Abstract
We report an unusual case of retained metallic intraocular foreign body (IOFB) presenting with acute retinal necrosis (ARN). A healthy young man presented with signs of ARN including hypopyon, dense vitritis, and peripheral retinal necrosis following alleged history of trauma with a high-velocity projectile. After initial management of ARN with systemic antivirals, a retained metallic IOFB was identified and subsequently removed surgically. The patient was followed up for 12 months postoperatively and retained excellent vision without recurrence of the ARN. The diagnosis of an IOFB in a case with associated inflammation can be challenging. A strong clinical suspicion with proper investigations can achieve optimum results.
Keywords: Acute retina necrosis, intraocular foreign body, penetrating trauma
Acute retinal necrosis (ARN) is a uveitic syndrome caused by the herpes group of viruses. The American Uveitis Society criteria for diagnosis are focal, well-demarcated areas of retinal necrosis located in the peripheral retina; rapid, circumferential progression of necrosis; evidence of occlusive vasculopathy; and a prominent inflammatory reaction in the vitreous and anterior chamber.[1] ARN is diagnosed based on clinical features, while laboratory tests are only corroborative.
Dormant virus can be reactivated due to an immunocompromised state such as human immunodeficiency virus (HIV) infection, immunosuppressive, waning cell-mediated immunity, diabetes, and malignancy immunosupressive medication.[2] Trauma has been shown to reactivate virus in various situations.[3]
Retained metallic intraocular foreign body (IOFB) needs surgical removal at the earliest. We present a rare case of retained IOFB masquerading as ARN following trauma with a projectile, which was managed successfully with good outcomes.
Case Report
A 28-year-old healthy man presented to us with the complaint of defective vision, redness, and pain in the right eye for 3 days, following an injury with a high-velocity iron projectile when using a hammer and chisel. On examination, the best-corrected visual acuity was 6/36 and 6/6 in the right and left eye, respectively. Anterior segment evaluation of the right eye revealed circumcorneal congestion, intense anterior chamber reaction, streak hypopyon, sluggishly reacting pupil, and clear lens. There was no evidence of a wound of entry in the cornea or the sclera. On fundus examination, there was Grade 2 vitritis with minimal inferior vitreous hemorrhage minimal inferior vitreous hemorrhage, obscuring clear visualisation. Peripheral retina showed 360° circumferential whitening and occlusive vasculitis with hemorrhages, especially in the inferior quadrant suggestive of retinitis [Fig. 1]. An ultrasound was performed but did not pick up any IOFB. Left eye evaluation was within normal limits.
Figure 1.
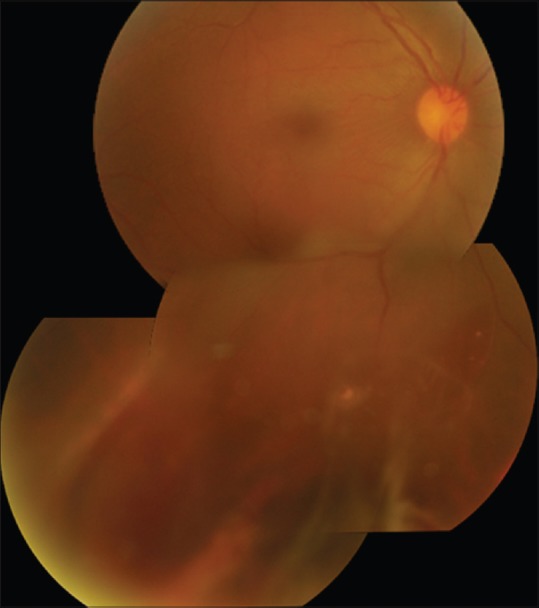
Fundus photo montage showing vitritis, peripheral retinitis patches, and retinal hemorrhages
Based on the typical clinical characteristics, a diagnosis of ARN was made. Routine baseline investigations (complete blood counts, Mantoux, ELISA for HIV, and Treponema pallidum particle agglutination assay) were negative. Therapy for ARN was initiated with intravenous acyclovir 500 mg twice daily along with topical prednisolone and cycloplegics. From the 4th day, as there was decrease in anterior and posterior chamber reaction with resolution of retinitis, as evident by sharpening of borders, he was shifted to oral valacyclovir 1000 mg thrice daily and prednisolone 40 mg once a day in tapering doses. After 8 days, vision improved to 6/6. There was reduction in anterior chamber reaction, disappearance of hypopyon, clearing vitritis, and resolving peripheral retinitis. An area of linear tract-like chorioretinal scarring was noted in the inferotemporal quadrant, leading to the suspicion of retained IOFB. Ultrasound B scan evaluation showed a high reflective echo in the inferior quadrant suggestive of an IOFB [Fig. 2a]; computed tomography (CT) scan of orbit confirmed the presence of 3 mm × 2 mm metallic intraocular foreign body at 6 o’clock position anteriorly in the eye [Fig. 2b and c].
