Dox) and NP(Ei)).
| NP Formulations |
Initial DOPA:Dox:Ei Molar Ratio |
Actual drug Loading (wt%) |
Actual Dox:Ei Ratio (wt/wt) |
Particle Size (nm) Determined by DLS |
|
|---|---|---|---|---|---|
| Ei | Dox | ||||
| NP(Ei→Dox) | 1:1:3 | 0.5 | 1.4 | 1:0.37 | 73.9±2.0 |
| NP(Ei→Dox) | 2:1:3 | 0.6 | 1.6 | 1:0.38 | 73.0±1.7 |
| NP(Ei→Dox) | 3:1:3 | 1.8 | 1.8 | 1:1.02 | 74.0±0.7 |
| NP(Ei→Dox) | 5:1:3 | 2.1 | 1.9 | 1:1.09 | 72.8±1.9 |
| NP(Ei→Dox) | 10:1:3 | 2.2 | 1.9 | 1:1.15 | 74.8±3.1 |
| NP(Ei→Dox) | 10:1:6 | 3.5 | 1.9 | 1:1.83 | 76.9±1.7 |
| NP(Dox) | 5:1:0 | N/A | 3.8 | 1:0 | 75.3±5.6 |
| NP(Ei) | 5:0:1 | 3.6 | N/A | 0:1 | 74.0±1.7 |
Notes: For particle size and PDI, data represent mean ± standard deviation, n = 3; and N/A means “not applicable”.
Figure 3.
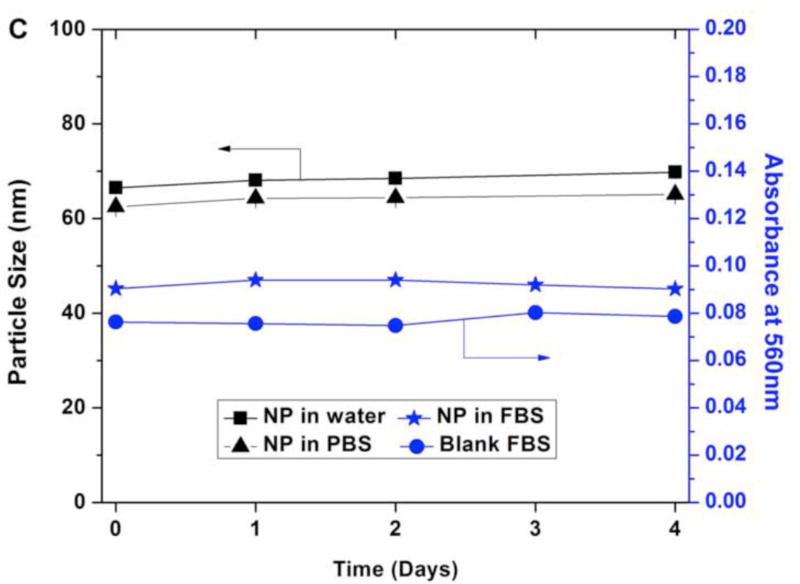
(A) TEM images for sequential delivery nanoparticle NP(Ei→Dox) with a DOPA:Dox:Ei molar ratio of 5:1:3, scale bar 100 nm; and (B) particle size distribution for NP(Ei→Dox) with a DOPA:Dox:Ei molar ratio of 5:1:3 measured using ImageJ. Data represent mean ± standard deviation, n=64; (C) stability evaluation of NP(Ei→Dox) by monitoring particle size in water and PBS, and absorbance of NP(Ei→Dox) in FBS at 560 nm. 0.1 ml of 10 mg/ml NP was mixed with 1 ml of FBS to evaluate its stability.
The stability of NP(Ei→Dox) was evaluated in the three different media of water, PBS, and FBS. The size of NP(Ei→Dox) was stable over 4 days in both water and PBS, indicating good stability in a physiological condition (Fig. 3C). To assess the stability of the NPs in serum, an absorbance-based approach was used since proteins in serum might induce particle aggregation and it would interfere with the particle size measurements.(Fang et al., 2010) The absorbance of NP(Ei→Dox) in FBS at 560 nm was reported to be suitable for detecting the formation of NP aggregates.(Dehaini et al., 2016; Fang et al., 2010; Hu et al., 2011) If NPs were not stable in FBS, then the aggregation of NPs would be detected by the increment in absorbance at 560 nm. The absorbance value stayed almost the same for 4 days (Fig. 3C), indicating the stability of the NPs in the serum. Based on the observations of almost constant NP sizes and absorbance values, NP(Ei→Dox) is expected to be stable under in vivo conditions.
