Abstract
Objective
We present our experience of microvascular decompression (MVD) for glossopharyngeal neuralgia (GPN) and evaluate the postoperative outcomes in accordance with four different operative techniques during MVD.
Methods
In total, 30 patients with intractable primary typical GPN who underwent MVD without rhizotomy and were followed for more than 2 years were included in the analysis. Each MVD was performed using one of four different surgical techniques: interposition of Teflon pieces, transposition of offending vessels using Teflon pieces, transposition of offending vessels using a fibrin-glue-coated Teflon sling, and removal of offending veins.
Results
The posterior inferior cerebellar artery was responsible for neurovascular compression in 27 of 30 (90%) patients, either by itself or in combination with other vessels. The location of compression on the glossopharyngeal nerve varied; the root entry zone (REZ) only (63.3%) was most common, followed by both the REZ and distal portion (26.7%) and the distal portion alone (10.0%). In terms of detailed surgical techniques during MVD, the offending vessels were transposed in 24 (80%) patients, either using additional insulation, offered by Teflon pieces (15 patients), or using a fibrin glue-coated Teflon sling (9 patients). Simple insertion of Teflon pieces and removal of a small vein were also performed in five and one patient, respectively. During the 2 years following MVD, 29 of 30 (96.7%) patients were asymptomatic or experienced only occasional pain that did not require medication. Temporary hemodynamic instability occurred in two patients during MVD, and seven patients experienced transient postoperative complications. Neither persistent morbidity nor mortality was reported.
Conclusion
This study demonstrates that MVD without rhizotomy is a safe and effective treatment option for GPN.
Keywords: Glossopharyngeal nerve diseases, Neuralgia, Microvascular decompression surgery, Polytetrafluoroethylene
INTRODUCTION
Glossopharyngeal neuralgia (GPN) is a rare cranial rhizopathic disorder characterized by unilateral severe, paroxysmal episodes of neuralgic pain in the sensory territory of the glossopharyngeal nerve, it can be triggered by swallowing, yawning, and sneezing5,7,19,32,33,39). Its incidence is reported to be 0.2–1.3% of that of trigeminal neuralgia (TN)18,19,28,39). GPN and TN share similar pain characteristics, and yet the intensity of GPN seems to be greater than that of TN. Additionally, it can be associated with hemodynamic instability that can lead to life-threatening syncopal episodes7,32,33,36). As GPN is more medically intractable than TN, approximately half of all patients eventually require an operation for pain relief despite newly introduced anti-epileptic drugs9,10,14). Microvascular decompression (MVD) is an invasive therapeutic modality for cranial rhizopathies that is generally considered risky and technically challenging because of the limited working space and potential complications associated with over-retraction of the cerebellum along with viable lower cranial nerves18). Given its rarity, only a limited number of reports on the long-term results of MVD for GPN is available7,15,19,26,28,40). Considering the relatively low success rate (55%) following stereotactic radiosurgery, MVD should be considered a better alternative despite its possible drawbacks16,22).
The aim of this study was to evaluate surgical outcomes and compare immediate and long-term clinical outcomes following MVD without a rhizotomy for GPN. Surgical results according to four different surgical techniques for GPN were also evaluated.
MATERIALS AND METHODS
Patient selection and preoperative evaluation
In total, 44 surgical procedures in 42 patients with intractable GPN were performed using MVD and gamma knife radiosurgery (GKS) between March 1996 and December 2016 at our institute. One patient with a petrosal meningioma, which caused intractable secondary GPN was managed with GKS and excluded from the analysis. Among the 41 patients with intractable primary GPN, 38 underwent MVD without rhizotomy, of whom 30 with follow-up periods of 2 years or longer were selected for the analysis. For the diagnosis of GPN, the characteristics of pain were evaluated according to the International Classification of Headache Disorders9) (Table 1). The following factors precluded MVD for GPN: advanced age (>80 years), poor general condition, and severe systemic diseases (severe restrictive lung disease, unstable ischemic heart disease, arrhythmia, congestive heart failure, stroke, and renal impairment).
Table 1.
Diagnostic criteria of glossopharyngeal neuralgia9)
|
ICHD: The International Classification of Headache Disorders
In addition to the glossopharyngeal nerve, GPN may also involve branches of the vagal nerve leading to bradycardia and syncope that may necessitate pacemaker placement in select patients. Although not recognized by the current diagnostic criteria for GPN, vagally mediated symptoms may occur in patients without a history of neuralgic pain8,27) In this study vagally mediated symptoms, including bradycardia, dyspnea, and syncope, were investigated, and the medical histories of patients, including hypertension and diabetes, were carefully reviewed.
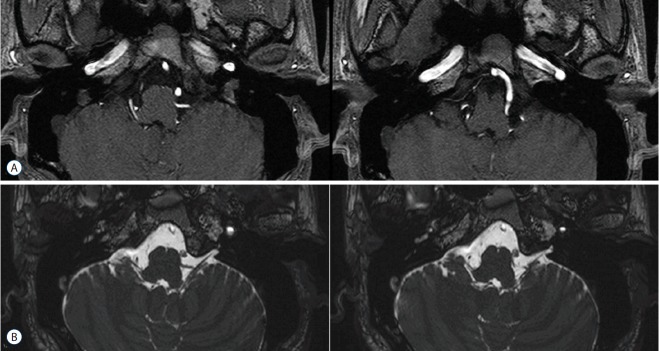
A 1.5 T (Signa EXCITE; GE Healthcare, Waukesha, WI, USA) or 3.0 T magnetic resonance imaging (MRI) system (Achieva 3.0T TX; Philips Healthcare, Best, the Netherlands) or multi-detector computed tomography angiography (Brilliance; Philips Healthcare) was used to detect compressing vessels or concomitant lesions near the root entry zone (REZ) of the glossopharyngeal nerve. The vascular anatomy causing neuralgic pain and the relationships between offending vessels and causative nerves were demonstrated (Fig. 1). All patients underwent preoperative pure-tone audiometry (Orbiter 922; Madsen Electronics, Minneapolis, MN, USA), speech audiometry (Orbiter 922; Madsen Electronics, Minneapolis, MN, USA), and impedance audiometry (Madsen Otoflex-100; GN Otometrics, Taastrup, Denmark) to identify any pre-existing hearing impairments or tinnitus.
