Abstract
Purpose:
The aim is to compare the outcome of nonendoscopic endonasal dacryocystorhinostomy (NEN DCR) with external DCR (EXT-DCR) in the treatment of nasolacrimal duct obstruction (NLDO) in children.
Methods:
A retrospective, comparative chart analysis of all consecutive children <16 years after EXT-DCR or NEN-DCR between June 2012 and February 2016.
Results:
Seventy-one children (79 eyes) underwent DCR in the study, of which 37 children (40 eyes) underwent EXT-DCR and 34 (39 eyes) NEN-DCR. Mean age of both groups (8.7 vs. 7.7 years) was comparable. Etiologically, persistent congenital NLDO was the most common indication (50% vs. 72%), followed by acquired and secondary NLDO. Mean duration was shorter for NEN-DCR (47 vs. 37 min; P = 0.0021). Mitomycin C 0.04% was used more often in NEN-DCR (10% vs. 56.41%). Success after primary EXT-DCR was 100% as compared to 75% for primary NEN-DCR at median follow-up of 12 and 16 months respectively. At revision, the main cause of failure was granuloma (60%). After revision, all eyes were symptom-free at a median follow-up of 9.5 months.
Conclusion:
Primary NEN-DCR has a poorer outcome than EXT-DCR in the treatment of NLDO in children and is more likely to need a revision procedure.
Keywords: External dacryocystorhinostomy, nasolacrimal duct obstruction in children, nonendoscopic endonasal dacryocystorhinostomy
Nasolacrimal duct obstruction (NLDO) in children is usually congenital. Massage of the lacrimal sac is the main-stay of treatment in early life and often leads to spontaneous resolution. In children with persistent NLDO, a graded surgical approach is adopted, beginning with one or more attempts at probing and irrigation with or without intubation and balloon catheter dilatation.[1,2,3,4,5] Dacryocystorhinostomy (DCR) is the preferred treatment for those children who fail the above minimally-invasive procedures.[1,2,3,4,5]
First described by Toti in 1904, external DCR (EXT-DCR) involves an external skin incision with a success rate of 80%–95%.[6] While structural and functional outcomes are excellent, wound complications such as hypertrophic scarring, epiblepharon formation, scar dehiscence, and medial ectropion, especially in children, remain a concern.[7] The endonasal approach, though first described in 1893 by Caldwell, was limited by poor visibility of the endonasal anatomy during surgery and hence rarely used.[8] With the advent of improved instrumentation and endoscopes, endonasal DCR gained popularity owing to the benefits of minimal perioperative morbidity, the absence of a cutaneous scar, and early rehabilitation.[9] Disadvantages of endonasal DCR include a long learning curve, significantly longer operating time, especially to set up the equipment, and the need for expensive instrumentation.[9] In this scenario, nonendoscopic endonasal DCR (NEN-DCR) offers the best of both worlds, i.e., retains the benefits of an endonasal approach and can be done without using expensive video endoscope or LASER systems and has a shorter learning curve.[9] While there are reports showing good outcome of NEN-DCR in adults, its outcome in children is not reported.[9] The purpose of this study was to compare the outcome of NEN-DCR with the eternal gold standard technique of EXT-DCR in the treatment of NLDO in children.
Methods
This was a retrospective, comparative interventional study of all consecutive children ≤16 years of age who underwent EXT-DCR or NEN-DCR between June 2012 and February 2016. Only children with a minimum of 3 months of follow-up were included in the study. The study adhered to the Declaration of Helsinki 1975 and was approved by the Institutional Ethics Committee. A diagnosis of NLDO was established after a comprehensive examination of children who presented with symptoms of tearing and/or discharge with or without matted lashes and/or had regurgitation on pressure over the lacrimal sac. In all children, a detailed history helped to determine the onset and duration of epiphora and discharge from the parents. DCR was offered as definitive surgery to only those who had persistent congenital NLDO (CNLDO) after one or more failed attempts of probing with or without silicone intubation, older children with acquired NLDO and those with secondary NLDO. The decision to do either EXT-DCR or NEN-DCR was taken by the parents of the children after they were counseled about the risk and benefit of each procedure.