Figure 2.
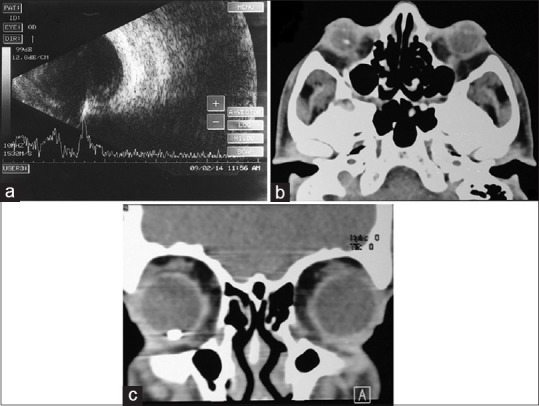
(a) Ultrasound B scan of right eye, longitudinal scan showing a high-reflective echo with back shadowing inferiorly suggestive of intraocular foreign body. (b) Computed tomography scan transverse section showing a hyperdense opacity in right eye suggestive of intraocular foreign body. (c) Computed tomography scan coronal section showing the intraocular foreign body at 6 o’clock position
He underwent pars plana vitrectomy 15 days after presentation during which an encapsulated IOFB was noted at 6 o’clock position just posterior to the ora serrata [Fig. 3a]. The encapsulation was dissected with the vitreous cutter [Fig. 3b] and the dislodged metallic IOFB [Fig. 3c] was elevated with an intraocular magnet and exteriorized through an enlarged superotemporal sclerotomy [Fig. 3d]. The IOFB was oval shaped, 6 mm long with maximum breadth of 2 mm. Cryo was applied posterior to the superonasal and superotemporal enlarged sclerotomy, and around the area of IOFB impaction at 6 o’clock position. There was minimal retinal scarring at the ora in the region of the IOFB. The ARN appeared to be resolved, with no active retinitis seen intraoperatively. The crystalline lens was preserved and remained clear till last follow-up. During surgery, on examining carefully, a minute, self-sealed scleral puncture 2 mm long was noted 2 mm from the limbus at the 6 o’clock meridian. Routine postoperative treatment was given with continued antivirals for 21 days. The ARN resolved completely, without any additional areas of scarring. At his last visit, 12 months later, he maintained 6/6 vision with a normal anterior chamber and stable fundus.
Figure 3.
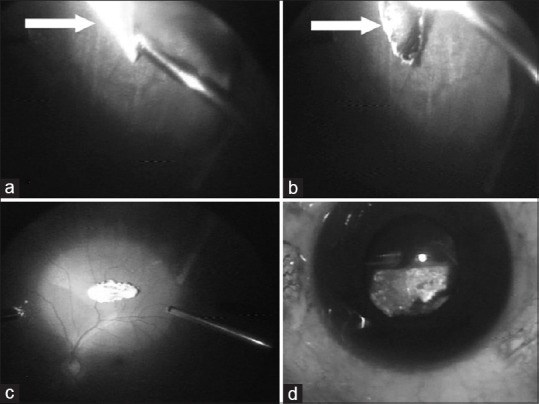
(a) Intraoperative photograph showing the encapsulated intraocular foreign body at 6 o’clock position near pars plana. (b) The encapsulation being cut with a cutter to free the foreign body. (c) Intraocular foreign body dislodged into the vitreous cavity. (d) Intraocular foreign body being exteriorized from the eye
Discussion
We present a case of ARN where the latent virus was reactivated by trauma and the presence of a metallic foreign body in the vitreous cavity. Due to the vitreous inflammation and hypopyon at initial presentation, the IOFB was missed. Fortunately, the ARN responded very well to systemic acyclovir, enabling us to visualize the unusual pigmented tract-like chorioretinal scarring, and peaked our already high suspicion of an IOFB. Targeted ultrasound imaging and a CT scan lead to the identification of the IOFB.