Fig. 1.
Magnetic resonance image of the left posterior inferior cerebellar artery in contact with the root entry zone of the left glossopharyngeal nerve. A: Three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA). B: Three-dimensional fast imaging employing steady-state acquisition (3D-FIESTA).
Surgical procedure
All patients underwent retromastoid lateral suboccipital craniotomy under general anesthesia in the lateral park bench position with the side of the desired MVD placed up. Computed tomography (CT) navigation-guided craniotomy was used to expose the margin of the sigmoid sinus from its beginning to the region behind the mastoid tip and to reduce the risks of bleeding from the sigmoid sinus25). Intraoperative brainstem auditory evoked potentials and sensory evoked potentials (Viasys Healthcare, Conshohocken, PA, USA) were monitored throughout the operation to check the function of the brainstem and cranial nerves. All operations were performed by a single surgeon.
A C-shaped dural incision was made and reflected over the sigmoid sinus. The arachnoid membrane was dissected carefully to allow cerebrospinal fluid (CSF) drainage and to expose the lower cranial nerves and the vessels responsible for GPN. A retractor with a 2-mm-wide tip was applied intermittently during the arachnoid dissection. Several small Tachosil (Nycomed, Linz, Austria) pieces, Teflon pieces (Bard PTFE FELT, Tempe, AZ, USA), and fibrin glue (Tisseel; Baxter Healthcare Corp., Glendale, CA, USA) were prepared. After performing arachnoid dissections around the glossopharyngeal-vagal complex, the relationship between the offending vessel and the REZ of the glossopharyngeal or vagal nerve was visualized using an operative microscope (OPMI Pentero; Carl Zeiss, Oberkocken, Germany) and neuroendoscope (Karl Storz GmbH, Tuttlingen, Germany) if needed. Gentle mobilization of the offending vessels was attempted, and the perforating arteries to the brainstem were also thoroughly investigated and carefully protected21).
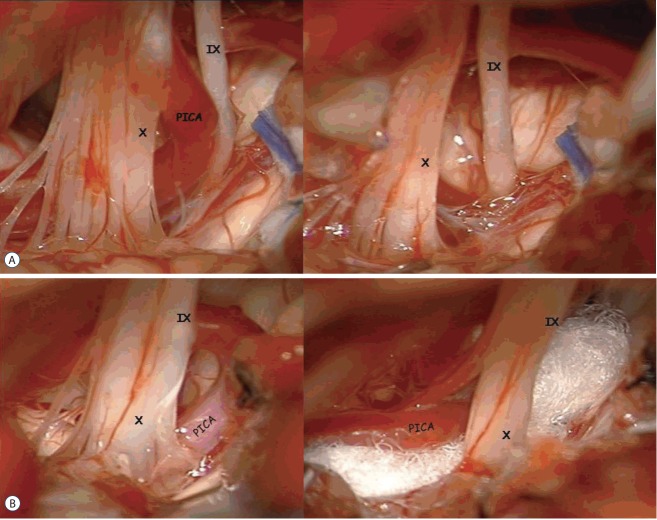
Each MVD was performed using one of four different surgical techniques: interposition of Teflon pieces, transposition of offending vessels using Teflon pieces, transposition of offending vessels using a fibrin glue-coated Teflon sling, and removal of offending veins (Fig. 2).
Fig. 2.
Intraoperative images of microvascular decompression of the glossopharyngeal root entry zone from an offending artery in patients with glossopharyngeal neuralgia. A: Transposition using a fibrin glue-coated Teflon sling retraction. B: Interposition using Teflon pieces. PICA: posterior inferior cerebellar artery.
Transposition of offending vessels using Teflon pieces was performed to keep the offending vessels away from the REZ of the 9th and 10th cranial nerves. Teflon pieces were used to hold offending vessels on the brain stem surface and were fixated with several small pieces of Tachosil and fibrin glue. Transposition of offending vessels using a fibrin glue-coated Teflon sling was applied in select patients whose offending vessels were difficult to transpose by the usual method for transposition using Teflon pieces. A fibrin glue-coated Teflon sling was crafted by the surgeon. Several Teflon threads were manually twisted together using fibrin glue to form an elongated strand. The bioglue enabled a smoother, more flexible, and lint-free Teflon sling. The coating procedure using bioglue increased not only the manipulability of the Teflon sling but also the tensile force of the strand. Using a microscope, the sling was carefully looped around the offending vessels, which were typically repositioned superiorly and laterally from the nerves, and was stitched onto the petrous dural wall using 5–0 black silk sutures. Careful inspection was necessary to avoid kinking or stretching of small perforating vessels before stitching the sling on the dura. The transposed vessel was further secured using fibrin glue to ensure that the REZ of the 9th and 10th cranial nerves was free from any offending vessels21).