All surgeries for NEN-DCR were performed by a single surgeon (SR) under general anesthesia. After the introduction of the light pipe through the upper punctum, a nasal speculum with a guard and 5 cm long blades was placed in the nostril. Under direct visualization, the area showing maximum transillumination on the lateral nasal wall was infiltrated with 2% lidocaine with epinephrine (1 in 200,000) till mucosal blanching was evident. The nasal cavity was packed with gauze soaked in 0.05% oxymetazoline for decongestion. A myringotomy sickle knife was used to make a C-shaped incision on the lateral nasal mucosa showing maximal transillumination effect with hinge placed posteriorly. The nasal mucosal flap was excised with Weil-Blakesley forceps. The osteotomy was begun by removing the thin lacrimal bone on the posterior half of the lower lacrimal sac. The thick bone of the frontal process of the maxilla was sequentially removed to the level of the common internal punctum. The medial wall of the lacrimal sac was incised with a myringotomy sickle knife while the sac was tented by a light pipe and a large, posteriorly hinged lacrimal mucosal flap was created. The posterior lacrimal mucosal flap was either fixed with fibrin glue or trimmed short to avoid reclosure of the marsupialized sac. Irrigation was done to check for the patency of the drainage system. Mitomycin C (MMC) 0.04% was applied at the ostium under direct visualization for 3 minutes in select cases that included secondary NLDO, fibrosed lacrimal sac, and inadequate-sized mucosal flaps. Bicanalicular silicone tubes were used in all cases. Neurosurgical cotton soaked with triamcinolone acetonide was guided along the tubes and placed at the osteotomy site.
EXT-DCR was performed by one of the two surgeons. After induction of general anesthesia, local infiltration with 2% lidocaine with epinephrine (1 in 200,000) was done in the lacrimal sac region. The nasal cavity was packed with gauze soaked in 0.05% oxymetazoline nasal drops. The skin incision was made with no. 15 Bard-Parker blade along the anterior lacrimal crest with the superior extent below the medial canthal tendon. Orbicularis muscle fibers were separated, and bleeders were cauterized. The periosteum was incised and reflected to expose the lacrimal sac. The osteotomy was begun by fracturing the thin lacrimal bone and enlarged by using serial-sized bone rongeurs. Care was taken to avoid extending the superior edge of the osteotomy beyond the medial canthal tendon in children. The lacrimal sac was incised with no. 11 Bard-Parker blade while a lacrimal probe was passed through the lower punctum to tent the sac wall. H-shaped incisions on the lacrimal sac and nasal mucosa were done to fashion anterior and posterior flaps. The posterior flaps were excised. An end-to-end anastomosis of anterior flaps was done with the suture passed through the orbicularis muscle to facilitate flap. Adjuvants such as MMC and bicanalicular intubation were used at the discretion of the surgeon. Skin and orbicularis were sutured in a single layer with interrupted 6-0 polygalactyl sutures.
Routine postoperative visits included day 1, week 2, 6, and months 3, 6, 9, and 12 after surgery. Postoperative medications included a nasal decongestant, corticosteroid nasal spray, and saline douche in the first 4 weeks in both groups. Children with a history of acute dacryocystitis received perioperative oral systemic antibiotics. Parents were specifically asked about epiphora at each visit and fluorescein dye disappearance test (FDDT) was done to detect if there was a delay in dye wash-out. Irrigation of the lacrimal passage was possible only in those children who underwent an examination under anesthesia. Primary outcome measure was the functional success after EXT and NEN-DCR and this was determined by an absence of tearing noted by parent (s) and no delay in the FDDT. Secondary outcome measure was anatomical patency on irrigation. Bicanalicular tubes had to be removed under anesthesia approximately 6–8 weeks after surgery and/or earlier if there was a spontaneous extrusion.
Results
In all, 71 children (79 eyes) underwent DCR in the study period. Of these, 37 children (40 eyes) underwent EXT-DCR and the remaining 34 (39 eyes) NEN-DCR. Mean age of both groups (8.7 vs. 7.7 years) was comparable [Table 1]. Males predominated and the right eye was more often affected in both groups. Etiologically, persistent CNLDO was the most common indication for DCR in both groups (28 vs. 20; P = 0.06). Interestingly as many as 18 children in EXT-DCR group and 9 children in NEN-DCR group were found to have acquired NLDO (P = 0.058) and these children did not have a history of trauma or an associated craniofacial anomaly. Two children in EXT-DCR and three in NEN-DCR group had a prior history of acute dacryocystitis, and this had resolved at presentation. One child in the EXT-DCR group underwent revision surgery after a failed prior EXT-DCR.
Table 1.
