Abstract
The aim of this study was to investigate the incidence, predictors and outcome of prosthesis-patient mismatch (PPM) following transcatheter aortic valve replacement (TAVR). A total of 30 articles incorporating 4,691 patients were identified. The pooled incidences of overall, moderate and severe PPM following TAVR were 33.0%, 25.0% and 11.0% respectively. Medtronic CoreValve (MCV) had lower incidence of overall (32% vs: 40%, P < 0.0001) and moderate (23% vs 32%, P < 0.0001) than Edwards Sapien (ESV). PPM was associated with a younger age, smaller annulus diameter and lower left ventricular ejection fraction in comparison with those patients without PPM. Post-dilation (OR, 0.51, 95% CI, 0.38 to 0.68, p < 0.001) during TAVR would decrease the incidence of PPM. Although PPM was common after TAVR, no significant differences were observed both in short- and mid-term all-cause mortality (30 day: OR: 1.1, 95% CI, 0.70 to 1.73 and 2 year: OR: 1.01, 95% CI, 0.74 to 1.38) between patients with PPM and those without PPM. In conclusion, despite being common after TAVR, the incidence of PPM was lower than that of surgical aortic valve replacement (SAVR) and decreased with the experience accumulating, and PPM was not seen to impact on short- and mid-term survival, regardless of its magnitude.
Introduction
Prosthesis-patient mismatch (PPM) is present when the prosthetic valve is too small in relation to body size and was first described by Rahimtoola1. The severity of PPM was evaluated by the indexed effective orifice area, PPM was defined if indexed EOA was less than 0.85 cm2/m2, moderate PPM defined as ≥0.65 cm2/m2 and ≤0.85 cm2/m2, and severe PPM defined as <0.65 cm2/m2 1. Several studies have reported that the prevalence of PPM after surgical aortic valve replacement (SAVR) ranged from 20% to 70%2, and the higher pressure gradients observed in PPM3 results in reduced reverse remodeling4. Besides, two recent meta-analysis reported that moderate and severe PPM after SAVR was associated with higher overall mortality5,6.
Transcatheter aortic valve replacement have developed rapidly as an alternative technique to treating patients with severe aortic stenosis. And Increasing evidence demonstrated TAVR have comparable results in patients with intermediate surgical risk, compared to SAVR7,8. Thus, the incidence and outcome of PPM after TAVR is concerned when TAVR extended to patients with intermediate surgical risk. However, controversial has intensified on the incidence, predictors and outcome of PPM after TAVR9–11. Therefore, we performed a systematic review and meta-analysis to comprehensively and quantitatively investigate the incidence, predictors, preventive approaches and outcome of PPM.
Results
Literature search and study selection
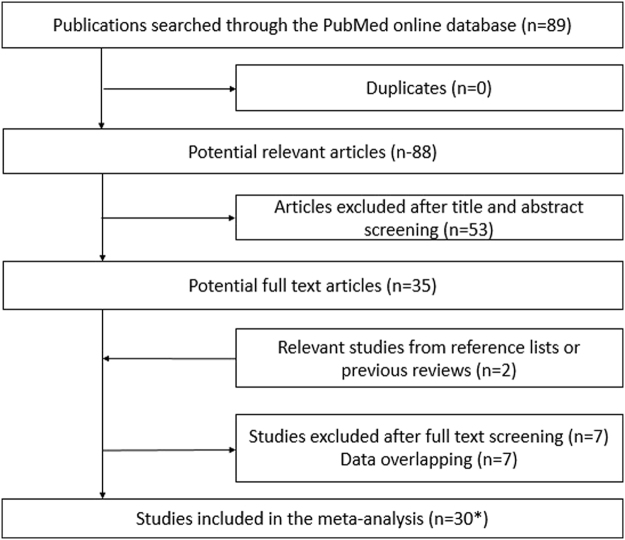
The process of study selection was illustrated in Fig. 1. There were 35 studies left after screening the titles and abstracts. After removing overlapping data, a total of 30 studies9–37 incorporating 4,691 patients were eligible. There was 1 study11 reporting two clusters of patients including randomized and non-randomized clinical trial which was regarded as two independent studies. The characteristics of the included overall studies were shown in Supplemental Table 1.
Figure 1.
Flow diagram of citation research and selection. *Indicate that one study reporting two clusters of patients including randomized and non-randomized clinical trial which was regarded as two independent studies.
Quality assessment
The quality of eligible cohort studies were assessed using the NOS scale, while quality of single-arm studies were evaluated by using Cross-Sectional/Prevalence Study Quality. Overall quality of these eligible studies was good.
Incidence of PPM and subgroup analysis
The pooled incidence of overall, moderate and severe PPM after TAVR was 33.0%, 25.0% and 11.0% separately (Supplemental Table 2). Compared with Edwards Sapien valve (ESV), Medtronic CoreValve (MCV) was associated with lower occurrence rate of overall (MCV: 32% vs ESV: 40%, P < 0.0001), moderate (23% vs 32%, P < 0.0001) but not severe PPM (10% vs 12%, P = 0.15) (Supplemental Table 2).