Interposition using Teflon pieces and fibrin glue was also performed in select patients whose offending vessels were tethered to the REZ or brain stem, on account of short perforating vessels originating directly from the offending vessels. In such patients, the offending vessels cannot be transposed safely. Several pieces of Teflon were applied to the small space between the offending vessel and the REZ of the 9th and 10th cranial nerves. The specific compression caused by short perforating vessels deserves special attention, because any slight stretching can disrupt the perforating vessels. The interposed Teflon pieces were then fixated using fibrin glue.
Any vein thought to be a causative vessel was removed, and the coagulated vein was always divided to prevent re-canalization.
Pre- and postoperative evaluation of pain
The preoperative pain status of individual patients was evaluated, and the severity of neuralgic pain was assessed using a visual analog scale (VAS). To evaluate postoperative outcomes, the pain before and after the operation was assessed using the Barrow Neurological Institute (BNI) pain intensity score30) (Table 2).
Table 2.
The Barrow Neurological Institute (BNI) pain intensity score
| Score | Pain description |
|---|---|
| Class I | No pain, no medication required |
| Class II | Occasional pain, no medication required |
| Class III | Some pain, adequately controlled with medication |
| Class IV | Some pain, not adequately controlled with medication |
| Class V | Severe pain or no pain relief with medication |
We defined a follow-up period of 2 years or longer as long term. Patients were contacted by telephone to obtain long-term follow-up information regarding symptom relief, complications, functional outcomes, and patient satisfaction.
Statistical analyses
Statistical analyses were performed using SPSS software (ver. 20; IBM SPSS Statistics, Armonk, NY, USA). Categorical variables are expressed as proportions and continuous variables as means and standard deviations. Clinical outcomes were evaluated according to surgical management, use of medications, pain recurrence, offending vessels, and postoperative complications. Descriptive statistics were used for patient data, and the grades of preoperative and postoperative pain were analyzed using the Wilcoxon signed-rank test. p values <0.05 were considered to indicate statistical significance.
RESULTS
Demographic data
Of the 30 patients with GPN, the mean age at the time of surgery was 52.4 (range, 31–72) years, 19 (63.3%) were males, and 21 (70%) presented with left-sided neuralgic pain. The mean duration of symptoms was 39.7 (1–132) months, and the mean follow-up duration was 68.5 (25–251) months after surgery. Three patients (10.0%) experienced TN concurrently with GPN, and in four patients (13.3%) GPN had been misdiagnosed as TN prior to visiting our institution.
Concomitant medical diseases included hypertension (8 patients), diabetes mellitus (4 patients), and cardiac disease (1 patient). One patient experienced preoperative dysphonia. No patient experienced any preoperative vagally mediated symptoms, including bradycardia, dyspnea, and syncope. Two patients experienced temporary arrhythmias along with unstable blood pressure during the operation. Atherosclerotic changes in the posterior inferior cerebellar artery (PICA) were observed during surgery in one case. Three patients harbored an incidental tumor, but none of these tumors was related to the etiology of the GPN.
Operative findings
Neurovascular compression was found in all patients, and the causative vessel was identified more frequently as a single artery than multiple arteries. The most common offending vessel was the PICA, as a single artery causing GPN, in 21 (70%) patients, followed by multiple arteries in 7 (23.3%) patients, and vein alone in 1 (3.3%) patient (Table 3).
Table 3.
Distribution of the offending vessels
| Offending vessel | No. of patients* | |
|---|---|---|
| Single artery | 21/30 (70.0%) | |
| PICA | 21 | |
| VA | 0 | |
|
| ||
| Multiple arteries | 7/30 (23.3%) | |
| PICA+AICA | 1 | |
| PICA+AICA+VA | 1 | |
| PICA+VA | 2 | |
| PICA+SCA | 1 | |
| Small arteries | 2 | |
| Vein only | 1/30 (3.3%) | |
|
| ||
| Vein+artery (PICA) | 1/30 (3.3%) | |
Percentages do not total 100% because percentages have been rounded off.
PICA: posteroinferior cerebellar artery, VA: vertebral artery, AICA: anteroinferior cerebellar artery, SCA: superior cerebellar artery
The locations of neurovascular compression were variable and included the REZ only, the REZ plus distal part of the nerve, or the distal part of the nerve only. The compression was located solely on the REZ in 19 (63.3%) patients, on both the REZ and distal part of the glossopharyngeal nerve in 8 patients, and solely on the distal part of the glossopharyngeal nerve in 3 patients. Among the 19 compressions solely on the REZ, 14 were located on the conjoint area between the glossopharyngeal and vagal REZ, 3 exclusively on the glossopharyngeal REZ, and 2 on the vagal REZ. The site of compression, however, did not cause any significant difference in clinical manifestations or short-term and long-term surgical outcomes (p=0.068, p=0.203; Table 4).
Table 4.
Distribution of the compression sites
| Compression site | No. of patients |
|---|---|
| REZ only | 19/30 (63.3%) |
| Glossopharyngeal and vagal REZ | 14 |
| Glossopharyngeal REZ | 3 |
| Vagal REZ | 2 |
|
| |
| REZ and distal part of the glossopharyngeal nerve | 8/30 (26.7%) |
|
| |
| Distal part of the glossopharyngeal nerve | 3/30 (10.0%) |
REZ, root entry zone
Among the four different surgical techniques, transposition using Teflon pieces was applied in 15 (50.0%) patients, and transposition using a fibrin glue-coated Teflon sling was applied in 9 (30.0%; Table 5). The decompression technique was selected according to the type of vascular conflict (p=0.003, Wilcoxon rank test). The type of decompression technique was not related to short-term or long-term surgical outcomes or complications (p=0.477, p=0.619, and p=0.717, respectively).