Comparison of the baseline and other characteristics of external and nonendoscopic endonasal dacryocystorhinostomy
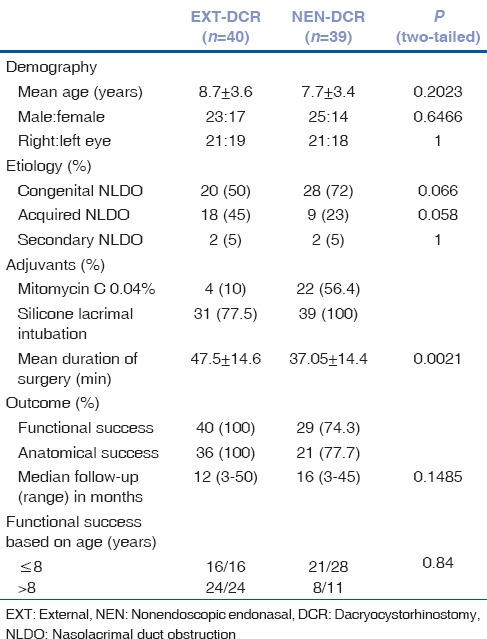
NEN-DCR was the relatively shorter surgical procedure with a mean duration of surgery recorded as 37.05 ± 14.48 min versus 47.5 ± 14.68 min for EXT-DCR (P = 0.0021). Silicone intubation was used in 31/40 cases of EXT-DCR and as standard procedure in all (100%) cases of NEN-DCR. MMC 0.04% was used more frequently in NEN-DCR as compared to EXT-DCR (56.41% vs. 10%).
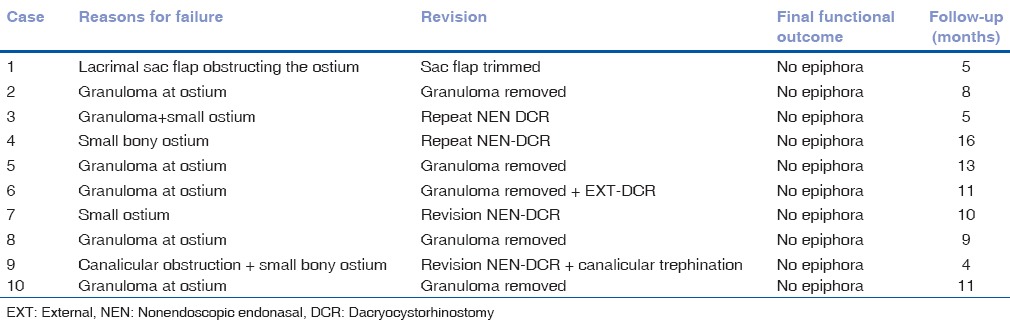
The median follow up of EXT-DCR was 12 versus 16 months for NEN-DCR (P = 0.41). All children who underwent EXT-DCR had the successful functional outcome (100% success). In contrast, as many as 10 (25.64%) children after primary NEN-DCR had persistent epiphora and underwent minor/major revision. The most common cause of failure after NEN-DCR was a granuloma at the ostium found in 6/10 cases [Table 2]. Five of these children underwent removal of the granuloma and were eventually symptom-free. Other causes of failure included a small ostium in 4/10 and canalicular obstruction and a mucosal flap obstructing the ostium in one case each. These needed a major revision surgery. All 10 children who underwent revision were eventually relieved of epiphora at a median follow-up of 9.5 months (range 4–16 months).
Table 2.
Cause of failure in primary nonendoscopic endonasal dacryocystorhinostomy
Subgroup analysis
The median age for all children with CNLDO was younger at 7.05 + 2.91 years versus 10.29 + 3.54 years for acquired NLDO (P = 0.0001). Eight children out of 48 CNLDO failed versus 3 of 31 in those with acquired NLDO (P = 0.52). The outcome in the subgroup of children <8 versus >8 years was compared to understand if age at primary surgery was a determinant of the outcome [Table 1]. The EXT-DCR group had more (28; 60%) children who were >8 years than the NEN-DCR (21; 53%) group. However, success after NEN-DCR in <8 and >8 year age groups was not statistically significant (P = 0.84; Chi-square test).
Discussion
DCR is the intervention of last resort in children with persistent CNLDO and is reported to have good (83%–93%) outcome.[3,10] The outcome of NEN-DCR in the subgroup of NLDO in children is unknown. In this study, comparison of the outcome after primary EXT-DCR and NEN-DCR in children with NLDO showed an absolute success (100%) in the EXT-DCR group versus 75% in NEN-DCR group. All primary failures of NEN-DCR were eventually symptom-free after revision.