3.2 Encapsulation Efficiency and Release Kinetics for Co-Delivery Nanoparticles
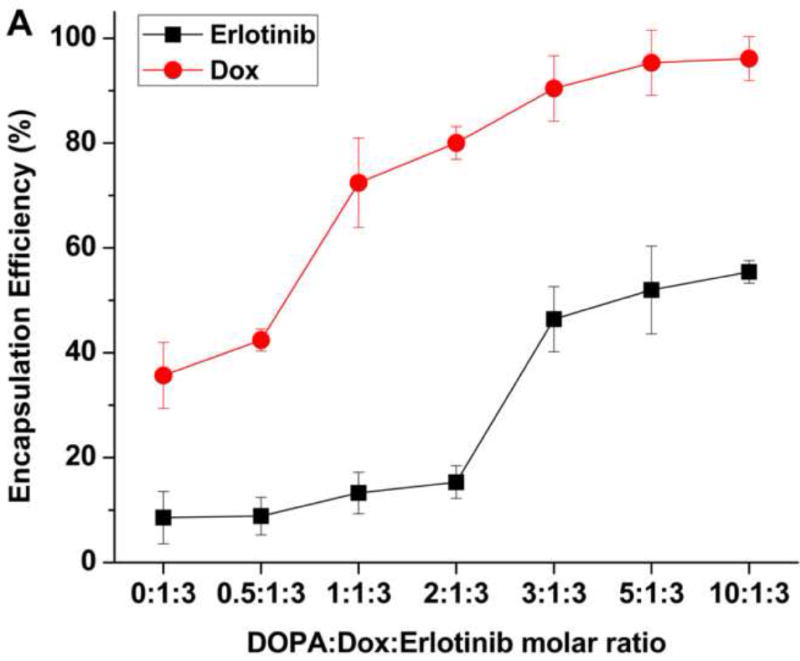
The drug encapsulation efficiencies (EE) of Ei and Dox were examined with respect to DOPA:Dox:Ei molar ratio (Fig. 4A). As expected, the EE of Dox increased with an increase in the molar ratio of DOPA to Dox, and reached a maximum value when a DOPA:Dox:Ei ratio is greater than 5:1:3, suggesting the full complexation of DOPA and Dox. It was interesting to note that the EE of Ei also showed its similar dependence on DOPA, suggesting that both Dox and Ei form complexes with DOPA. The complexation of Ei and DOPA was confirmed by 1H NMR (Fig. S5 and S6). The ionizable group of Ei predicted by Marvin software (ChemAxon) is quinazoline. The peaks assigned to quinazoline were shifted when Ei was complexed with DOPA (Fig. S5 and S6). In addition, the complexation of both Dox and Ei with DOPA was also studied using 1H NMR (Fig. S7). Similar peak shift and peak reduction were observed from Ei and Dox, indicating that both Dox and Ei were complexed in a similar manner to their separate complexation with DOPA. However, it was also noted that the EE of Ei is almost a half of the EE of Dox. However, in the absence of Dox, the EE of Ei could reach ~90% when DOPA was used. It was also found that when either Dox or Ei was complexed with DOPA, a 1:1 molar ratio of Dox (or Ei) to DOPA was enough to give ~90% encapsulation efficiency (EE) of Dox or Ei. It is speculated that when both Dox and Ei are present, Dox interferes with the complexation of Ei with DOPA. To ensure the maximum drug encapsulations of Dox and Ei at approximately the same weight ratio, a DOPA: Dox: Ei molar ratio was kept constant at 5:1:3 for the rest of the evaluations.