Table 5.
Surgical techniques used for microvascular decompression
| Decompression technique | No. of patients |
|---|---|
| Transposition using Teflon pieces | 15/30 (50.0%) |
| Transposition using a fibrin glue-coated Teflon sling retraction | 9/30 (30.0%) |
| Interposition using Teflon pieces | 5/30 (16.7%) |
| Coagulation and division | 1/30 (3.3%) |
A concurrent tumor, a lipoma was detected during MVD in one patient, in whom transposition of the causative vessel was performed. Although the lipoma was in close contact with the REZ of the glossopharyngeal nerve, it was not removed because the causative vessel was observed and the lipoma alone was not thought to be the cause of the neuralgic pain4). The neuralgic pain was markedly reduced immediately after surgery and resolved gradually over 6 months. In the patient with a petrosal meningioma detected on diagnostic MRI, the tumor was partially removed, in addition to MVD, and the remaining tumor was treated further with GKS. A vestibular schwannoma incidentally detected on diagnostic MRI was treated with GKS 6 months after treatment of the neuralgic pain with MVD. Given that the neuralgic pain disappeared completely after MVD, the tumor per se did not seem to have been the cause of the pain.
Postoperative outcomes
Of the 30 patients, 26 (86.7%) were pain-free immediately after surgery, the remaining 4 (13.3%) experienced residual pain, although the intensity of the pain was reduced markedly. By the 1-month follow-up, the residual pain was less frequent and less severe than that observed preoperatively. Three of the four patients no longer required medication (BNI score of I–II). However, the one remaining patient complained of recurrent throat pain that was triggered by swallowing (BNI score of IV), even though his otalgic pain had disappeared and neuralgic pain decreased somewhat, at 1 month after surgery. He also complained of trigeminal neuralgic pain that was tolerable before glossopharyngeal MVD. In this particular patient, the suspected vessel causing GPN was transposed with a fibrin-glue coated Teflon sling, and the securely transposed offending vessel was confirmed by postoperative MRI. At 9 months after MVD, GKS was used to control the sustained pain on both the glossopharyngeal and trigeminal nerves simultaneously. At 3 years after the surgery, his pain still remained and had to be controlled by medications and occasional percutaneous sympathetic blocks. One patient who was treated by interposition with Teflon pieces became pain free immediately after surgery; however, at 3 years after the surgery he experienced occasional pain that did not require medication (Table 6).
Table 6.
Short- and long-term surgical outcomes
| BNI pain intensity score | Short-term outcome* | Long-term outcome† |
|---|---|---|
| I | 26 (86.7%) | 25 (83.3%) |
| II | 0 | 4 (13.3%) |
| III | 3 (10.0%) | 0 |
| IV | 1 (3.3%) | 1 (3.3%) |
| V | 0 | 0 |
The number of patients immediately after surgery.
The number of patients after at least 2 years of follow-up.
BNI, Barrow Neurological Institute
TN was concurrent with GPN in 3 (10.0%) patients. MVD was performed to decompress both the trigeminal and glossopharyngeal nerves in two patients, both of whom became pain-free following the MVD. The remaining patient who had undergone MVD for GPN developed recurrent glossopharyngeal pain as well as aggravated TN. GKS targeting both nerves was performed 9 months after MVD, which alleviated the pain greatly.
Complications
No mortality or serious morbidity was observed in the patients (Table 7). Two patients experienced transient postoperative dysphagia and/or dysphonia. One patient complained of decreased hearing postoperatively, and his hearing disturbance failed to recover. Vagally mediated complications, such as hypotension, bradycardia, abdominal pain, and indigestion, were not observed in any patient. CSF rhinorrhea developed in two patients during the early postoperative stage but was controlled with lumbar CSF drainage for 1 week. One patient experienced brain swelling of unknown cause at the end of the operation but recovered after conservative treatment with no neurological deficits.
Table 7.
Postoperative complications
| Complication | No. of patients |
|---|---|
| Morbidity | |
| None | 23/30 (76.7%) |
| Transient dry cough | 1/30 (3.3%) |
| Transient dysphonia/dysphagia | 2/30 (6.7%) |
| Decreased hearing | 1/30 (3.3%) |
| Cerebrospinal fluid leakage | 2/30 (6.6%) |
| Brain swelling | 1/30 (3.3%) |
| Mortality | 0 |
DISCUSSION
We evaluated the therapeutic effects of MVD in patients with medically intractable primary GPN. It is considered that transposition of the offending vessels is ideal to treat patients with cranial rhozophthic diseases if a MVD procedure is needed18,23). However, the transposition technique can be applicable in select patients with GPN. In this series, we divided patients with GPN into four groups according to the surgical technique used for decompression. Additionally, decompression-associated surgical outcomes were compared in 30 patients with follow-up periods of 2 years or longer.
MVD with or without rhizotomy
Since Dandy’s description of surgical section of the glossopharyngeal nerve to treat GPN, destruction of the glossopharyngeal nerve has long been the gold standard for a cure and is still an applicable therapeutic option for patients with intractable GPN5,16,32,34,35). To date, various procedures including rhizotomy have been adopted for the treatment of GPN19,20,31). In 1981, when surgical microscopes were unavailable, a report was published on 129 GPN patients, in whom various surgical procedures for GPN such as rhizotomy, avulsion, or sectioning of the glossopharyngeal nerve as well as the upper rootlets of the vagus nerve were carried out31). Of those cases, 110 patients obtained good pain relief, but 13 patients suffered recurrence at 2–6 weeks after surgery. The most common postoperative complication was swallowing disturbance, which occurred in 25 (19.4%) patients, with a 5% perioperative mortality rate.