Latent virus can be reactivated due to many stimuli including trauma. Thomas et al.[3] described that mechanical trauma was associated with a significantly higher risk of zoster in involved area. They suggest that traumatic stimulation of nerve may trigger viral reactivation in the dorsal root ganglion.
Thompson et al.[4] report three cases of ARN, caused by reactivation of congenital herpes simplex infection following blunt trauma. In this series, two patients had trauma to ipsilateral malar region and one had a corneal abrasion. There was no penetrating trauma in either of these cases. Svozílková et al.[5] report a case of blunt trauma to the eye with suspected ocular ischemic syndrome. On treatment with steroids and vasoactive drugs, the patient developed bilateral ARN 4 weeks later. Despite aggressive treatment, vision was lost in both the eyes. They hypothesize that trauma led to the induction, and corticosteroid therapy promoted the reactivation of the latent virus. In a series of ARN, Tran et al.[6] report one case who had sustained a head injury followed by virus reactivation and ARN 1 week later, but his eye had not been injured.
Park et al.[7] report a case of a 32-year-old male who developed ARN 2 days after surgery for removal of an iron IOFB. In our case, the ARN was the presenting feature, and IOFB was detected only after inflammation resolved.
We postulate that penetrating trauma resulted in viral reactivation in our case. As the patient was immunocompetent, the degree of inflammation was severe. The resultant significant anterior chamber and vitreous reaction, associated circumferential retinitis, and lack of an obvious wound of entry with normal intraocular pressure led to a delay in diagnosis of the IOFB. In our case, resolution of ARN occurred without significant scarring. However, the linear scar with RPE hyperplasia, which is not a feature of ARN, along with the history of projectile injury, leads us to suspect and search for an IOFB, which was subsequently located and successfully removed surgically.
Conclusion
This case shows that a high level of suspicion must be maintained when patients present with a typical history of trauma with a high-velocity projectile, even in unusual scenarios. Judicious medical treatment of ARN with subsequent surgical removal of IOFB may result in optimal visual and anatomical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117:663–7. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham ET, Jr, Wong RW, Takakura A, Downes KM, Zierhut M. Necrotizing herpetic retinitis. Ocul Immunol Inflamm. 2014;22:167–9. doi: 10.3109/09273948.2014.925378. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SL, Wheeler JG, Hall AJ. Case-control study of the effect of mechanical trauma on the risk of herpes zoster. BMJ. 2004;328:439. doi: 10.1136/bmj.37991.511829.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WS, Culbertson WW, Smiddy WE, Robertson JE, Rosenbaum JT. Acute retinal necrosis caused by reactivation of herpes simplex virus type 2. Am J Ophthalmol. 1994;118:205–11. doi: 10.1016/s0002-9394(14)72900-9. [DOI] [PubMed] [Google Scholar]
- 5.Svozílková P, Ríhová E, Diblík P, Kuthan P, Kovarík Z, Kalvodová B, et al. Varicella zoster virus acute retinal necrosis following eye contusion: Case report. Virol J. 2005;2:77. doi: 10.1186/1743-422X-2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran TH, Stanescu D, Caspers-Velu L, Rozenberg F, Liesnard C, Gaudric A, et al. Clinical characteristics of acute HSV-2 retinal necrosis. Am J Ophthalmol. 2004;137:872–9. doi: 10.1016/j.ajo.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Park SW, Byon IS, Park HJ, Lee JE, Oum BS. A case of presumed acute retinal necrosis after intraocular foreign body injury. Clin Ophthalmol. 2013;7:545–8. doi: 10.2147/OPTH.S42175. [DOI] [PMC free article] [PubMed] [Google Scholar]