Figure 4.
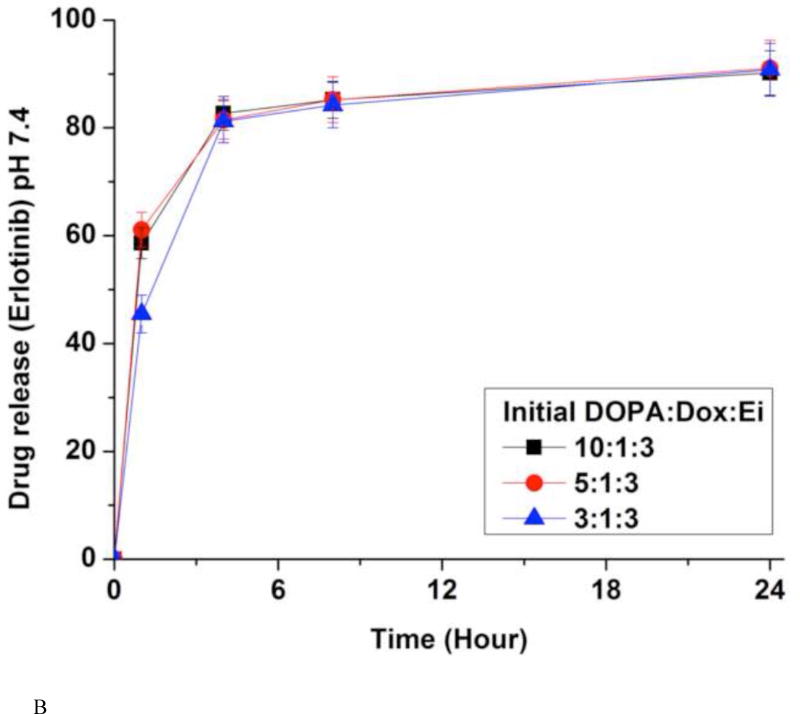
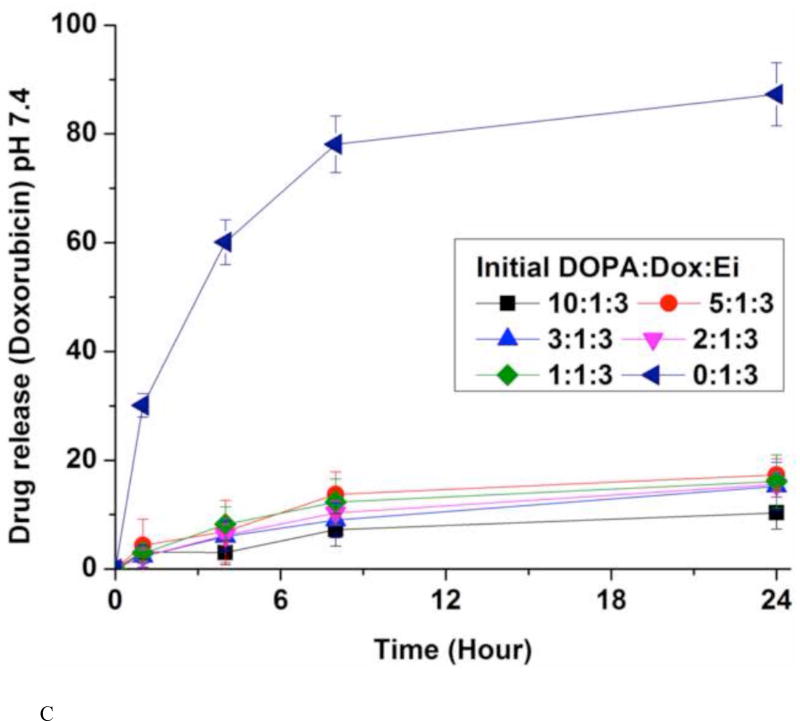
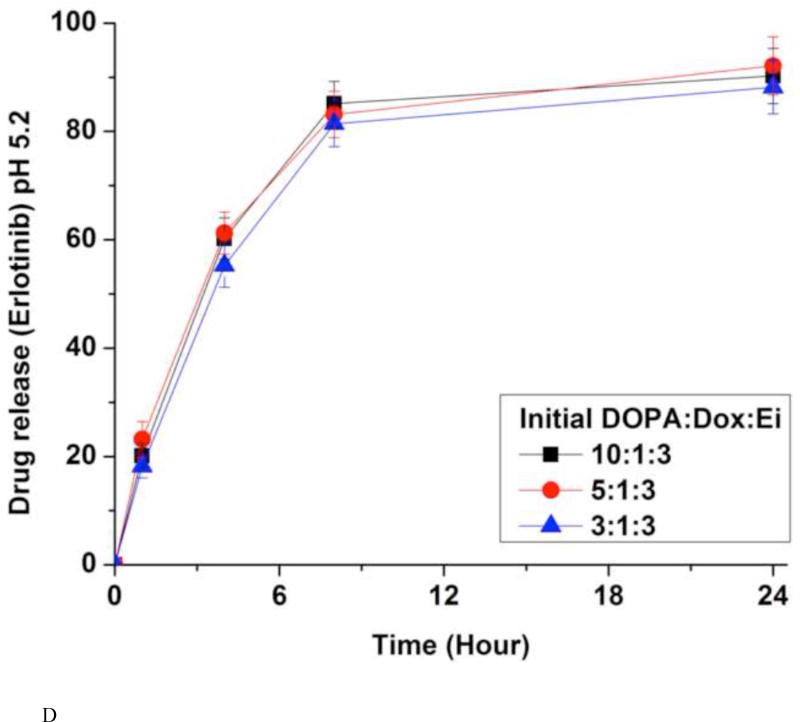
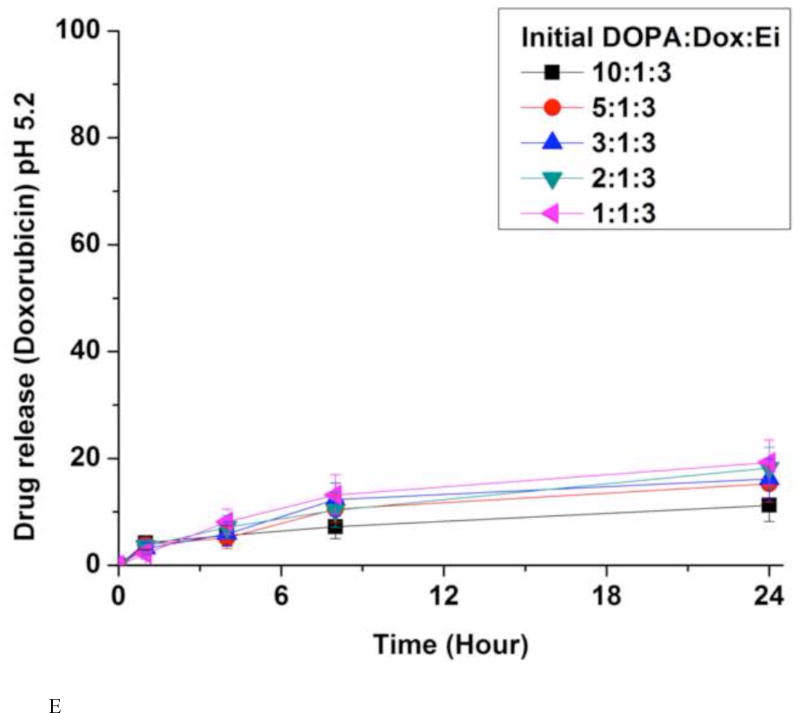
(A) Encapsulation efficiencies (EE) of erlotinib (Ei) and doxorubicin (Dox). Data represent mean ± standard deviation, n = 3; In vitro release kinetics of Ei from NPs with different initial DOPA:Dox:E ratios in PBS at 37 °C and pH 7.4 (B) and pH 5.2 (D); In vitro release kinetics of Dox from NPs with different initial DOPA:Dox:Ei ratios in PBS at 37 °C and pH 7.4 (C) and pH 5.2 (E). For NPs with DOPA:Dox:Ei ratios of 2:1:3 and 1:1:3, due to the low EE there were little Ei loaded, they were not evaluated for Ei release. The amounts of Dox and Ei remaining inside NP(Ei→Dox) were measured at 1 hr, 4 hrs, 8 hrs, and 24 hrs using fluorescence plate reader and HPLC, respectively. Data represent mean ± standard deviation, n = 3.
The in vitro release kinetics of Ei and Dox was investigated by dialysis against PBS at the physiological temperature (37 °C) and pH (7.4) conditions (Fig. 4B and 4C). The quantities of Ei encapsulated inside the NPs were determined from HPLC by dissolving an aliquot of NPs in acetonitrile. Dox was measured in a fluorescence plate reader (485 nm/600 nm). About 80% of Ei was released within 4 hours while less than 20% of Dox was released up to 24 hours. However, in the absence of DOPA, Dox was released with a much faster rate (Fig. 4C). The slow release kinetics of Dox was derived from the ion-pairing of Dox and DOPA while the NPs without DOPA released Dox much faster. DOPA is deemed to help increase the hydrophobicity and molar volume of the ion-pair complex in comparison with Dox alone.