Abstract
Objective
Acute subdural hematoma (ASDH) is generally considered a condition that should be managed surgically. However, some patients initially receive conservative treatment, a subset of whom require surgical intervention later. This study aimed to evaluate the predictors of delayed surgical intervention in ASDH patients who are initially managed conservatively.
Methods
From January 2007 to December 2015, 842 patients diagnosed with ASDH were treated at our institution. Among them, 158 patients with convexity ASDH were initially treated conservatively. Patients were divided into a delayed surgery group and a conservative group. Demographic characteristics, past medication and medical histories, and radiological and laboratory data were collected by retrospective chart review. Independent risk factors were identified with univariate and multivariate analyses.
Results
Twenty-eight patients (17.7%) underwent delayed surgical intervention. Their mean age was 69.0 years, and 82.1% were male. Hypertension, diabetes mellitus, and heart disease prevalence and use of anti-platelet agents did not significantly differ from the conservative group. However, age (p=0.024), previous cerebral infarction history (p=0.026), increased maximal hematoma thickness (p<0.001), midline shifting (p=0.001) and accompanying subarachnoid hemorrhage (p=0.022) on initial brain computed tomography (CT) scan, low hemoglobin level (p<0.001), high leukocyte count (p=0.004), and low glucose level (p=0.002) were significantly associated with delayed surgical intervention. In multivariate analysis, increased maximal hematoma thickness (odds ratio [OR]=1.279, 95% confidence interval [CI] 1.075–1.521; p=0.006), low hemoglobin level (OR=0.673, 95% CI 0.467–0.970; p=0.034), and high leukocyte count (OR=1.142, 95% CI 1.024–1.272; p=0.017) were independent risk factors for delayed surgical intervention.
Conclusion
Due to the high likelihood of delayed surgical intervention among minimal ASDH patients with a thicker hematoma on initial brain CT, lower hemoglobin level, and higher leukocyte count, these patients should receive more careful observation.
Keywords: Hematoma, Subdural, Surgical procedure, Operative, Conservative treatment, Risk factors, Outcome
INTRODUCTION
Acute subdural hematoma (ASDH) generally considered a condition that should be managed surgically, but in some cases may be treated conservatively1). Minimally symptomatic ASDH patients do not always require early surgical evacuation3). In minimal ASDH patients, initial treatment is typically determined according to surgical guidelines and the surgeon’s experience2). With advances of radiological diagnostic tools, the number of patients with minimal subdural hematoma (SDH) who are treated conservatively is increasing. However, some ASDH patients managed with conservative treatment require surgical intervention later28). Hemorrhage resulting from injury of the bridging veins is liquefied with micro-bleeding and progresses to symptomatic chronic subdural hematoma (CSDH) as expanding8). Patients with progression to CSDH require surgical decompression such as craniotomy and Burr hole trephination with or without irrigation and drainage8). Therefore, it is important to know the conditions when the SDH progresses and who will require surgical intervention. The purpose of this study was to identify predictable risk factors that are associated with ASDH patients who are initially managed conservatively undergoing delayed surgical intervention.
MATERIALS AND METHODS
We retrospectively collected data from 842 patients diagnosed with SDH who were treated at the neurosurgery department of our tertiary medical center between January 2007 and December 2015. Two hundred fifty-seven patients underwent emergency craniectomy and hematoma evacuation. Cases of minimal falx or subtentorial hemorrhage (n=280), which commonly does not require surgery, were not included. Patients younger than 15 years old (n=37) or who received surgical intervention within 7 days after diagnosis (n=47) were excluded. Sixty-three patients who died or refused treatment were also excluded (Fig. 1).
Fig. 1.