Other subgroup analysis were presented in Supplemental Table 2. Generally, a reduced trend in the occurrence rate of overall, moderate and severe PPM was seen in patients who were referred to TAVR later (later vs early, overall: 31% vs 38%, P = 0.001; moderate: 23% vs 26%, P = 0.12; severe: 10% vs 15%, P = 0.0005). Patients with higher risk (Logistic EuroSCORE > 20%) had similar incidence of overall, moderate and severe PPM with patients with lower risk (higher vs lower, overall: 30% vs 35%, P = 0.08; moderate: 24% vs 23%, P = 0.54; severe: 12% vs 10%, P = 0.11). The results of pooled estimate above were stable, albeit significant heterogeneity available in some of them.
TAVR vs SAVR
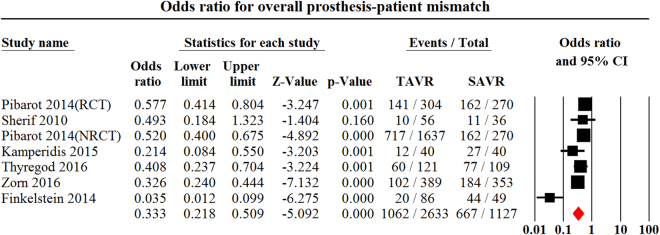
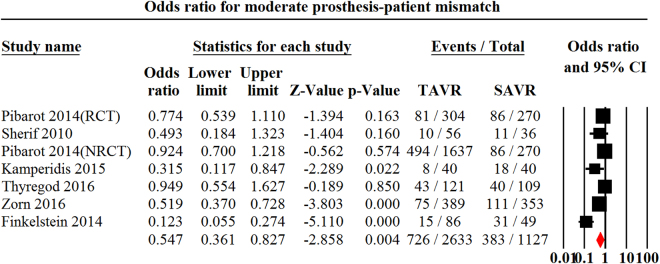
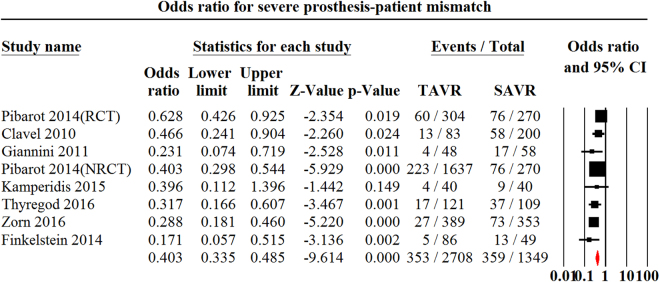
There were 711,13,26,31,33,34, 711,13,26,31,33,34 and 811,21,22,26,31,33,34 studies recruiting 3,760, 3,760 and 4,057 patients that reported the incidences of overall, moderate and severe PPM after TAVR compared to SAVR respectively. TAVR had lower incidence of overall (OR, 0.33, 95% CI, 0.22 to 0.51, P < 0.001, I2 = 81.4, egger’s = 0.09, Fig. 2), moderate (OR, 0.55, 95% CI, 0.36 to 0.83, P = 0.003, I2 = 80.0, egger’s = 0.13, Fig. 3) and severe PPM (OR, 0.39, 95% CI, 0.33 to 0.48, P < 0.001, I2 = 36.0, egger’s = 0.17, Fig. 4) than SAVR.
Figure 2.
Odds ratio for overall Prosthesis-Patient Mismatch Comparing Transcatheter Aortic Valve replacement with Surgical Aortic Valve Replacement. PPM indicates Prosthesis-Patient Mismatch; TAVR indicates Transcatheter Aortic Valve Replacement.
Figure 3.
Odds ratio for moderate Prosthesis-Patient Mismatch Comparing Transcatheter Aortic Valve replacement with Surgical Aortic Valve Replacement. PPM indicates Prosthesis-Patient Mismatch; TAVR indicates Transcatheter Aortic Valve Replacement.
Figure 4.
Odds ratio for severe Prosthesis-Patient Mismatch Comparing Transcatheter Aortic Valve replacement with Surgical Aortic Valve Replacement. PPM indicates Prosthesis-Patient Mismatch; TAVR indicates Transcatheter Aortic Valve Replacement.