(Gaudana et al., 2011; Meyer and Manning, 1998; Pinkerton et al., 2013; Song et al., 2016) The fast dissociation of the Ei/DOPA complex at pH 7.4 is deemed to be responsible for its fast release. The pKa value of Ei is estimated to be 4.6 (predicated by Marvin software (ChemAxon)). This means that Ei is not likely kept protonated at pH 7.4 when protonation is required for ion-pairing.(Pinkerton et al., 2013; Song et al., 2016) Thus the Ei/DOPA complex is speculated to be fast dissociated at pH 7.4 carried out for the release study. This speculation was verified from the result of a much slower release rate of Ei at pH 3, where the Ei/DOPA complex should be more stable than at pH 7 (Fig. S8). The difference in the release kinetics allowed for the desired sequential delivery of Ei and Dox. In addition, the effects of different DOPA:Dox:Ei ratios on Dox and Ei release kinetics were examined. It was found that the ratio did not affect the release kinetics. The amount of DOPA changed the encapsulation efficiency, but did not change the release kinetics. The NPs with DOPA:Dox:Ei ratios of 1:1:3 and 2:1:3 were not examined for Ei release kinetics due to their low Ei encapsulation efficiency (Fig. 4B). The Ei and Dox releases at pH 5.2 were also performed to simulate the microenvironment around tumor and intracellular conditions (Fig. 4D and 4E). It was found that the release profiles of Dox and Ei were very similar to those obtained at pH 7.4.