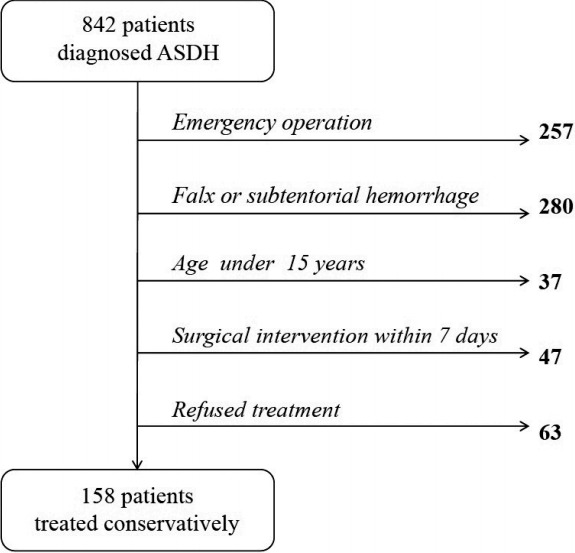
Flow chart of eligible patients included in the study. ASHD, acute subdural hematoma.
Finally, 158 patients were enrolled in the study. All patients were admitted to the neurosurgical department and initially treated conservatively. Anti-epileptic drugs were administered to all patients, and osmotic diuretics were given when needed. Anti-platelet agents and anticoagulation agents were halted at the time of diagnosis. If patents demonstrated coagulopathy on initial laboratory findings, such as low platelet count or prolonged international normalized ratio (INR), the issue was corrected with platelet and fresh frozen plasma transfusion or IV administration of vitamin K.
Patients’ medical histories were retrospectively collected by chart review. Past medical comorbidities, medication, and trauma histories were reviewed. Radiological and laboratory studies were performed at the time of diagnosis, and follow-up brain computed tomography (CT) scanning was performed routinely within the first 8 hours and every 1 week unless neurological deterioration was observed.
Radiological findings were obtained by chart review. All films were interpreted by a radiologist when the patient was admitted and confirmed by neurosurgeons. On the initial brain CT scan, intracranial injuries accompanying SDH, such as intraparenchymal hemorrhage or subarachnoid hemorrhage, were evaluated, and maximal hematoma thickness and midline shifting were measured.
Surgery was performed when follow-up brain CT scan revealed hematoma progression or a mass effect appeared such as headache aggravation or development of a neurological deficit. All such patients underwent Burr hole trephination and drainage with or without irrigation under general or local anesthesia.
Chi-square and Fisher’s exact test were utilized to analyze categorical data, and numerical variables were compared with Student’s t-test. Statistical differences were considered significant for p values less than 0.05. Finally, to adjust for relationships among various factors, multivariate logistic regression was used to identify independent risk factors.
RESULTS
The 158 patients enrolled in this study were all initially treated conservatively. They were divided into two groups according to follow-up treatment: the conservative treatment group (n=130) and surgical treatment group (n=28). Patient characteristics are listed in Tables 1 and 2. All patients presented minor symptoms, such as headache, dizziness, nausea, or vomiting. No definite neurologic deficit was observed. Patients’ mean age was 62.93 years; 112 patients (70.9%) were male, 46 (29.1%) were female. Twenty-eight patients (17.7%) demonstrated progression of SDH on follow-up brain CT and underwent surgical intervention. The mean interval between initial diagnosis to operation was 13.9 days.
Table 1.
Characteristics, past medical and medication histories of conservative group and surgical group
| Variable | Conservative (n=130) | Surgery (n=28) | p-value |
|---|---|---|---|
| Age | 61.62±15.65 | 69.00±15.01 | 0.024 |
|
| |||
| Sex (male) | 89: 41 (68.5) | 23: 5 (82.1) | 0.148 |
|
| |||
| Comorbidity | |||
| HTN | 53 (40.8) | 16 (57.1) | 0.113 |
| DM | 33 (25.4) | 7 (25.0) | 0.966 |
| Heart disease | 13 (10.0) | 4 (14.3) | 0.506 |
| Prev. infarction | 6 (4.6) | 5 (17.9) | 0.026 |
|
| |||
| Anti-PLT | 18 (13.8) | 5 (17.9) | 0.563 |
|
| |||
| Anticoagulant | 1 (0.8) | 1 (3.6) | 0.324 |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated. HTN: hypertension, DM: diabetes mellitus, PLT: platelet
Table 2.