Predictive and preventive factors
In order to investigate the predictors of PPM, we pooled 149–12,15,17,19,20,23,28,31–33 cohort studies incorporating 1,454 patients suffering from PPM and 2,324 patients free from PPM using the univariate analysis method (Table 1). Patients with PPM were younger than those patients without PPM (PPM: 80.8 yrs vs No-PPM: 82.5 yrs, p < 0.001). The PPM group was associated with larger body surface area (BSA) (PPM: 1.85 m2 vs No-PPM: 1.74 m2, p < 0.001), larger body mass index (BMI) (PPM: 28.1 kg/m2 vs No-PPM: 25.9 kg/m2, p < 0.001), smaller aortic annulus diameters (PPM: 21.5 mm vs No-PPM: 21.8 mm, p = 0.03), lower baseline left ventricular ejection fraction (LVEF) (52.7 vs 55.7, p < 0.001), smaller baseline EOA (PPM: 0.63 cm2 vs No-PPM: 0.68 cm2, p = 0.005) and smaller baseline indexed EOA (0.32 cm2/m2 vs 0.38 cm2/m2, p < 0.001) in comparison with that of No-PPM group. Other variables were insignificant and presented in Table 1.
Table 1.
Pooled characteristics of patients with and without Prosthesis-Patient Mismatch.
| Characteristics | PPM | No-PPM | Number of studies | Number of patients | P value |
|---|---|---|---|---|---|
| Age | 80.8 ± 0.8 | 82.5 ± 0.7 | 149–12,15,17,19,20,23,28,31–33 | 3,778 | <0.001 |
| Male, n/total (%) | 591/1454 (40.6%) | 1151/2324 (49.5%) | 109–12,15,17,19,20,23 | 3,778 | <0.001 |
| BSA (m2) | 1.85 ± 0.02 | 1.74 ± 0.01 | 109–12,15,17,19,20,23 | 3,290 | <0.001 |
| BMI (Kg/m2) | 28.1 ± 0.5 | 25.9 ± 0.4 | 710–12,15,17,20 | 3,389 | <0.001 |
| Logistic EuroSCORE (%) | 21.6 ± 2.2 | 21.8 ± 2.1 | 89–12,15,20,23 | 3,469 | 0.634 |
| Previous MI, n/total (%) | 267/973 (27.4%) | 320/1373 (23.3%) | 511,12,15,20 | 2,346 | 0.29 |
| Aortic annulus diameters(mm) | 21.5 ± 0.5 | 21.8 ± 0.5 | 910–12,15,17,19,20,23 | 3,498 | 0.03 |
| LVEF (%) | 52.7 ± 0.8 | 55.7 ± 0.9 | 711,12,15,19,20,23 | 2,586 | <0.001 |
| Mean gradient (mmHg) | 46.1 ± 1.0 | 46.3 ± 0.7 | 89,11,12,15,19,20,23 | 3,325 | 0.23 |
| EOA (cm2) | 0.63 ± 0.02 | 0.68 ± 0.02 | 59,12,15,19,20 | 1,151 | 0.005 |
| Indexed EOA (cm2/m2) | 0.32 ± 0.01 | 0.38 ± 0.01 | 412,15,20,23 | 1,107 | <0.001 |
| Post-balloon dilation | 119/345 (34.5%) | 724/1553 (46.6%) | 311,19,32 | 1,898 | <0.001 |
| Discharge | |||||
| LVEF (%) | 56.2 ± 0.9 | 56.8 ± 0.7 | 310,20,23 | 772 | 0.36 |
| Mean gradient (mmHg) | 11.3 ± 1.0 | 8.6 ± 1.0 | 69,10,12,19,20,23 | 1,023 | <0.001 |
| EOA (cm2) | 1.25 ± 0.06 | 1.86 ± 0.06 | 310,12,19 | 698 | <0.001 |
| Indexed EOA (cm2/m2) | 0.69 ± 0.03 | 1.09 ± 0.04 | 410,12,19,20 | 862 | <0.001 |
Abbreviations: BSA body surface area; MI myocardial infarction; EOA effective orifice area; LVEF left ventricular ejection fraction.
Only optimal implant position17 (univariate, p = 0.015) and post-dilation11,19,32 (univariate, OR, 0.51, 95% CI, 0.38 to 0.68, I2 = 0, p < 0.001) were reported to be associated with reduced incidence of PPM.
Outcome of PPM
Pooled studies revealed that patients with PPM were associated with higher mean transvalvular gradient, EOA and indexed EOA after TAVR, compared to those patients without PPM (Table 1). Additionally, no significant difference was noted between patients with PPM and those without PPM in both short-term and mid-term all-cause mortality (PPM vs No-PPM: 30 day: OR: 1.17, 95% CI, 0.75 to 1.84, I2 = 0; 1 year: OR: 1.14, 95% CI, 0.92 to 1.42, I2 = 12.5 and 2 years: OR: 0.98, 95% CI, 0.72 to 1.32, I2 = 4.1) (Table 2). The direction of the results above kept consistent when omitting individual studies from the analysis. Furthermore, we found that patients with moderate and severe PPM were not associated with higher risk of 1-year (moderate, OR: 1.0, 95% CI, 0.77 to 1.29, I2 = 0; severe, OR: 1.93, 95% CI, 0.76 to 4.91, I2 = 81.9) and 2-year all-cause mortality (moderate, OR: 1.05, 95% CI, 0.72 to 1.54, I2 = 5; severe, OR: 1.95, 95% CI, 0.38 to 9.91) respectively in comparison with that of PPM (Table 2).