3.3 Cytotoxicity of sequential co-delivery nanoparticles
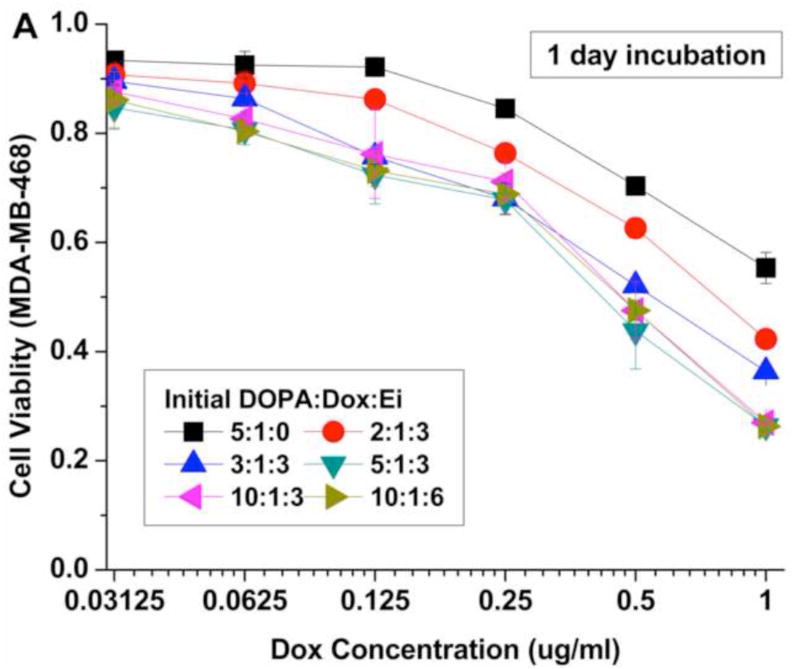
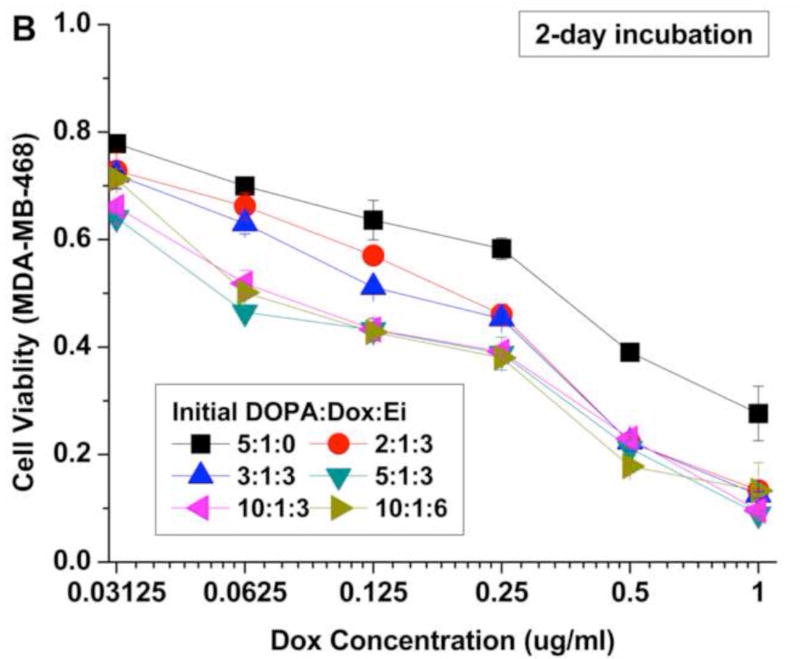
The cytotoxic efficacy of the NPs was temporally examined in vitro using the MDA-MB-468 cell line as a model of basal A type of TNBC (Fig. 5). To evaluate the reported enhanced efficacy of sequential and controlled delivery of Ei and Dox, the cells were treated with NPs with different initial DOPA:Dox:Ei molar ratios. The cell viability was assessed by the MTS assay with 1-day and 2-day incubation periods after Dox addition (Fig. 5A and B). The cytotoxic efficacy of the NPs was noticeably enhanced with an increase in the DOPA:Dox:Ei molar ratios for 2 days. The enhanced cytotoxic efficacy is attributed to the sensitization of MDA-MB-468 cells by the sequential delivery of Ei followed by Dox. Among the DOPA-Dox-Ei molar ratio evaluated, the 5:1:3 ratio is deemed to be an optimal ratio for maximizing drug encapsulation efficiency. In the case of 2:1:3, the encapsulation efficiency of Ei was too low to inhibit EGFR (Fig. 4A). Also extra DOPA (i.e. 10:1:3) did not increase drug loadings for Dox and Ei, and thus its cytotoxic efficacy was about the same as that of the DOPA:Dox:Ei ratio of 5:1:3. The polymer, PLA-PEG, and the lipid, DOPA, were also tested for in vitro toxicity to examine the toxicity derived from the carrier. Indeed, both PLA-PEG and DOPA did not exhibit toxicity to cell. (Fig. S9)
Figure 5.
Cell viability for MDA-MB-468 cells after 24 hrs and 48 hrs treatments with nanoparticles with different initial DOPA:Dox:Ei molar ratios. Data represent mean ± standard deviation, n = 3.