In the current ‘era of microsurgery’, Taha and Tew34) reported long-term results of surgical treatment of GPN in 1995; 16 procedures were performed: 2 percutaneous thermal rhizotomies, 2 extracranial sections of the superior laryngeal nerve, and 12 intracranial glossopharyngeal and upper vagal rhizotomies (4 with MVD and 8 without MVD). Of the 12 patients who underwent intracranial sectioning of the 9th and 10th cranial nerves, two developed dysphagia, one experienced transient hoarseness, and two developed frequent coughing episodes. Taha et al.35) evaluated morbidity after rhizotomy and proposed that intraoperative monitoring of the rostral vagal rootlets is an important technique for minimizing the complications of upper vagal rhizotomy. However, all such procedures necessitate partial sacrifice of the glossopharyngeal nerve and, in most cases, also involve destruction of at least part of the vagus nerve19). Many reports have claimed that section of the glossopharyngeal nerve and rostral vagal rootlets can be a good treatment option for GPN without serious neurological deficits19,34). However, it cannot be ingnored that any interruption of the vagus nerve, especially the upper rootlets, may result in various consequences, such as dysarthria and dysphagia, because the upper rootlets contribute fibers to the superior and recurrent laryngeal nerves7,34,38). In our study, rhizotomy of the 9th and 10th cranial nerves was never performed, and successful outcomes were achieved by the MVD per se. In our opinion, based on our own results, rhizotomy does not need to be considered as the first-line option, except for some limited cases that preclude MVD.
Vascular conflict versus thickened arachnoid membrane
Neurovascular compression of TN was noted, even in patients with typical TN at the time of surgery, in only approximately 80–90% of cases24). Furthermore, in some cases of TN, no offending vessel was detected, and the thickened arachnoid membrane around the nerves had created abnormal tension on the trigeminal REZ, resulting in deformity of the nerves1,24). In most cases of GPN, the vertebral artery (VA) becomes distorted and shifts the PICA, which directly compresses the rootlets of the glossopharyngeal and vagus nerves19). Based on the intraoperative findings in our series, the VA itself was not responsible for compression in any case. Dolichoectatic VA causes the compression in hemifacial spasm, but it is seldom involved in GPN due to the rarity of GPN and anatomical differences between the facial and glossopharyngeal nerve12,21). Veins, on the other hand, were identified as the offending vessels in our series, either alone or together with an artery; they appeared to be engorged, probably because of disrupted drainage.
In this study, neurovascular contact at the REZ or distal nerve was noted in all of the patients, but there was no case in whom adhesion of the thickened arachnoid membrane was the solitary cause of the compression. In two cases, the arachnoid membrane was responsible for the compression, yet only secondarily to the tortuous PICA, which was the primary culprit. The anatomical positions of the 9th and 10th nerves may explain why a thickened arachnoid membrane is rare.
Intraoperative cardiac arrhythmia and instability
GPN is sometimes associated with cardiac dysarrythmia and instability2,7,29,32,33,36). Riley et al.29) were the first to describe asystole and convulsive seizures in the context of GPN, in two cases. Among 217 patients with GPN, four presented with syncope. It has been suggested that the grossly estimated frequency of syncope in GPN is approximately 20%37), which makes it quite a rare event considering that the overall incidence of GPN is 0.7/100.000 per year8,17,37). In our study, two patients experienced temporary arrhythmias and blood pressure fluctuations during surgery. In one case, while the left PICA as the offending vessel was mobilized from both the glossopharyngeal and vagal REZ, arrhythmia and blood pressure fluctuations developed. However, after removal of the retractor over the cerebellum, the patient’s vital signs stabilized with no additional medication needed from the anesthesiologists. In the other patient, transient cardiac arrhythmia developed but disappeared immediately after removal of the retractor over the cerebellum. These changes may have been due to the asymmetric autonomic distribution of the vagus nerves13). In contrast to the symmetrically paired somatic and sensory functions of all other cranial nerves with central innervation from both nucleus tractus solitarius, the left vagus nerve is the major regulator of the left heart, and it may increase cardiac output and stroke volume13). In addition, insertion of a fairly large piece, or many pieces, of Teflon between the offending vessels and the vagus nerve could have been another cause of the cardiac dysarrythmia and instability.
Distribution of compression sites
Dandy first postulated a nerve grooved or bent by an artery to be the cause of ‘tic douloureux’, and in 1977, Laha and Jannetta20) first reported favorable results of MVD for GPN and postulated that compression must be located at the REZ of the cranial nerve for it to cause symptoms11,39). De Ridder et al.6) concluded that in their experience of five MVDs, decompression of the CNS segment and not the REZ resulted in marked long-term improvement of the symptoms. In our study, offending vessels were compressing both the REZ and the distal part of the glossopharyngeal nerve in 8 (26.7%) patients and only the distal part of the nerve in 3 (10.0%) patients, consistent with a previous suggestion that compression can occur at any point along the cranial nerve, not only at the REZ6).
Surgical outcomes
The largest retrospective study on MVD of GPN to date reported in 2002 enrolled 217 patients with mean follow-up duration of 4 years26). In that study, the patients were treated with MVD whenever possible; however, the detailed MVD methods were not described. The results showed that complete pain relief without medication was observed in 145 (67%) patients immediately and in 121 (58%) patients over the long term. In that series, complications, including permanent cranial nerve palsy, developed in 62 (28.5%) patients, a rather high rate. However, they suggested that MVD seemed to be the most efficacious treatment option for GPN.