Table 2.
Pooled impact of Prosthesis-Patient Mismatch on all-cause mortality after transcatheter aortic valve replacement.
| Subgroup | No. of studies | No. of patients | ORs | I2 for heterogeneity | Model | P for Egger’s |
|---|---|---|---|---|---|---|
| Outcome of overall PPM on al-cause mortality | ||||||
| 30 days | 109,10,12,15,19,23,28,33 | 3,209 | 1.17 (0.75–1.84) | 0 | Fixed | 0.36 |
| 1 year | 810,11,15,20,23,28,33 | 3,125 | 1.14 (0.92–1.42) | 12.5 | Fixed | 0.37 |
| 2 years | 610,11,15,23,28,31 | 1,077 | 0.98 (0.72–1.32) | 4.1 | Fixed | 0.94 |
| Outcome of moderate PPM on al-cause mortality | ||||||
| 1 year | 410,11,15 | 2,061 | 1.00 (0.77–1.29) | 0 | Fixed | 0.32 |
| 2 years | 310,11,15 | 646 | 1.05 (0.72–1.54) | 5 | Fixed | 0.43 |
| Outcome of severe PPM on all-cause mortality | ||||||
| 1 year | 510,11,15,33 | 2,041 | 1.93 (0.76–4.91) | 81.9 | Random | 0.46 |
| 2 years | 410,11,15,31 | 653 | 1.95 (0.38–9.91) | 86.6 | Random | 0.60 |
Abbreviations: PPM Prosthesis-Patient Mismatch; OR Odds Ratio.
Discussion
The main results of the present meta-analysis and systematic review were: 1) more than thirty percent of patients underwent TAVR may encounter PPM; 2) MCV had lower prevalence of overall and moderate PPM than ESV; 3) TAVR was associated with reduced risk for the incidence of overall, moderate and severe PPM in comparison with SAVR; 4) baseline larger BMI, smaller aortic annulus diameters, lower LVEF and smaller baseline EOA were associated higher risk for PPM; 5) post-dilation during TAVR was associated with reduced risk for PPM; 6) compared with No-PPM, PPM was not associated with higher short- and mid-term all-cause mortality, regardless of its magnitude.
PPM is a frequent phenomenon after SAVR with the reported incidence ranging from 20% to 70%2,11, while, the impact of PPM on patients’ prognosis is still controversial5,6,38. Promisingly, TAVR was associated with lower risk in the prevalence of overall, moderate and severe PPM referring to that of SAVR in the present meta-analysis. Additionally, the incidence of PPM was significant lower after TAVR in patients with smaller native aortic annulus (<20 mm)11,16,39 but not in patients with larger aortic annulus (≥20 mm)39. This difference may be explained by a less frequent occupation of native aortic annulus due to absence of sewing ring11,40 which resulted in better hemodynamic results21,41. Furthermore, patients underwent SAVR always have more selected size of valve, while patients underwent TAVR have limited size of valve. Besides, oversizing in aortic annulus diameter is commonly recommend in TAVR. These may jointly result in a larger transcatheter valve being inserted in smaller individuals with a small annulus thus may reducing the occurrence of severe PPM13,21,22.
However, limited definitive data exists for PPM in the setting of TAVR, and interest in this area is intensifying. The pooled incidence of PPM following TAVR was 33%, while the combined prevalence of moderate and severe PPM was 25% and 11% respectively in our meta-analysis. The definition of PPM in our eligible studies was based on measured EOA indexed to BSA. However, regarding the relationship between left ventricular output tract diameter (LVOTd) and EOA, the accuracy measurements of LVOTd is vital for the reporting prevalence of PPM. In our study, the incidence of PPM was higher in the method using proximal to leaflet, in comparison with that using underneath stent (proximal to leaflet 46.0% vs underneath stent 36.0%, P = 0.002).While, Clavel et al.42 revealed that LVOTd being measured underneath the stent was more stable and reduplicated than that proximal to prosthesis cusps. And it was the way underneath the stent that correlated better with transthoracic gradient42. Therefore, the definition of PPM according to LVOTd being measured underneath the stent may be more accurate than that being measured proximal to prosthesis cusps.
In our study, we found that MCV had lower incidence of overall and moderate PPM than ESV. Consistently, the pooled results showed that MCV9,12–14,17,18,22,31,33,37 had lower mean trans-vavular mean gradient than ESV9–11,14,16,19–21,23,25,32 (MCV, 9.3 mmHg vs ESV, 10.2 mmHg, P < 0.001). Additionally, the randomized CHOICE trial43 also revealed that MCV was associated with lower trans-valvular mean gradient than ESV (MCV vs ESV, 8 mmHg vs 9 mmHg, p = 0.004), albeit no reported data on the comparison of PPM. This difference may be attributed to the different design. As we all known, MCV is a supra-annular design with an hour-glass shape stent and a flared inflow. A self-expanding design may confer greater conformity to the native valve and left ventricular outflow tract morphology than the intra-annular design of ESV14. Therefore, when patients with high risk for PPM, a self-expandable MCV may be a preferential choice.