Radiologic and laboratory findings of conservative group and surgical group
| Variable | Conservative (n=130) | Surgery (n=28) | p-value |
|---|---|---|---|
| Radiology | |||
| Thickness | 5.78±3.27 | 11.54±5.16 | <0.001 |
| Midline shift | 1.08±2.36 | 3.32±3.13 | 0.001 |
| Bilaterality | 23 (17.7) | 6 (21.4) | 0.643 |
| SAH | 42 (32.3) | 3 (10.7) | 0.022 |
| ICH | 22 (16.9) | 4 (14.3) | 1 |
|
| |||
| Laboratory | |||
| Leukocyte | 5.82±5.63 | 9.00±4.83 | 0.004 |
| Hemoglobin | 13.56±1.78 | 12.18±2.07 | <0.001 |
| Platelet | 216.87±186.78 | 175.00±63.91 | 0.244 |
| Glucose | 157.65±59.36 | 132.64±27.51 | 0.002 |
| INR | 1.14±1.08 | 1.00±0.01 | 0.52 |
Values are presented as mean±standard deviation or number (%). SAH: subarachnoid hemorrhage, ICH: intracerebral hemorrhage, INR: international normalized ratio
Patients in the surgical group were older than those in the conservative group (mean age 69.00 vs. 61.62 years, p=0.024). However, there was no significant difference in sex distribution, with 89 males and 41 females in the conservative group and 23 males and 5 females in the surgical group (p=0.229).
In total, 69 patients had hypertension: 53 in the conservative group and 16 in the surgical group, with no significant difference between groups (p=0.113). Rates of diabetes mellitus (DM) and cardiac disease history did not differ between the groups (p=0.966 and p=0.506), while history of cerebral infarction significantly more in surgical group (p=0.026). Medication rates did not differ between the two groups. Eighteen patients in the conservative group and 5 in surgical group were taking anti-platelet agents. Two patients were on anti-coagulant agents, one in each group.
The initial brain CT scans of patients in surgical group showed greater in maximal thickness (11.54 vs. 5.78 mm, p<0.001) and more midline shifting to the opposite hemisphere compared to the conservative group (3.32 vs. 1.08 mm, p=0.001).
On initial brain CT scan, 45 patients had subarachnoid hemorrhage, and 26 patients had intraparenchymal hemorrhage. The conservative group included a significantly higher proportion of patients with subarachnoid hemorrhage (42 vs. 3, p=0.022), but no difference was seen in intraparenchymal hemorrhage (22 vs. 4, p=1).
Among the initial laboratory findings, prothrombin time (INR) and platelet count did not differ between the two groups (p=0.522 and p=0.279). However, initial hemoglobin was negatively correlated with surgical intervention (12.25 vs. 13.53, p=0.001). Leukocyte count was also higher in the surgical group than in the conservative group (89.0 vs. 58.6, p=0.005). By contrast, initial glucose was lower in the surgical group (134.6 vs. 157.3, p=0.005).
To correct for confounding factors, we performed a multivariate logistic regression analysis including possible risk factors with p values less than 0.01 on univariate analysis. Multivariate logistic regression analysis identified initial SDH thickness (odds ratio [OR]=1.279, 95% confidence interval [CI] 1.075–1.521; p=0.006) and leukocyte count (OR=1.142, 95% CI 1.024–1.272; p=0.017; Table 3) as independent risk factors for delayed surgical intervention. Additionally, initial hemoglobin was confirmed as an independent negative predictor of hematoma progression (OR=0.673, 95% CI 0.467–0.970; p=0.034). Age (p=0.692), history of cerebral infarction (p=0.993), midline shifting (p=0.191), accompanying SAH (p=0.747), and glucose level (p=0.254) were not significantly associated with surgical intervention.
Table 3.