In the next largest study of intractable GPN, which enrolled 47 patients, a successful postoperative outcome was achieved in 46 (97.9%) patients immediately and in 29 (96.5%) patients long term (10.5–17.5 years)32). The offending vessels were mostly repositioned from the nerve using a Teflon sling; however, in a few cases that involved stretching or the risk of kinking of a perforator vessel, a Teflon sponge was placed between the nerve and vessel. In this series, permanent cranial nerve palsy was observed in 5 (17.2%) patients with no mortality.
Another report demonstrated relatively good outcomes in 31 patients, with good postoperative outcomes in all patients immediately and in 28 (90.3%) patients over the long term7). Compressive arteries were dissected and maintained far away from the REZ by interposing small pieces of Teflon, and compressive veins were removed by electrocoagulation and division. There were no mortalities, and no permanent deficits were observed except for transient cranial nerve palsy, which developed in 10 (32.2%) patients.
The present study enrolled 30 patients, and the results revealed good surgical outcomes in the short-term and long-term in 29 patients (96.7%, BNI scores of I–III). Medication-free results (BNI scores of I–II) immediately and over the long term were achieved in 26 (86.6%) and 29 (96.7%) patients, respectively. One patient who was a singer/song writer suffered dysphasia and dysphonia preoperatively, in addition to a neuralgic pain attack, all of which disappeared completely within 6 months after the surgery. He regained his ability to sing professionally.
Seven patients (23.3%) experienced postoperative complications, none of which lasted more than 3 months. No serious postoperative neurological deficit or mortality was observed. In the current study, the decompression techniques were selected depending on the type of vascular conflict. As mentioned previously, the offending vessels were transposed in 80% of the patients. Using the interposition technique, more Teflon pieces would be needed to achieve proper decompression. An excessive number of Teflon pieces may cause Teflon granulomas, known to cause iatrogenic irritation of the lower cranial nerves3,32). Our series showed no iatrogenic irritation of the lower cranial nerves, and we consider that this was mainly because of the transposition technique, which minimized the use of Teflon pieces.
Postoperative follow-up
As Laha and Jannetta20) noted, repositioning a tortuous or arteriosclerotic VA away from the REZ by insertion of Teflon pieces can precipitate a brainstem infarction caused by trauma to the perforating arteries. Many perforating vessels surrounding cranial nerves are physiological end arteries with no significant collateral supply and thus should not be interrupted by surgical procedures19). Furthermore, when the Teflon pieces are interposed between the VA or PICA and the REZs of the glossopharyngeal and vagus nerves, they may inversely recompress the REZs of these nerves, particularly when the offending vessels are tortuous and arteriosclerotic. Consequently, insertion of fairly large pieces can easily cause a similar effect on neural and vascular structures when troublesome offending vessels need to be repositioned19). Additionally, the main cause of recurrence is adhesion between the offending vessels and the nerve at its REZ, caused by the inserted pieces19). To resolve this problem and to prevent any surgical complication associated with the recompression caused by the inserted pieces, transposition of the offending vessels may be preferred over inserting pieces of Teflon.
In the current study, interposition using Teflon pieces had to be performed in five patients. In those patients, brainstem perforators from the offending vessels were restrained by transposition of the offending vessel. No recurrence during the follow-up period was noted in the patients who underwent the interposition technique. These patients may need further follow-up to evaluate their long-term outcomes. The present study suggests that the type of decompression technique does not affect the clinical outcome. Considering that excessive insertion of Teflon pieces may cause unnecessary pulsating pressure on the REZ, transposition of the offending vessels would be a more definitive technique to prevent recurrence. We consider that long-term outcomes of GPN are important; however, we suggest that long-term follow-up is not necessarily essential for a patient if the causative vessels were transposed securely, and pain has not recurred in 6 months, because recurrence may be less likely in such cases.
TN versus GPN
GPN is clearly distinguishable from TN based on the distribution of neuralgic pain, although the pain characteristics are similar. Of the 30 patients with primary GPN in our series, 3 (10%) were also diagnosed with TN. This condition accounts for 10–46.7% of GPN cases and 0.3–0.5% of TN cases31). TN does not cause GPN or vice versa.
In case 1, the patient suffered from TN and became pain-free after MVD performed at another institute 3 years previously. However, he later started to complain of a different neuralgic pain that was consistent with GPN. His GPN was treated successfully with another MVD. Case 2 was primarily diagnosed with GPN, but the patient also complained of pain in the trigeminal territory. During MVD, offending vessels were observed on both the REZ of the glossopharyngeal and trigeminal nerves. Following decompression of both nerves, the patient became asymptomatic. Case 3 underwent MVD only for GPN, which only partially alleviated the GPN, and he complained of a substantial degree of pain in the trigeminal territory. He ended up undergoing GKS on both the glossopharyngeal and trigeminal nerves. On retrospective consideration, although he developed both pain entities, the pain from TN might have been masked by more intense pain from the GPN. We believe that these three cases provide a valuable lesson: a diagnosis of GPN does not always exclude concomitant TN, and a thorough preoperative assessment of pain distribution cannot be overemphasized.
Apart from the three cases described above, another four patients had GPN that had been misdiagnosed as TN before they visited our institute. One of these patients had undergone MVD for TN, which, needless to say, did not help his GPN pain at all. Two patients allegedly received an alcohol injection at the trigeminal nerve ganglion which not only failed to alleviate GPN pain but also caused a permanent complication: anesthesia dolorosa on the trigeminal territory. MVD for GPN in these two patients was eventually successful, but their anesthesia dolorosa persisted, causing substantial facial pain. As mentioned, differentiation of GPN from TN is based on thorough and careful history taking. Some patients use rather vague terms when describing the location and factors that set off the pain. For example, for ‘pain in the face when eating’, it should be discerned whether ‘eating’ means chewing or swallowing. The former would infer TN but the latter GPN. The option of GPN must be considered before performing surgery for TN, especially when the surgery is a destructive one.