Interestingly, PPM was not associated with increased short-and mid-term all-cause mortality following TAVR in the present meta-analysis, which was in line with that from the Placement of AoRTic TraNscathetER Valves (PARTNER) trial11. Overall, moderate and severe PPM did not show a detrimental effect on short- and mid-term survival in our meta-analysis; a relationship was shown, however, in some studies, with severe PPM predicting mid-term mortality in a multivariable analysis15,32 and in a subgroup analysis of the PARTNER trial that excluded patients with aortic regurgitation11. This is in contrast to established data from the surgical literature, with moderate and severe PPM following SAVR shown to have a higher mortality in pooled data from 34 and 58 studies recruiting 27,186 and 40,381 patients respectively5,6. This paradox may be related to the influence of individual preoperative characteristics and baseline comorbidities38,40. Patients undergoing TAVR thus far have been older than that of SAVR, potentially with lower basic activity requirements, and multiple comorbidities that compete with the influence of PPM. Furthermore, Price et al.38 and Dayan et al.5 reported that patients older than 70 years with PPM following SAVR were not susceptible to impaired survival and congestive heart failure regardless of LV function and LV mass regression. Additionally, compared with SAVR, TAVR was demonstrated to have better hemodynamic characteristics resulting in less impairment of coronary flow reserve which may be another key factor to explain the difference11,21,22. Previous studies also reported that extracorporeal circulation during SAVR was associated with increased risk for systemic inflammatory response syndrome and myocardial infarction, which would impair perioperative survival and impede LV function recovery44,45. This would also explain the different prognosis effect of PPM between SAVR and TAVR. Nonetheless, patients with PPM has been shown to impede the improvement of LV mass regression and NYHA class after TAVR, but that the extent of such an improvement varies widely between individuals post procedure11,12. Therefore, with the extension of TAVR to younger patients, the detrimental effect of PPM following TAVR could conceivably become apparent.
In our study, we found that patients with more severely stenotic aortic valves were more likely to encounter PPM. When the transcatheter valve was implanted in a severely stenotic native aortic valve especially that with heavy leaflet calcification, incomplete expansion of the stent frame may occur and result in PPM46. Under such circumstances, post-dilation, an additional procedure could be considered to reduce the risk of PPM, especially in the presence of substantial paravalvular leak46. Previous study reported there was no significant difference on the incidence of post-dilation between patients with prior balloon valvulopasty and without prior balloon valvuloplasty, thus whether prior balloon valvuloplasty before TAVR would influence the occurrence rate of PPM should be evaluated in further studies47. Moreover, because there are limited sizes of prostheses available for a wide range of annular sizes, patients with larger BSA were susceptible to a higher occurrence rate of PPM which is in lines with that in SAVR48. The present meta-analysis found there was significant correlation between annulus diameter and the prevalence of PPM, which was consistent with PARTNER trail39. Therefore, an optimized method for measuring annular size was a crucial approach to reducing the occurrence of PPM39. Regarding the method of sizing the annulus diameters before TAVR, 3D echocardiography and 3D multidetector CT were superior to 2D echocardiography that could decrease the incidence of PPM19,37. In addition, Jilaihawi et al.17 demonstrated that optimal position of the implanted valve (defined in this early study as 5–10 mm below the native non-coronary cusp) was associated with a lower incidence of PPM with the MCV device. Based on this potential relationship, we should strive to obtain an optimal position by selecting suitable projection direction and careful deployment.
Limitation
There were several limitations in the present systematic review and meta-analysis. Firstly, although significant heterogeneity was noted, subgroup analysis including study design, patients’ recruitment time and time to define PPM was conducted to reveal the source of heterogeneity. While, the result of sensitivity analysis confirmed the robustness of our pooled estimate. Secondly, cumulative survival data was sparse and we relied on binary survival data to generate ORs rather than Hazard Ratios (HR). Although the combined ORs referring to mortality did not exclude the influence of potential mediators, the unadjusted pooled ORs can be elucidated as the entire causal effects of PPM, which might be more clinically relevant. Thirdly, the TAVR series had relatively short follow-up compared to SAVR series, so the results comparing the incidence of PPM between TAVR and SAVR should be validated by further studies. Finally, the preventive measures of PPM presented here were based on isolated studies and would therefore require further validation in future large-scale prospective studies.
Conclusion
In the presented TAVR was associated with a significantly lower risk of overall, moderate and severe PPM compared with SAVR. Reported frequencies of overall and moderate PPM following TAVR-MCV was lower than that of TAVR-ESV. Although PPM after TAVR did not display a detrimental effect on short- and mid-term outcomes, regardless of the severity of PPM, the impact on long-term outcomes, particularly relevant to the increasingly younger TAVR population, remains to be elucidated. Until such data is available, it seems reasonable to strive to optimize TAVR hemodynamic performance and reduce the occurrence of PPM.