Also a lower Dox-to-Ei ratio was evaluated to examine whether a Dox-to-Ei weight ratio of 1:1 is enough to sensitize the cells. In order to obtain a Dox:Ei loading ratio of 1:2 inside NPs, an initial DOPA:Dox:Ei ratio of 10:1:6 was used. As a result, an actual Dox:Ei loading ratio of 1:1.8 (wt/wt) was obtained (Table 1). The cytotoxic efficacy results showed an insignificant difference between the two actual Dox:Ei loading ratios, indicating that the actual 1:1 loading is enough to sensitize the cells. In addition, based on our results shown in Fig. S1, the simultaneous co-application of free Ei and Dox did not show a significant synergistic cytotoxic effect compared with Dox alone (Fig. S1). Therefore, it can be concluded that the enhanced efficacy of NP(Ei→Dox) (initial DOPA:Dox:Ei molar ratios of 2:1:3, 3:1:3, 5:1:3, 10:1:3, and 10:1:3) over NP(Dox) (initial DOPA:Dox:Ei molar ratio of 5:1:0) was derived from the desired drug release order, which dynamically rewrote the singling pathway and induced Dox sensitization. These results successfully demonstrate that it is possible to enhance the cytotoxic efficacy of Dox while minimizing its dosage in order to reduce its side effects including cardiomyopathy by controlling the release order and time between the two drugs.
3.4 In vivo biodistribution
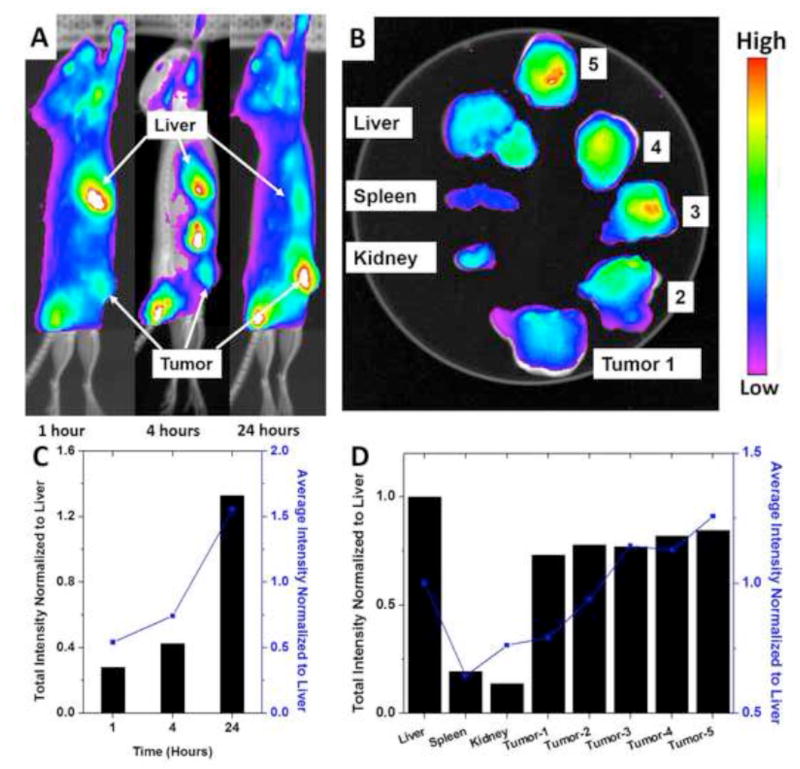
A bio-distribution study was conducted in order to examine the potential of this NP design for therapeutic applications. For real-time optical monitoring of in vivo distribution of NPs, time-dependent excretion profile, and tumor accumulation, Cy5.5 was labeled to the terminus of PLA. Then the Cy 5.5-labeled PLA was physically blended with PLA-PEG at a 10:90 (wt:wt) ratio for the formation of near infrared fluorophore (NIRF) NPs using nanoprecipitation. A syngeneic orthotopic breast tumor model was used for the fluorescent biodistribution test. NPs were administrated to the mice through tail vein injection. The mice were imaged at 1 hour, 4 hours, and 24 hours after the injection (Fig. 6A and 6C). The NPs started to accumulate inside the tumor within 1 hour and continued to accumulate up to 24 hours. However, the NIFR intensity at the liver was very high at 1 hour and continued to decrease with time, indicating that NPs located at the liver were steadily excreted. At 24 hours, the NIRF intensity at the tumor was highest throughout the whole body. In addition, the fluorescent intensities at the tumor were quantified using ImageJ and were normalized to the fluorescent intensities measured at the liver. It is clear that the fluorescent intensities at the tumor over the liver steadily increased from 1 hour to 24 hours, showing NP accumulation at the tumor site. After the 15-day post injection, the mice were euthanized and major organs were recovered for ex vivo imaging (Fig. 6B and 6D). Then the tumor was sliced into five pieces. It was noticeable that the high fluorescent intensities were still detectable from the dissected tumor compared to the major organs, indicating that NPs stayed inside the tumor while the NPs in the major organs of liver, spleen, and kidney were cleared out. The accumulation of NPs at tumor site is attributed to the EPR effect. The fast growth of tumor requires a large amount of nutrients supplied from blood. Therefore, there would be many newly formed blood vessels around the tumor. However, in many cases, such blood vessels could have leaky vascular structure and thus NPs with appropriate sizes could extravasate into the tumor and get trapped inside.(Maeda et al., 2000; Peer et al., 2007) Such specific localization of the NPs at the tumor site is anticipated to help enhance the therapeutic efficacy and reduce the systemic toxicity of the therapeutic agents by differentiating tumor and healthy tissues.