This study had some limitations, including its retrospective design and relatively small number of patients. Thus, further large-scale, prospective, comparative studies are required. The duration of follow-up was not long enough, even though the patient population was restricted to those followed-up for at least 2 years.
CONCLUSION
We suggest that MVD with no rhizotomy procedure is a safe and effective treatment modality with a low complication rate.
References
- 1.Baechli H, Gratzl O. Microvascular decompression in trigeminal neuralgia with no vascular compression. Eur Surg Res. 2007;39:51–57. doi: 10.1159/000098436. [DOI] [PubMed] [Google Scholar]
- 2.Barbash GI, Keren G, Korczyn AD, Sharpless NS, Chayen M, Copperman Y, et al. Mechanisms of syncope in glossopharyngeal neuralgia. Electroencephalogr Clin Neurophysiol. 1986;63:231–235. doi: 10.1016/0013-4694(86)90089-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Lee S, Lui T, Yeh Y, Chen T, Tzaan W. Teflon granuloma after microvascular decompression for trigeminal neuralgia. Surg Neurol. 2000;53:281–287. doi: 10.1016/s0090-3019(00)00169-5. [DOI] [PubMed] [Google Scholar]
- 4.Choi MS, Kim YI, Ahn YH. Lipoma causing glossopharyngeal neuralgia: a case report and review of literature. J Korean Neurosurg Soc. 2014;56:149–151. doi: 10.3340/jkns.2014.56.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandy WE. Glossopharyngeal neuralgia (tic douloureux): Its diagnosis and treatment. Arch Surg. 1927;15:198–214. [Google Scholar]
- 6.De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L. Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery. 2002;51:427–434. doi: 10.1097/00006123-200208000-00023. discussion 433–434. [DOI] [PubMed] [Google Scholar]
- 7.Ferroli P, Fioravanti A, Schiariti M, Tringali G, Franzini A, Calbucci F, et al. Microvascular decompression for glossopharyngeal neuralgia: a long-term retrospectic review of the Milan-Bologna experience in 31 consecutive cases. Acta Neurochir (Wien) 2009;151:1245–1250. doi: 10.1007/s00701-009-0330-5. [DOI] [PubMed] [Google Scholar]
- 8.Gadient PM, Smith JH. The neuralgias: diagnosis and management. Curr Neurol Neurosci Rep. 2014;14:459. doi: 10.1007/s11910-014-0459-3. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz M, Horowitz M, Ochs M, Carrau R, Kassam A. Trigeminal neuralgia and glossopharyngeal neuralgia: two orofacial pain syndromes encountered by dentists. J Am Dent Assoc. 2004;135:1427–1433. doi: 10.14219/jada.archive.2004.0052. quiz 1468. [DOI] [PubMed] [Google Scholar]
- 11.Jannetta PJ. Neurovascular compression in cranial nerve and systemic disease. Ann Surg. 1980;192:518–525. doi: 10.1097/00000658-198010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm: Operative techniques and results in 47 patients. J Neurosurg. 1977;47:321–328. doi: 10.3171/jns.1977.47.3.0321. [DOI] [PubMed] [Google Scholar]
- 13.Jannetta PJ, Segal R, Wolfson SK., Jr Neurogenic hypertension: etiology and surgical treatment. I Observations in 53 patients. Ann Surg. 1985;201:391–398. doi: 10.1097/00000658-198503000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabatas S, Karasu A, Civelek E, Sabanci AP, Hepgul KT, Teng YD. Microvascular decompression as a surgical management for trigeminal neuralgia: long-term follow-up and review of the literature. Neurosurg Rev. 2009;32:87–93. doi: 10.1007/s10143-008-0171-3. discussion 93–94. [DOI] [PubMed] [Google Scholar]
- 15.Kandan SR, Khan S, Jeyaretna DS, Lhatoo S, Patel NK, Coakham HB. Neuralgia of the glossopharyngeal and vagal nerves: long-term outcome following surgical treatment and literature review. Br J Neurosurg. 2010;24:441–446. doi: 10.3109/02688697.2010.487131. [DOI] [PubMed] [Google Scholar]
- 16.Kano H, Urgosik D, Liscak R, Pollock BE, Cohen-Inbar O, Sheehan JP, et al. Stereotactic radiosurgery for idiopathic glossopharyngeal neuralgia: an international multicenter study. J Neurosurg. 2016;125(Suppl 1):147–153. doi: 10.3171/2016.7.GKS161523. [DOI] [PubMed] [Google Scholar]
- 17.Katusic S, Williams DB, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of glossopharyngeal neuralgia, rochester, minnesota, 1945–1984. Neuroepidemiology. 1991;10:266–275. doi: 10.1159/000110283. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima M, Matsushima T, Inoue T, Mineta T, Masuoka J, Hirakawa N. Microvascular decompression for glossopharyngeal neuralgia through the transcondylar fossa (supracondylar transjugular tubercle) approach. Neurosurgery. 2010;66(6 Suppl Operative):275–280. doi: 10.1227/01.NEU.0000369662.36524.CF. discussion 280. [DOI] [PubMed] [Google Scholar]
- 19.Kondo A. Follow-up results of using microvascular decompression for treatment of glossopharyngeal neuralgia. J Neurosurg. 1998;88:221–225. doi: 10.3171/jns.1998.88.2.0221. [DOI] [PubMed] [Google Scholar]