Methods
Registration
Our systematic review was registered online in PROSPERO (registration number: CRD42014015518).
Data sources and study selection
The PubMed online database (between January 1, 2002 and Oct 19, 2016) was searched by using the following search strategy: (prosthesis patient mismatch OR patient prosthesis mismatch) AND (percutaneous OR transcatheter OR transfemoral OR transapical OR transartery) AND (aortic valve) AND (replacement OR implantation). Besides, reference lists of pertinent articles were also screened manually for potentially relevant citations. All citations were initially screened at the level of title and abstract. And then full-length articles were retrieved for further evaluation. Two authors screened citations separately. If controversies existed, a discussion was made to achieve consensus.
Study inclusion criteria
Articles were included if they 1) reported the specific number or incidence of PPM; 2) presented the predictive factors or preventive measures of PPM; 3) displayed the particular number of death or survival curve of PPM; 4) were human studies and published in English. The exclusion criteria were abstract, editorials, reviews and case reports. The process of study selection was illustrated in Fig. 1.
Data extraction and quality assessment
Two authors (YBL and YJL) independently extracted the specific characteristics from eligible studies including author, PPM definition, baseline profiles and mortality. If mortality or number of death was not presented directly, we used digitizing software (Engauge Digtizer 4.1) to gain data from survival cure49. PPM in the present meta-analysis was defined if indexed EOA was less than 0.85 cm2/m2, moderate PPM defined as ≥0.65 cm2/m2 and ≤0.85 cm2/m2, and severe PPM defined as <0.65 cm2/m2. A table was designed to record the data. Study quality was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS)50.
Data synthesis and analysis
Pooled incidences and odds ratios (OR) were acquired using the Comprehensive Meta-Analysis Software Version 2. Heterogeneity was assessed by calculating the I2 statistic and its P value. If the I2 statistic was more than 50% and its P value was less than 0.05, a random-effects model was used to obtain the combined effect estimates. Two-sided P values of 0.05 were considered statistically significant.
Publications bias analysis
Publication bias was evaluated by visual inspection of the symmetry of the funnel plot and by Egger’s test. Two-sided P values of 0.05 in the Egger’s test were not associated with significant publication bias. Besides, if the number of pooled studies was small, publication bias was not performed.
Sensitivity and subgroup analysis
We performed sensitivity analysis by discarding one study at a time and repeating the meta-analysis to examine the robustness of the pooled results. Sensitivity analysis was not conducted unless there were sufficient studies to gain the pooled estimate. Additionally, subgroup analysis was carried out according to the valve type, study design, mean Logistic EuroSCORE, patients’ recruitment time and the time point where PPM was analyzed to explore the source of heterogeneity.
Present systematic review and meta-analysis was conducted and reported according to the recommendations of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group51.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The work was supported by the Science and Technology Support Plan (grant numbers: 2016FZ0078, Sichuan, China), the Youth Science and Technology Innovation Team (grant numbers: 2017TD0004, Sichuan, China), the Health and Family Planning Commission Popularization Application Project (grant numbers: 2017PJ004, Sichuan, China) and the National Key Research and Development Plan (grant numbers: 2016YFC1102200, China).
Author Contributions
M.C. and Y.F. designed the study. Y.B.L., Y.J.L., J.L.L., X.W. and J.Y.T. extracted, analyzed and interpreted the data. Y.B.L., T.Y.X. and Y.N.X. drafted the manuscript. M.C., Y.F. and Z.G.Z. revised the manuscript. All authors approve the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yan-biao Liao and Yi-jian Li contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15396-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuan Feng, Email: fynotebook@hotmail.com.
Mao Chen, Email: hmaochen@foxmail.com.