Persistent CNLDO was the most common etiology in both EXT- and NEN-DCR groups in this study. Interestingly, 45% of children in the EXT-DCR group had acquired NLDO compared to 23% in NEN-DCR group (P = 0.058). In all of these children, the parents had noted a recent onset of epiphora and the children were devoid of craniofacial anomalies and/or a history of trauma. While the literature abounds with information on CNLDO, there is a dearth of information on acquired NLDO in children. The entity of acquired NLDO in children finds mention by Jones et al. in a series of pediatric endoscopic DCR where 3/34 children <14 years had acquired NLDO.[11] Jones et al. reported the comparable outcome in congenital versus acquired NLDO in children.[11] As there was a preponderance of children with acquired NLDO in the EXT-DCR group, a subgroup analysis of the outcome based on etiology found 8 of 48 eyes with CNLDO failed versus 3 of 31 with acquired NLDO (P = 0.52). Therefore, the better outcome in the EXT-DCR group could not be attributed to the etiology of NLDO. Unfortunately, the pathogenesis of an acquired NLDO in children is unknown and needs further evaluation.
Pediatric DCR differs from adult DCR in several ways. Anatomically, incomplete pneumatization of the agger nasi and an underdeveloped maxilla make the nasal cavity roomy in children, offering easier access to the site of surgery. In contrast, the nasal cavity in children is different from adults in having a narrower vestibule and shorter vertical height with lower skull base.[12,13,14] These characteristics with a septal deviation, if present, can make visualization and surgery difficult in children.[12,13,14] Finally, children are known to have an exaggerated wound healing response compared to adults. All the above necessitate a more careful approach in pediatric DCR.[12,13,14]
The outcome of endoscopic endonasal DCR in children has ranged between 75% and 94%.[11,12,13,14,15] Jones et al. reported success in 22/29 (75%) children with CNLDO.[11] Significantly improved outcomes have been reported in pediatric endoscopic endonasal DCR by other authors in recent times.[12,13,14,15] Mann and Wormald opined that anastomosis of the nasal and lacrimal mucosa in DCR leads to healing by primary intention with minimal granulation and shrinkage of the ostium.[16] This is significant as our surgical technique in NEN-DCR involved removal of the nasal mucosa and opening of the lacrimal sac to fashion a large posterior mucosal flap. Healing in NEN-DCR thus happens by secondary intention and the subsequent granulation tissue may result in a granuloma or lead to shrinkage or obstruction at the ostium. In contrast, the excellent response to EXT-DCR in children may be attributed to the primary intention healing consequent to the mucosal anastomosis achieved. This, we believe, is the most significant revelation of our study and resulted in a modification of the surgical technique of NEN-DCR at our institution. Currently, the author fashions a nasal mucosal flap which is retained and closely approximates the large lacrimal sac flap in NEN-DCR. Whether this modification will result in outcomes comparable to that of EXT-DCR remains to be seen.
Celenk et al. have reported that a minimally invasive septal surgery was needed in 21/83 (25%) of pediatric endoscopic endonasal DCR to enhance visualization.[14] Despite the limitation posed by a narrow nasal cavity, most surgeons do not recommend septoplasty in children.[14] However, a minimal submucosal resection is sometimes done to facilitate access.[14] None of the NEN-DCR performed on the children in our study needed a septal surgery. In the author's experience, septal deviations associated with NLDO have never posed difficulties in access in NEN-DCR. This unique and significant advantage is intrinsic to the surgical technique of NEN-DCR, wherein the long-bladed (5 cm) nasal speculum with a guard allows adequate access to the surgical site, thus obviating the need for additional septal surgeries.
In children, topical MMC has been reported to benefit the repair of choanal atresia,[17] management of laryngeal and tracheal stenosis,[18] and more recently in preventing the recurrence in caustic esophageal strictures.[19,20] No complications were observed in any of these studies.[17,18,19,20] In another report, Dolmetsch et al. observed no adverse effects after MMC in 71 children in nonlaser endoscopic endonasal DCR.[15]
Limitations of our study are primarily related to its retrospective design and sample size. In our experience, primary NEN-DCR has a poorer outcome than EXT-DCR in the treatment of NLDO in children and is more likely to require a revision procedure.
Conclusion
Primary NEN-DCR has a poorer outcome than primary EXT-DCR in the treatment of NLDO in children and is more likely to need a revision procedure. All primary failures of NEN-DCR were eventually symptom-free after single revision.