Result of multivariate analysis
| Variable | p-value | Odds ratio | 95% confidence interval |
|---|---|---|---|
| Thickness | 0.006 | 1.279 | 1.075–1.521 |
| Leukocyte | 0.017 | 1.142 | 1.024–1.272 |
| Hemoglobin | 0.034 | 0.673 | 0.467–0.970 |
DISCUSSION
A few previous reports have investigated the risk factors of delayed surgical intervention in initially conservatively treated traumatic ASDH patients (Table 4). According to previous studies, 12.6% of ASDH patients developed CSDH that required delayed surgical intervention. In this study, 28 patients (17.7%) showed progression of SDH and required surgical decompression. They presented aggravation of headache, dysarthria, gait disturbance, or subjective motor weakness. These patients underwent Burr hole trephination and drainage under general or local anesthesia an average of 13.9 days after admission, and none showed neurological deterioration after surgery.
Table 4.
Previous reports on progression of hematoma in initially conservatively treated ASDH patients
| Study | Country | Study population | Surgical group | Country | Risk factors | Not risk factor |
|---|---|---|---|---|---|---|
| Laviv and Rappaport (2014)10) | Istrael | 95 | 43 (45.2) | Israel | IHD HTN ACE-inhibitor Anticoagulant Clopidogrel Size of SDH |
DM Bilaterality |
| Lee et al. (2015)11) | Korea | 117 | 16 (13.7) | Korea | Age Midline shifting Hematoma depth Hounsfield Unit |
HTN DM SAH H. contusion Bilaterality Midline shifting Aspirin, clopidogrel Warfarin |
| Kim et al. (2014)9) | Korea | 98 | 34 (34.7) | Korea | Thickness Hematoma volume Midline shifting H. contusion SAH |
Sex Combined hemorrhage Warfarin |
| Han et al. (2014)7) | Korea | 277 | 20 (7.2) | Korea | HTN DM Cb. Infarction Anti-PLT Location (convexity) Encephalomalacia |
Age Sex Gcs Anticoagulant Anti-PLT |
| Bajsarowicz et al. (2015)1) | Canada USA | 647 | 42 (6.5) | Canada USA | Prev. fall Alcohol Location (convexity) Thickness Midline shifting |
Age Sex INR Thickness Midline shifting |
Values are presented as number (%). ASDH: acute subdural hematoma, IHD: ischemic heart disease, HTN: hypertension, ACE: angiotensin-converting-enzyme, DM: diabetes mellitus, H.: hemorrhagic, Cb.: cerebral, PLT: platelet, GCS: Glasgow coma scale, Prev.: previous
Older patients with minimal ASDH in elderly were more likely to undergo delayed surgery than younger patients. Physiology factors in the brain, such as low elasticity, vulnerable bridging veins, and atrophy, makes older people more susceptible to head injury12,15). In the present study, mean age was significantly higher in the surgical group than the conservative group (69.00 vs. 61.62 years) in univariate analysis (p=0.024) but not multivariate analysis. These findings suggest that aging is not an independent factor, but may be related to other co-morbidities or physiological factors2,5,6,12).
Previous studies have reported conflicting results regarding whether hypertension influences the risk of delayed surgery. In the present study, hypertension demonstrated no significant correlation with delayed surgical intervention. These findings may suggest that hypertension is not an independent risk factor for SDH progression. But some previous studies showed the relationship between hypertension and delayed surgery, future research should focus on risk factors influenced by hypertension7,10). Previous studies have also reported conflicting results on the association between DM and SDH progression7,9–11). Han et al.7) suggested that DM vasculopathy may play a role in re-bleeding in SDH, while DM was found not to be a risk factor for the progression of ASDH to CSDH. In DM patients, it has been proposed that higher blood osmotic pressure induces hyperviscosity and increases platelet aggregation, thus potentially decreasing re-bleeding in SDH patients25,29).