References
- 1.Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–24. doi: 10.1161/01.CIR.58.1.20. [DOI] [PubMed] [Google Scholar]
- 2.Dumesnil JG, Pibarot P. Prosthesis-patient mismatch: an update. Curr cardiol rep. 2011;13:250–257. doi: 10.1007/s11886-011-0172-7. [DOI] [PubMed] [Google Scholar]
- 3.Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36:1131–1141. doi: 10.1016/S0735-1097(00)00859-7. [DOI] [PubMed] [Google Scholar]
- 4.Tasca G, et al. Impact of valve prosthesis-patient mismatch on left ventricular mass regression following aortic valve replacement. Ann thorac surg. 2005;79:505–510. doi: 10.1016/j.athoracsur.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Dayan V, et al. Predictors and Outcomes of Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc Imaging. 2016;9:924–33. doi: 10.1016/j.jcmg.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Head SJ, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33:1518–29. doi: 10.1093/eurheartj/ehs003. [DOI] [PubMed] [Google Scholar]
- 7.Sondergaard, L. et al. Two-Year Outcomes in Patients With Severe Aortic Valve Stenosis Randomized to Transcatheter Versus Surgical Aortic Valve Replacement: The All-Comers Nordic Aortic Valve Intervention Randomized Clinical Trial. Circulation Cardiovasc Interv. 9, 10.1161/CIRCINTERVENTIONS.115.003665 (2016). [DOI] [PubMed]
- 8.Leon MB, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 9.Bleiziffer S, et al. Incidence and impact of prosthesis-patient mismatch after transcatheter aortic valve implantation. J Heart Valve Dis. 2013;22:309–316. [PubMed] [Google Scholar]
- 10.Kukucka M, et al. Patient-prosthesis mismatch after transapical aortic valve implantation: incidence and impact on survival. J Thorac Cardiovasc Surg. 2013;145:391–397. doi: 10.1016/j.jtcvs.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Pibarot P, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort–a analysis. J Am Coll Cardiol. 2014;64:1323–1334. doi: 10.1016/j.jacc.2014.06.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzikas A, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the medtronic CoreValve system in patients with aortic stenosis. Am J Cardiol. 2010;106:255–260. doi: 10.1016/j.amjcard.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Sherif MA, et al. Early hemodynamic and neurohormonal response after transcatheter aortic valve implantation. Am Heart J. 2010;160:862–869. doi: 10.1016/j.ahj.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Nombela-Franco L, et al. Comparison of hemodynamic performance of self-expandable CoreValve versus balloon-expandable Edwards SAPIEN aortic valves inserted by catheter for aortic stenosis. Am J Cardiol. 2013;111:1026–1033. doi: 10.1016/j.amjcard.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Garcia AJ, et al. Incidence and clinical outcome of prosthesis-patient mismatch after transcatheter aortic valve implantation with the CoreValve prosthesis. Int J Cardiol. 2013;167:1074–1076. doi: 10.1016/j.ijcard.2012.10.062. [DOI] [PubMed] [Google Scholar]
- 16.Kalavrouziotis D, et al. Transcatheter aortic valve implantation in patients with severe aortic stenosis and small aortic annulus. J Am Coll Cardiol. 2011;58:1016–1024. doi: 10.1016/j.jacc.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Jilaihawi H, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the Medtronic-Corevalve bioprosthesis. Eur Heart J. 2010;31:857–864. doi: 10.1093/eurheartj/ehp537. [DOI] [PubMed] [Google Scholar]
- 18.Gotzmann M, Lindstaedt M, Bojara W, Mugge A, Germing A. Hemodynamic results and changes in myocardial function after transcatheter aortic valve implantation. Am Heart J. 2010;159:926–932. doi: 10.1016/j.ahj.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Freeman M, et al. Multidetector CT predictors of prosthesis-patient mismatch in transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2013;7:248–255. doi: 10.1016/j.jcct.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Ewe SH, et al. Hemodynamic and clinical impact of prosthesis-patient mismatch after transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;58:1910–1918. doi: 10.1016/j.jacc.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Clavel MA, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 22.Giannini C, et al. Left ventricular reverse remodeling in percutaneous and surgical aortic bioprostheses: an echocardiographic study. J Am Soc Echocardiogr. 2011;24:28–36. doi: 10.1016/j.echo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Van Linden A, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation using the Edwards SAPIEN prosthesis. Thorac Cardiovasc Surg. 2013;61:414–420. doi: 10.1055/s-0032-1311534. [DOI] [PubMed] [Google Scholar]
- 24.Seiffert M, et al. Impact of patient-prosthesis mismatch after transcatheter aortic valve-in-valve implantation in degenerated bioprostheses. J Thorac Cardiovasc Surg. 2012;143:617–624. doi: 10.1016/j.jtcvs.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Del Trigo M, et al. Self-expanding Portico Valve Versus Balloon-expandable SAPIEN XT Valve in Patients With Small Aortic Annuli: Comparison of Hemodynamic Performance. Rev Esp Cardiol (Engl Ed). 2016;69:501–508. doi: 10.1016/j.recesp.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Kamperidis V, et al. Surgical sutureless and transcatheter aortic valves: hemodynamic performance and clinical outcomes in propensity score-matched high-risk populations with severe aortic stenosis. JACC Cardiovasc Interv. 2015;8:670–677. doi: 10.1016/j.jcin.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Laflamme J, et al. Incidence and risk factors of hemolysis after transcatheter aortic valve implantation with a balloon-expandable valve. Am J Cardiol. 2015;115:1574–1579. doi: 10.1016/j.amjcard.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 28.Poulin, F. et al. Impact of Prosthesis-Patient Mismatch on Left Ventricular Myocardial Mechanics After Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 5, 10.1161/JAHA.115.002866 (2016). [DOI] [PMC free article] [PubMed]
