We describe the evolution of subinternal limiting membrane (ILM) hemorrhage associated with anemic retinopathy on fundus autofluorescence (FAF) and spectral domain optical coherence tomography (OCT). A 27-year-old male with megaloblastic anemia complained of defective vision in the right eye (RE). Fundus examination revealed multiple Roth spots bilaterally suggestive of anemic retinopathy. Preretinal hemorrhage was seen over the fovea and temporally in the RE which appeared hypoautofluorescent on FAF and as hyperreflective echoes beneath an elevated ILM on OCT. Hemorrhages resolved following treatment with blood transfusion, folate, and Vitamin B12 supplements. Sub-ILM hemorrhage transformed into a yellow spot at macula which was hyperautofluorescent on FAF and hyperreflective on OCT and then resolved leaving a small sub-ILM hyporeflective cavity. Recent sub-ILM hemorrhage is hypoautofluorescent as fresh blood has no autofluorescence while altered blood is hyperautofluorescent due to bilirubin in the degraded heme which is an endogenous fluorophore.
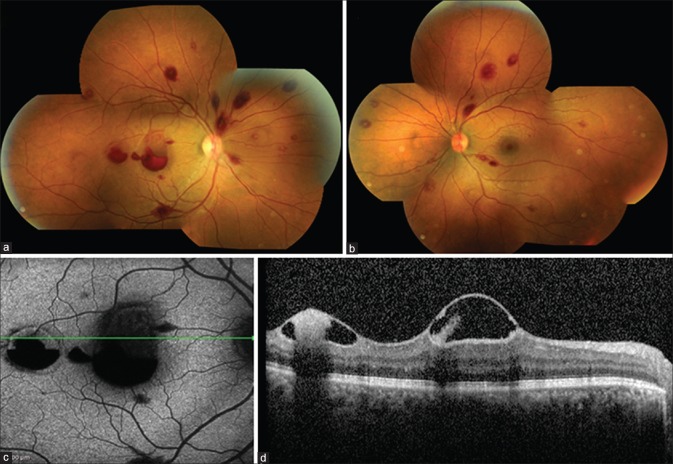
A 27-year-old male patient with megaloblastic anemia developed sudden visual loss in the right eye (RE). Fundus examination showed bilateral Roth spots [Fig. 1a and b]. RE had preretinal hemorrhage located foveally and temporally, which appeared hypoautofluorescent on fundus autofluorescence (FAF) and as hyperreflective deposits under an elevated internal limiting membrane (ILM) on spectral domain optical coherence tomography (OCT) [Fig. 1c and d]. Roth spots reduced following treatment with Vitamin B12 and folate supplements and blood transfusion [Fig. 2a and b]. Sub-ILM hemorrhage first evolved into a yellow spot at macula [Fig. 2c] which showed hyperautofluorescence on FAF and hyperreflectivity on OCT [Fig. 2d and e] and then resolved leaving a small sub-ILM hyporeflective cavity [Fig. 3a and b].
Figure 1.
At presentation, fundus photograph (a and b) of the right eye and the left eye showing multiple Roth spots. The right eye had preretinal hemorrhage over the fovea and temporally. Fundus autofluorescence of the right eye (c) showing hypoautofluorescence corresponding to the hemorrhages. Optical coherence tomography of the right eye (d) showing elevated hyperreflective internal limiting membrane over the fovea and temporally with subinternal limiting membrane hyperreflective deposits within a hyporeflective cavity due to plasma erythrocyte separation
Figure 2.
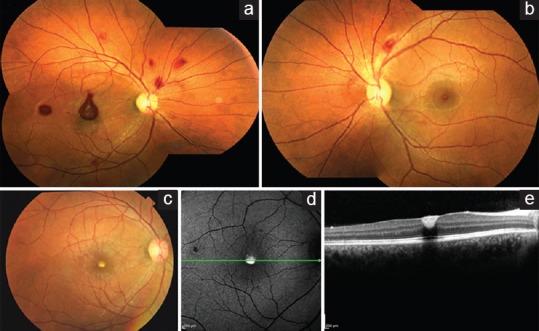
Fundus photograph of the right eye (a) and the left eye (b) at 2 weeks showing decrease in hemorrhages. Fundus photograph of the right eye at 6 weeks (c) showing complete resolution of hemorrhages and a yellowish spot at the macula. Corresponding fundus autofluorescence (d) showing hyperautofluorescence and optical coherence tomography (e) showing flattened foveal contour with subinternal limiting membrane hyperreflective deposits
Figure 3.
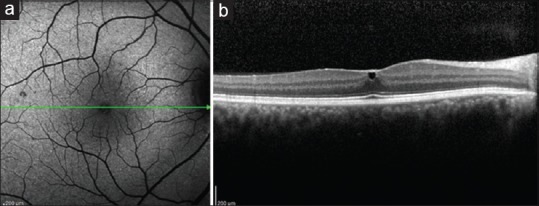
Fundus autofluorescence (a) of the right eye at 1 year showing normal foveal autofluorescence and optical coherence tomography (b) showing small subinternal limiting membrane hyporeflective cavity
Discussion
Newer investigations such as FAF and OCT enable accurate evaluation of anemic retinopathy (AR), a condition characterized by hemorrhages involving all retinal layers.[1] We describe the evolution of sub-ILM hemorrhage associated with AR on FAF, which has not been previously reported. FAF imaging is used to provide density map of lipofuscin, the predominant fluorophore in retinal pigment epithelium (RPE). Other fluorophores associated with accumulation of fluid, blood, or melanolipofuscin granules in various pathologies can also contribute to autofluorescence.[2] Recent retinal hemorrhage is hypoautofluorescent as fresh blood has no autofluorescence whereas devitalized yellowish hemorrhage is hyperautofluorescent. Hyperautofluorescence in subretinal hemorrhage results from the toxic autofluorescent compounds formed through iron-catalyzed free radical attack on the lipids in the photoreceptor outer segments.[3] However, in sub-ILM hemorrhage, the hyperautofluorescence is observed intraretinally rather than at the RPE. We attribute this to bilirubin, an endogenous fluorophore formed from hemoglobin degradation over time.[4,5] As its excitation occurs at 470 nm and emission at approximately 520 nm, its autofluorescence can be captured by spectralis OCT with BluePeak system (Heidelberg Engineering, Germany) which use an excitation wavelength of 488 nm and detect emission wavelength of 500–700 nm.[2,5] Finally, with clearing of heme, the hyperautofluorescence resolved.
Conclusion
Evolution of sub-ILM hemorrhage associated with AR can be documented with FAF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lam S, Lam BL. Bilateral retinal hemorrhages from megaloblastic anemia: Case report and review of literature. Ann Ophthalmol. 1992;24:86–90. [PubMed] [Google Scholar]
- 2.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: Review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 3.Sawa M, Ober MD, Spaide RF. Autofluorescence and retinal pigment epithelial atrophy after subretinal hemorrhage. Retina. 2006;26:119–20. doi: 10.1097/00006982-200601000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Harris GS. Subinternal limiting lamina hemorrhages associated with sudden vascular pressure rise: Yellow preretinal hemorrhages. Dev Ophthalmol. 1981;2:53–8. doi: 10.1159/000395303. [DOI] [PubMed] [Google Scholar]
- 5.Glushko V, Thaler M, Ros M. The fluorescence of bilirubin upon interaction with human erythrocyte ghosts. Biochim Biophys Acta. 1982;719:65–73. doi: 10.1016/0304-4165(82)90308-7. [DOI] [PubMed] [Google Scholar]