We expected that history of cardiovascular or cerebrovascular disease and usage of anti-coagulant or anti-platelet agents would influence delayed surgery. However, only history of cerebrovascular disease significantly differed between the two groups. Cerebral infarction leads to a reduction in brain parenchyma volume, creating a larger subdural space. Consequently, patients are at greater risk of SDH progression19). Use of anti-platelet agents was shown to increase the risk of morbidity and mortality in ASDH patients21). Among the previous studies on SDH progression, Kim et al.9), Lee et al.11), and Bajsarowicz et al.1) reported that use of anti-platelet agents does not influence delayed surgery. By contrast, Laviv and Rappaport10) and Tseng et al.26) reported that use of anti-platelet agents increases the risk of delayed surgery. Consistent with the former group, in the present study, use of anti-platelet agents did not increase the risk of delayed surgical intervention. Use of anti-platelet agents increases the risk of re-bleeding, is associated with liquefaction of SDH, and facilitates early redistribution of hematoma. Han et al.7) suggested that anti-platelet agents affect hematoma liquidity, allowing the hematoma to be more easily washed out by cerebrospinal fluid flow18,27). Cessation and correction of coagulation abnormality could play a role in reducing the risk of delayed surgery. Additionally, it is possible that patients on anti-platelet agents might have had a much thicker SDH requiring emergency surgical decompression or refused intensive treatment, leading them to be excluded from this study.
We found that greater SDH thickness and midline shifting were associated with delayed surgical intervention, and SDH thickness was an independent risk factor for SDH progression. Midline shifting is influenced by SDH thickness, and smaller initial SDH thickness could be considered to reflect greater stability. A thicker hematoma can stress the bridging veins more severely and make them vulnerable to tearing13), resulting in hematoma expansion. Through a combination of persistent inflammatory reaction, neoangiogenesis, collection of subdural fluid, and repeated coagulation and fibrinolysis, it the hematoma develops a neomembrane17,23,24). Consequently, initially large SDH require more time to be washed out and have a greater risk of transforming to chronic SDH.
We analyzed the accompanying intracranial injuries on all patients’ initial brain CT scan. Focal intraparenchymal hemorrhage was not associated with delayed surgery. However, concomitant subarachnoid hemorrhage was more frequently seen in the conservative group. Combined subarachnoid hemorrhage could make the parenchymal swelling. Brain swelling after head injury compresses the hematoma, leading to redistribution6). It is possible that an edematous cerebral condition might reduce the free subdural space between the skull and parenchyma, preventing the progression of SDH14).
In addition, we collected initial laboratory findings for all patients. Among the initial complete blood cell count, hemoglobin demonstrated an independent negative correlation with delayed surgery, and leukocytes demonstrated an independent positive correlation with surgery. Low hemoglobin count could be considered to reflect the aging process, but also indicates low viscosity of the blood. Viscosity has an important role in coagulation pathways. Low hemoglobin indicates low viscosity of the blood, and these patients have tendency for micro-bleeds and progression to CSDH20). CSDH is also a consequence of inflammatory reactions in hematoma23,24). Previous reports suggested that eosinophil granulation is associated with SDH membrane formation16,22,30). Another previous study indicated that the inflammatory reaction is the key role in progression of the CSDH. Leukocytes, eosinophils, macrophages and inflammatory cytokines were elevated and play the important role of progression. At this point of view, an elevated leukocyte count could be interpreted as a marker of inflammatory processes. Pro- and anti-inflammatory cytokines encourage the neoangiogenesis4). So patients with a high leukocyte count, more easily form a hematoma neomembrane. Moreover, leukocytes, especially neutrophils, are thought to mediate the exacerbation of secondary brain injury in intracerebral hemorrhage by releasing additional pro-inflammatory proteases that affect blood-brain barrier permeability31,32). Therefore, relieving the inflammatory condition might reduce the risk of delayed surgery and secondary brain injury4), and further studies should focus on the association of CSDH progression and inflammatory markers including not only leukocyte count but also specific cell types (eosinophils, neutrophils, and basophils) and other markers such as erythrocyte sedimentation rate and C-reactive protein.
Initial low glucose was also a risk factor for surgical intervention, which could be considered from the same point of view as hemoglobin. High glucose indicates high viscosity of the blood, which could facilitate hemostasis. This phenomenon could in turn prevent additional bleeding and halt the SDH expansion.