Figure 6.
(A) In vivo non-invasive NIRF images of time-dependent left lateral imaging of a tumor-bearing mouse at 1 hr, 4 hrs, and 24 hrs of post i.v. injection of Cy5.5-NP. Solid red arrows indicate the tumors. (B) Ex vivo NIRF imaging of major organs and tumors after 15 days of i.v. injection of Cy5.5-NP. (C) The quantification of total NIRF intensities and averaged NIRF intensities at tumor over the time. The intensities were normalized to liver. (D) The quantification of total NIRF intensities and averaged NIRF intensities at major organs and tumor sites after 15 days of i.v. injection of Cy5.5-NP. The intensities were normalized to liver.
Conclusions
In summary, a novel polymeric NP system was developed for the sequential delivery of Ei and Dox for enhanced therapeutic effects. The hydrophobic DOPA/Dox complex suitable for encapsulation in PLA-PEG polymer was successfully synthesized through ion pairing. The ion pairing increased the hydrophobicity and molar volume of Dox, and thus resulted in high encapsulation efficiency and slow release kinetics. The sequential delivery was validated through the drug release profiles of Ei and Dox. The sequential release of both drugs from the NPs successfully demonstrated enhanced therapeutic efficacy. Lastly, it was confirmed through biodistribution that this NP design could exhibit tumor targeting through passive EPR effect and stay inside the tumor site while other NPs delivered to organs started to be cleared out after the 15-day post injection. An in vivo animal model plans to be used to further study the treatment efficacy of the polymeric NP system.
Supplementary Material
Acknowledgments
This study was supported by the Marlene Harris-Ride Cincinnati Breast Cancer Foundation, and the authors appreciate their financial support.
References
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature biotechnology. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DS, Avila-Sakar A, Cheng Y. Visualizing proteins and macromolecular complexes by negative stain EM: from grid preparation to image acquisition. J Vis Exp. 2011:e3227. doi: 10.3791/3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KY, Silvestre OF, Huang X, Min KH, Howard GP, Hida N, Jin AJ, Carvajal N, Lee SW, Hong JI, Chen X. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano. 2014;8:4559–4570. doi: 10.1021/nn500085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaini D, Fang RH, Luk BT, Pang Z, Hu C-MJ, Kroll AV, Yu CL, Gao W, Zhang L. Ultra-small lipid-polymer hybrid nanoparticles for tumor-penetrating drug delivery. Nanoscale. 2016 doi: 10.1039/c6nr04091h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Aryal S, Hu C-MJ, Zhang L. Quick Synthesis of Lipid–Polymer Hybrid Nanoparticles with Low Polydispersity Using a Single-Step Sonication Method. Langmuir. 2010;26:16958–16962. doi: 10.1021/la103576a. [DOI] [PubMed] [Google Scholar]
- Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. International Journal of Pharmaceutics. 1989;55:R1–R4. [Google Scholar]
- Gaudana R, Parenky A, Vaishya R, Samanta SK, Mitra AK. Development and characterization of nanoparticulate formulation of a water soluble prodrug of dexamethasone by HIP complexation. Journal of Microencapsulation. 2011;28:10–20. doi: 10.3109/02652048.2010.520093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Lin CM, Xu Z, Miao L, Wang Y, Huang L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano. 2014;8:4996–5009. doi: 10.1021/nn5010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska CK, Riske KA, Marchi-Artzner V, Lehn J-M, Lipowsky R, Dimova R. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proceedings of the National Academy of Sciences. 2006;103:15841–15846. doi: 10.1073/pnas.0602766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Su Z, Xue L, Xu H, Zhang C. Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J Control Release. 2016;229:80–92. doi: 10.1016/j.jconrel.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, Yaffe MB. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand P, Lesieur S, Bochot A, Gref R, Raatjes W, Barratt G, Vauthier C. Influence of polymer behaviour in organic solution on the production of polylactide nanoparticles by nanoprecipitation. Int J Pharm. 2007;344:33–43. doi: 10.1016/j.ijpharm.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Lei G, MacDonald RC. Lipid Bilayer Vesicle Fusion: Intermediates Captured by High-Speed Microfluorescence Spectroscopy. Biophysical Journal. 