- 29.Spangenberg T, et al. Treatment of acquired von Willebrand syndrome in aortic stenosis with transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:692–700. doi: 10.1016/j.jcin.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Subban V, et al. Transcatheter valve-in-valve replacement of degenerated bioprosthetic aortic valves: a single Australian Centre experience. Cardiovasc Revasc Med. 2014;15:388–392. doi: 10.1016/j.carrev.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Thyregod HG, et al. No clinical effect of prosthesis-patient mismatch after transcatheter versus surgical aortic valve replacement in intermediate- and low-risk patients with severe aortic valve stenosis at mid-term follow-up: an analysis from the NOTION trial. Eur J Cardiothorac Surg. 2016;50:721–728. doi: 10.1093/ejcts/ezw095. [DOI] [PubMed] [Google Scholar]
- 32.Utsunomiya H, et al. Geometric changes in ventriculoaortic complex after transcatheter aortic valve replacement and its association with post-procedural prosthesis-patient mismatch: an intraprocedural 3D-TEE study. Eur Heart J Cardiovasc Imaging. 2017;18:1–10. doi: 10.1093/ehjci/jew039. [DOI] [PubMed] [Google Scholar]
- 33.Zorn GL, 3rd, et al. Prosthesis-patient mismatch in high-risk patients with severe aortic stenosis: A randomized trial of a self-expanding prosthesis. J Thorac Cardiovasc Surg. 2016;151:1014–1023.e3. doi: 10.1016/j.jtcvs.2015.10.070. [DOI] [PubMed] [Google Scholar]
- 34.Finkelstein A, et al. Hemodynamic performance and outcome of percutaneous versus surgical stentless bioprostheses for aortic stenosis with anticipated patient-prosthesis mismatch. J Thorac Cardiovasc Surg. 2014;147:1892–1899. doi: 10.1016/j.jtcvs.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Unbehaun A, et al. New 29-mm balloon-expandable prosthesis for transcatheter aortic valve implantation in large annuli. Ann Thorac Surg. 2013;95:1982–1990. doi: 10.1016/j.athoracsur.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt T, et al. Redo TAVI: initial experience at two German centres. EuroIntervention. 2016;12:875–82. doi: 10.4244/EIJV12I7A144. [DOI] [PubMed] [Google Scholar]
- 37.da Silva C, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation: impact of 2D-transthoracic echocardiography versus 3D-transesophageal echocardiography. Int J Cardiovasc Imaging. 2014;30:1549–57. doi: 10.1007/s10554-014-0510-0. [DOI] [PubMed] [Google Scholar]
- 38.Price JTH, Lam BK, Lapierre H, Mesana TG, Ruel M. The impact of prosthesis-patient mismatch after aortic valve replacement varies according to age at operation. Heart. 2014;100:1099–1106. doi: 10.1136/heartjnl-2013-305118. [DOI] [PubMed] [Google Scholar]
- 39.Rodes-Cabau J, et al. Impact of aortic annulus size on valve hemodynamics and clinical outcomes after transcatheter and surgical aortic valve replacement: insights from the PARTNER Trial. Circulation Cardiovasc Interv. 2014;7:701–711. doi: 10.1161/CIRCINTERVENTIONS.114.001681. [DOI] [PubMed] [Google Scholar]
- 40.Popma JJ, Khabbaz K. Prosthesis-patient mismatch after “high-risk” aortic valve replacement. J Am Coll Cardiol. 2014;64:1335–1338. doi: 10.1016/j.jacc.2014.07.952. [DOI] [PubMed] [Google Scholar]
- 41.Clavel MA, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883–1891. doi: 10.1016/j.jacc.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 42.Clavel MA, et al. Validation and characterization of transcatheter aortic valve effective orifice area measured by Doppler echocardiography. JACC Cardiovasc imaging. 2011;4:1053–1062. doi: 10.1016/j.jcmg.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Wahab M, et al. 1-Year Outcomes After Transcatheter Aortic Valve Replacement With Balloon-Expandable Versus Self-Expandable Valves: Results From the CHOICE Randomized Clinical Trial. J Am Coll Cardiol. 2015;66:791–800. doi: 10.1016/j.jacc.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Donndorf P, et al. Impact of closed minimal extracorporeal circulation on microvascular tissue perfusion during surgical aortic valve replacement: intravital imaging in a prospective randomized study. Interact Cardiov TH. 2014;19:211–217. doi: 10.1093/icvts/ivu131. [DOI] [PubMed] [Google Scholar]
- 45.Ozeren M, et al. Usefulness of elevated red cell distribution width for predicting systemic inflammatory response syndrome after extracorporeal circulation. Perfusion. 2015;30:580–586. doi: 10.1177/0267659114567138. [DOI] [PubMed] [Google Scholar]
- 46.Genereux P, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64:2605–2615. doi: 10.1016/j.jacc.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 47.Liao YB, et al. Meta-Analysis of the Effectiveness and Safety of Transcatheter Aortic Valve Implantation Without Balloon Predilation. Am J Cardiol. 2016;117:1629–1635. doi: 10.1016/j.amjcard.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 48.Mohty D, et al. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol. 2009;53:39–47. doi: 10.1016/j.jacc.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margulis AV, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.