- 20.Laha RK, Jannetta PJ. Glossopharyngeal neuralgia. J Neurosurg. 1977;47:316–320. doi: 10.3171/jns.1977.47.3.0316. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Park JS, Ahn YH. Bioglue-coated teflon sling technique in microvascular decompression for hemifacial spasm involving the vertebral artery. J Korean Neurosurg Soc. 2016;59:505–511. doi: 10.3340/jkns.2016.59.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Wang H, Fan Z, Fan Z. Complications in retrosigmoid cranial nerve surgery. Acta Otolaryngol. 2010;130:247–252. doi: 10.3109/00016480903092340. [DOI] [PubMed] [Google Scholar]
- 23.Matsushima T, Yamaguchi T, Inoue TK, Matsukado K, Fukui M. Recurrent trigeminal neuralgia after microvascular decompression using an interposing technique. Teflon felt adhesion and the sling retraction technique. Acta Neurochirur (Wien) 2000;142:557–561. doi: 10.1007/s007010050469. [DOI] [PubMed] [Google Scholar]
- 24.Meaney JF, Eldridge PR, Dunn LT, Nixon TE, Whitehouse GH, Miles JB. Demonstration of neurovascular compression in trigeminal neuralgia with magnetic resonance imaging. Comparison with surgical findings in 52 consecutive operative cases. J Neurosurg. 1995;83:799–805. doi: 10.3171/jns.1995.83.5.0799. [DOI] [PubMed] [Google Scholar]
- 25.Paleologos TS, Wadley JP, Kitchen ND, Thomas DG. Clinical utility and cost-effectiveness of interactive image-guided craniotomy: clinical comparison between conventional and image-guided meningioma surgery. Neurosurgery. 2000;47:40–47. doi: 10.1097/00006123-200007000-00010. discussion 47–48. [DOI] [PubMed] [Google Scholar]
- 26.Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery. 2002;50:705–710. doi: 10.1097/00006123-200204000-00004. discussion 710–711. [DOI] [PubMed] [Google Scholar]
- 27.Reddy K, Hobson D, Gomori A, Sutherland GR. Painless glossopharyngeal “neuralgia” with syncope: a case report and literature review. Neurosurgery. 1987;21:916–919. doi: 10.1227/00006123-198712000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64–68. doi: 10.1227/00006123-199501000-00008. discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 29.Riley HA, German WJ, Wortis H, Herbert C, Zahn D, Eichna L. Glossopharyngeal neuralgia initiating or associated with cardiac arrest. Trans Am Neurol Assoc. 1942;68:28–29. [Google Scholar]
- 30.Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47:1013–1019. doi: 10.1016/s0360-3016(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 31.Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia a study of 217 cases. Arch Neurol. 1981;38:201–205. doi: 10.1001/archneur.1981.00510040027002. [DOI] [PubMed] [Google Scholar]
- 32.Sampson JH, Grossi PM, Asaoka K, Fukushima T. Microvascular decompression for glossopharyngeal neuralgia: long-term effectiveness and complication avoidance. Neurosurgery. 2004;54:884–889. doi: 10.1227/01.neu.0000114142.98655.cc. discussion 889–890. [DOI] [PubMed] [Google Scholar]
- 33.St John JN. Glossopharyngeal neuralgia associated with syncope and seizures. Neurosurgery. 1982;10:380–383. doi: 10.1227/00006123-198203000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Taha JM, Tew JM., Jr Long-term results of surgical treatment of idiopathic neuralgias of the glossopharyngeal and vagal nerves. Neurosurgery. 1995;36:926–930. doi: 10.1227/00006123-199505000-00006. discussion 930–931. [DOI] [PubMed] [Google Scholar]
- 35.Taha JM, Tew JM, Jr, Keith RW, Payner TD. Intraoperative monitoring of the vagus nerve during intracranial glossopharyngeal and upper vagal rhizotomy: technical note. Neurosurgery. 1994;35:775–777. doi: 10.1227/00006123-199410000-00032. [DOI] [PubMed] [Google Scholar]
- 36.Tsuboi M, Suzuki K, Nagao S, Nishimoto A. Glossopharyngeal neuralgia with cardiac syncope. A case successfully treated by microvascular decompression. Surg Neurol. 1985;24:279–283. doi: 10.1016/0090-3019(85)90039-4. [DOI] [PubMed] [Google Scholar]
- 37.Varrasi C, Strigaro G, Prandi P, Comi C, Mula M, Monaco F, et al. Complex pattern of convulsive syncope in glossopharyngeal neuralgia: video/EEG report and short review. Epilepsy Behav. 2011;20:407–409. doi: 10.1016/j.yebeh.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Xiong NX, Zhao HY, Zhang FC, Liu RE. Vagoglossopharyngeal neuralgia treated by microvascular decompression and glossopharyngeal rhizotomy: clinical results of 21 cases. Stereotact Funct Neurosurg. 2012;90:45–50. doi: 10.1159/000333828. [DOI] [PubMed] [Google Scholar]
- 39.Yomo S, Arkha Y, Donnet A, Régis J. Gamma knife surgery for glossopharyngeal neuralgia: report of 2 cases. J Neurosurg. 2009;110:559–563. doi: 10.3171/2008.8.17641. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H, Zhang X, Zhu J, Tang YD, Li ST. Microvascular decompression for glossopharyngeal neuralgia: long-term follow up. World Neurosurg. 2017;102:151–156. doi: 10.1016/j.wneu.2017.02.106. [DOI] [PubMed] [Google Scholar]