2003;85:1585–1599. doi: 10.1016/S0006-3495(03)74590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YT, Qian XJ, Yu Y, Li ZH, Wu RY, Ji J, Jiao L, Li X, Kong PF, Chen WD, Feng GK, Deng R, Zhu XF. EGFR tyrosine kinase inhibitors promote pro-caspase-8 dimerization that sensitizes cancer cells to DNA-damaging therapy. Oncotarget. 2015;6:17491–17500. doi: 10.18632/oncotarget.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhou ZM, Wang XF, Xu J, Yang K, Cui Q, Chen X, Cao MY, Weng J, Zhang QQ. Formation of poly(L,D-lactide) spheres with controlled size by direct dialysis. Polymer. 2007;48:5767–5779. [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Meyer JD, Manning MC. Hydrophobic ion pairing: altering the solubility properties of biomolecules. Pharm Res. 1998;15:188–193. doi: 10.1023/a:1011998014474. [DOI] [PubMed] [Google Scholar]
- Miao L, Guo S, Zhang J, Kim WY, Huang L. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv Funct Mater. 2014;24:6601–6611. doi: 10.1002/adfm.201401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SW, Lee MJ, Deng ZJ, Dreaden EC, Siouve E, Shopsowitz KE, Shah NJ, Yaffe MB, Hammond PT. A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal. 2014;7:ra44. doi: 10.1126/scisignal.2005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Pacardo DB, Ligler FS, Gu Z. Programmable nanomedicine: synergistic and sequential drug delivery systems. Nanoscale. 2015;7:3381–3391. doi: 10.1039/c4nr07677j. [DOI] [PubMed] [Google Scholar]
- Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:1172–1181. [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Pinkerton NM, Grandeury A, Fisch A, Brozio J, Riebesehl BU, Prud'homme RK. Formation of stable nanocarriers by in situ ion pairing during block-copolymer-directed rapid precipitation. Mol Pharm. 2013;10:319–328. doi: 10.1021/mp300452g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Ren Y, Shi Z, Long L, Pu P, Sheng J, Yuan X, Kang C. Sequence-dependent synergistic inhibition of human glioma cell lines by combined temozolomide and miR-21 inhibitor gene therapy. Mol Pharm. 2012;9:2636–2645. doi: 10.1021/mp3002039. [DOI] [PubMed] [Google Scholar]
- Quintanar-Guerrero D, Allemann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm. 1998;24:1113–1128. doi: 10.3109/03639049809108571. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wang R, Gao L, Li K, Zhou X, Guo H, Liu C, Han D, Tian J, Ye Q, Hu YT, Sun D, Yuan X, Zhang N. Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. J Control Release. 2016;228:74–86. doi: 10.1016/j.jconrel.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Song YH, Shin E, Wang H, Nolan J, Low S, Parsons D, Zale S, Ashton S, Ashford M, Ali M, Thrasher D, Boylan N, Troiano G. A novel in situ hydrophobic ion paring (HIP) formulation strategy for clinical product selection of a nanoparticle drug delivery system. J Control Release. 2016;229:106–119. doi: 10.1016/j.jconrel.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Tang MH, Dou HJ, Sun K. One-step synthesis of dextran-based stable nanoparticles assisted by self-assembly. Polymer. 2006;47:728–734. [Google Scholar]
- Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, Waltz SE. [beta]-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene. 2011;30:3694–3704. doi: 10.1038/onc.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- Xu X, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, Shi J, Wu J, Kantoff PW, Lippard SJ, Langer R, Walker GC, Farokhzad OC. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc Natl Acad Sci U S A. 2013;110:18638–18643. doi: 10.1073/pnas.1303958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Granick S. How to Stabilize Phospholipid Liposomes (Using Nanoparticles) Nano Letters. 2006;6:694–698. doi: 10.1021/nl052455y. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu Z, Zhao Q, Huang J, Shen H, Zhang Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small. 2011;7:460–464. doi: 10.1002/smll.201001522. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Jiang M, Zhao JX, Zhou J, Chen DY. Hollow spheres from shell cross-linked, noncovalently connected micelles of carboxyl-terminated polybutadiene and poly(vinyl alcohol) in water. Macromolecules. 2004;37:1537–1543. [Google Scholar]
- Zhou Z, Badkas A, Stevenson M, Lee JY, Leung YK. Herceptin conjugated PLGA-PHis-PEG pH sensitive nanoparticles for targeted and controlled drug delivery. Int J Pharm. 2015;487:81–90. doi: 10.1016/j.ijpharm.2015.03.081. [DOI] [PubMed] [Google Scholar]
- Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA, Peace BE, Beauman SR, Collins MH, Waltz SE. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res. 2006;66:11967–11974. doi: 10.1158/0008-5472.CAN-06-2473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.