Financial support and sponsorship
This study was financially supported by Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Maini R, MacEwen CJ, Young JD. The natural history of epiphora in childhood. Eye (Lond) 1998;12(Pt 4):669–71. doi: 10.1038/eye.1998.166. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RA, Robb RM. The natural course of congenital obstruction of the nasolacrimal duct. J Pediatr Ophthalmol Strabismus. 1978;15:246–50. doi: 10.3928/0191-3913-19780701-14. [DOI] [PubMed] [Google Scholar]
- 3.Nowinski TS, Flanagan JC, Mauriello J. Pediatric dacryocystorhinostomy. Arch Ophthalmol. 1985;103:1226–8. doi: 10.1001/archopht.1985.01050080138035. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham MJ, Woog JJ. Endonasal endoscopic dacryocystorhinostomy in children. Arch Otolaryngol Head Neck Surg. 1998;124:328–33. doi: 10.1001/archotol.124.3.328. [DOI] [PubMed] [Google Scholar]
- 5.Struck HG, Weidlich R. Indications and prognosis of dacryocystorhinostomy in childhood. A clinical study 1970-2000. Ophthalmologe. 2001;98:560–3. doi: 10.1007/s003470170119. [DOI] [PubMed] [Google Scholar]
- 6.Toti A. Nuovo metodo conservatore di cura radicale delle suppurazioni croniche del sacco lacrimale (dacriocistorinostomia) Clin Mod Fir. 1904;10:385–7. [Google Scholar]
- 7.Preechawai P. Results of nonendoscopic endonasal dacryocystorhinostomy. Clin Ophthalmol. 2012;6:1297–301. doi: 10.2147/OPTH.S33030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell GW. Two new operations for obstruction of the nasal duct, with preservation of the canaliculi. Am J Ophthalmol. 1893;10:189–92. [Google Scholar]
- 9.Ganguly A, Videkar C, Goyal R, Rath S. Nonendoscopic endonasal dacryocystorhinostomy: Outcome in 134 eyes. Indian J Ophthalmol. 2016;64:211–5. doi: 10.4103/0301-4738.181749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welham RA, Hughes SM. Lacrimal surgery in children. Am J Ophthalmol. 1985;99:27–34. doi: 10.1016/s0002-9394(14)75862-3. [DOI] [PubMed] [Google Scholar]
- 11.Jones DT, Fajardo NF, Petersen RA, VanderVeen DK. Pediatric endoscopic dacryocystorhinostomy failures: Who and why? Laryngoscope. 2007;117:323–7. doi: 10.1097/01.mlg.0000250266.39362.1b. [DOI] [PubMed] [Google Scholar]
- 12.Komínek P, Cervenka S, Matousek P, Pniak T, Zeleník K. Primary pediatric endonasal dacryocystorhinostomy – A review of 58 procedures. Int J Pediatr Otorhinolaryngol. 2010;74:661–4. doi: 10.1016/j.ijporl.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Leibovitch I, Selva D, Tsirbas A, Greenrod E, Pater J, Wormald PJ. Paediatric endoscopic endonasal dacryocystorhinostomy in congenital nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2006;244:1250–4. doi: 10.1007/s00417-006-0273-y. [DOI] [PubMed] [Google Scholar]
- 14.Celenk F, Mumbuc S, Durucu C, Karatas ZA, Aytaç I, Baysal E, et al. Pediatric endonasal endoscopic dacryocystorhinostomy. Int J Pediatr Otorhinolaryngol. 2013;77:1259–62. doi: 10.1016/j.ijporl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Dolmetsch AM, Gallon MA, Holds JB. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in children. Ophthal Plast Reconstr Surg. 2008;24:390–3. doi: 10.1097/IOP.0b013e3181831f56. [DOI] [PubMed] [Google Scholar]
- 16.Mann BS, Wormald PJ. Endoscopic assessment of the dacryocystorhinostomy ostium after endoscopic surgery. Laryngoscope. 2006;116:1172–4. doi: 10.1097/01.mlg.0000218099.33523.19. [DOI] [PubMed] [Google Scholar]
- 17.McLeod IK, Brooks DB, Mair EA. Revision choanal atresia repair. Int J Pediatr Otorhinolaryngol. 2003;67:517–24. doi: 10.1016/s0165-5876(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 18.Senders CW. Use of mitomycin C in the pediatric airway. Curr Opin Otolaryngol Head Neck Surg. 2004;12:473–5. doi: 10.1097/01.moo.0000146708.64004.2b. [DOI] [PubMed] [Google Scholar]
- 19.Uhlen S, Fayoux P, Vachin F, Guimber D, Gottrand F, Turck D, et al. Mitomycin C: An alternative conservative treatment for refractory esophageal stricture in children? Endoscopy. 2006;38:404–7. doi: 10.1055/s-2006-925054. [DOI] [PubMed] [Google Scholar]
- 20.Olutoye OO, Shulman RJ, Cotton RT. Mitomycin C in the management of pediatric caustic esophageal strictures: A case report. J Pediatr Surg. 2006;41:e1–3. doi: 10.1016/j.jpedsurg.2005.12.051. [DOI] [PubMed] [Google Scholar]