CONCLUSION
In this study, 17.7% of initially conservatively treated ASDH patients developed CSDH and required delayed surgical intervention. Greater initial hematoma thickness, low hemoglobin, and high leukocyte count were identified as independent risk factors of hematoma progression. Therefore, patients with thicker hematomas on initial brain CT and low hemoglobin or high leukocyte count require more careful observation to prevent delayed surgical intervention.
References
- 1.Bajsarowicz P, Prakash I, Lamoureux J, Saluja RS, Feyz M, Maleki M, et al. Nonsurgical acute traumatic subdural hematoma: What is the risk? J Neurosurg. 2015;123:1176–1183. doi: 10.3171/2014.10.JNS141728. [DOI] [PubMed] [Google Scholar]
- 2.Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16–S24. discussion Si–iv. [PubMed] [Google Scholar]
- 3.Dent DL, Croce MA, Menke PG, Young BH, Hinson MS, Kudsk KA, et al. Prognostic factors after acute subdural hematoma. J Trauma. 1995;39:36–42. doi: 10.1097/00005373-199507000-00005. discussion 42–33. [DOI] [PubMed] [Google Scholar]
- 4.Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017;14:108. doi: 10.1186/s12974-017-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feliciano CE, De Jesús O. Conservative management outcomes of traumatic acute subdural hematomas. P R Health Sci J. 2008;27:220–223. [PubMed] [Google Scholar]
- 6.Fujimoto K, Otsuka T, Yoshizato K, Kuratsu J. Predictors of rapid spontaneous resolution of acute subdural hematoma. Clin Neurol Neurosurg. 2014;118:94–97. doi: 10.1016/j.clineuro.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Han SB, Choi SW, Song SH, Youm JY, Koh HS, Kim SH, et al. Prediction of chronic subdural hematoma in minor head trauma patients. Korean J Neurotrauma. 2014;10:106–111. doi: 10.13004/kjnt.2014.10.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong SI, Kim SO, Won YS, Kwon YJ, Choi CS. Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination. Korean J Neurotrauma. 2014;10:15–21. doi: 10.13004/kjnt.2014.10.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BJ, Park KJ, Park DH, Lim DJ, Kwon TH, Chung YG, et al. Risk factors of delayed surgical evacuation for initially nonoperative acute subdural hematomas following mild head injury. Acta Neurochir (Wien) 2014;156:1605–1613. doi: 10.1007/s00701-014-2151-4. [DOI] [PubMed] [Google Scholar]
- 10.Laviv Y, Rappaport ZH. Risk factors for development of significant chronic subdural hematoma following conservative treatment of acute subdural hemorrhage. Br J Neurosurg. 2014;28:733–738. doi: 10.3109/02688697.2014.918578. [DOI] [PubMed] [Google Scholar]
- 11.Lee JJ, Won Y, Yang T, Kim S, Choi CS, Yang J. Risk factors of chronic subdural hematoma progression after conservative management of cases with initially acute subdural hematoma. Korean J Neurotrauma. 2015;11:52–57. doi: 10.13004/kjnt.2015.11.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KS. The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Inj. 1998;12:595–603. doi: 10.1080/026990598122359. [DOI] [PubMed] [Google Scholar]
- 13.Lee KS, Shim JJ, Yoon SM, Doh JW, Yun IG, Bae HG. Acute-on-chronic subdural hematoma: not uncommon events. J Korean Neurosurg Soc. 2011;50:512–516. doi: 10.3340/jkns.2011.50.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637–645. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–381. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 16.Müller W, Firsching R. Significance of eosinophilic granulocytes in chronic subdural hematomas. Neurosurg Rev. 1990;13:305–308. doi: 10.1007/BF00346370. [DOI] [PubMed] [Google Scholar]
- 17.Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al. Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role of thrombomodulin in the mechanism. J Neurosurg. 2002;96:877–884. doi: 10.3171/jns.2002.96.5.0877. [DOI] [PubMed] [Google Scholar]
- 18.Niikawa S, Sugimoto S, Hattori T, Ohkuma A, Kimura T, Shinoda J, et al. Rapid resolution of acute subdural hematoma--report of four cases. Neurol Med Chir (Tokyo) 1989;29:820–824. doi: 10.2176/nmc.29.820. [DOI] [PubMed] [Google Scholar]
- 19.Okano A, Oya S, Fujisawa N, Tsuchiya T, Indo M, Nakamura T, et al. Analysis of risk factors for chronic subdural haematoma recurrence after burr hole surgery: optimal management of patients on antiplatelet therapy. Br J Neurosurg. 2014;28:204–208. doi: 10.3109/02688697.2013.829563. [DOI] [PubMed] [Google Scholar]
- 20.Roeloffzen WW, Kluin-Nelemans HC, Bosman L, de Wolf JT. Effects of red blood cells on hemostasis. Transfusion. 2010;50:1536–1544. doi: 10.1111/j.1537-2995.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 21.Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995;43:240–244. doi: 10.1111/j.1532-5415.1995.tb07329.x. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar C, Lakhtakia R, Gill SS, Sharma MC, Mahapatra AK, Mehta VS. Chronic subdural haematoma and the enigmatic eosinophil. Acta Neurochir (Wien) 2002;144:983–988. doi: 10.1007/s00701-002-0994-6. discussion 988. [DOI] [PubMed] [Google Scholar]
- 23.Stanisic M, Aasen AO, Pripp AH, Lindegaard KF, Ramm-Pettersen J, Lyngstadaas SP, et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm Res. 2012;61:845–852. doi: 10.1007/s00011-012-0476-0. [DOI] [PubMed] [Google Scholar]
- 24.Stanisic M, Lyngstadaas SP, Pripp AH, Aasen AO, Lindegaard KF, Ivanovic J, et al. Chemokines as markers of local inflammation and angiogenesis in patients with chronic subdural hematoma: a prospective study. Acta Neurochir (Wien) 2012;154:113–120. doi: 10.1007/s00701-011-1203-2. discussion 120. [DOI] [PubMed] [Google Scholar]
- 25.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63:1125–1129. doi: 10.1227/01.NEU.0000335782.60059.17. discussion 1129. [DOI] [PubMed] [Google Scholar]
- 26.Tseng JH, Tseng MY, Liu AJ, Lin WH, Hu HY, Hsiao SH. Risk factors for chronic subdural hematoma after a minor head injury in the elderly: a population-based study. Biomed Res Int. 2014;2014:218646. doi: 10.1155/2014/218646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsui EY, Fai Ma K, Cheung YK, Chan JH, Yuen MK. Rapid spontaneous resolution and redistribution of acute subdural hematoma in a patient with chronic alcoholism: a case report. Eur J Radiol. 2000;36:53–57. doi: 10.1016/s0720-048x(99)00167-9. [DOI] [PubMed] [Google Scholar]
- 28.Wong CW. Criteria for conservative treatment of supratentorial acute subdural haematomas. Acta Neurochir (Wien) 1995;135:38–43. doi: 10.1007/BF02307412. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98:1217–1221. doi: 10.3171/jns.2003.98.6.1217. [DOI] [PubMed] [Google Scholar]
- 30.Yamashima T, Kubota T, Yamamoto S. Eosinophil degranulation in the capsule of chronic subdural hematomas. J Neurosurg. 1985;62:257–260. doi: 10.3171/jns.1985.62.2.0257. [DOI] [PubMed] [Google Scholar]
- 31.Yu S, Arima H, Heeley E, Delcourt C, Krause M, Peng B, et al. White blood cell count and clinical outcomes after intracerebral hemorrhage: the interact2 trial. J Neurol Sci. 2016;361:112–116. doi: 10.1016/j.jns.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Ziai WC. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke. 2013;44(6 Suppl 1):S74–S78. doi: 10.1161/STROKEAHA.111.000662. [DOI] [PubMed] [Google Scholar]


