Abstract
Traumatic endophthalmitis is a devastating condition that can occur following an open globe injury and result in loss of vision. The use of prophylactic antibiotics is empirical as most surgeons fear complications associated with the same. No systematic review has been performed in English on the role of intravitreal/intracameral antibiotics in preventing traumatic endophthalmitis. We searched for randomized controlled trials and controlled clinical trials comparing intracameral/intravitreal antibiotics with placebos on PubMed, Google Scholar, Science Direct, and Cochrane Library using keywords open globe/trauma/penetrating/perforating injuries endophthalmitis. The last search was on 5 May 2017. We included patients of all ages with open globe injuries who received intracameral/intravitreal antibiotics, regardless of the dose. Quality of the trials was assessed using Cochrane collaboration tools to assess the risk of bias. The main outcome measures were endophthalmitis and visual acuity. We included three trials. Overall, intravitreal/intracameral antibiotics were noted to significantly reduce the occurrence of endophthalmitis in open globe injuries (relative risk [RR] 0.19, 95% confidence interval [CI] 0.06–0.57). The use of intravitreal/intracameral antibiotics did not have an effect in improving visual acuity (RR 1.17, 95% CI 0.61–2.23). Two trials (Narang 2003; Soheilan 2001) were observed to have no significant effect on visual acuity while another trial (Soheilan 2007) did not list visual acuity as part of its objectives. Intracameral/intravitreal antibiotics reduce the risk of endophthalmitis in open globe injuries; although, there was no improvement in the visual acuity. We, therefore, recommend the use of intravitreal/intracameral injections in open globe injuries to prevent this devastating complication.
Keywords: Intravitreal/intracameral antibiotics, traumatic endophthalmitis, prophylaxis
Description of the condition
Traumatic endophthalmitis is an urgent devastating ophthalmologic condition that can result in severe loss of vision. Endophthalmitis is a complication that can occur following open globe injury as bacteria can enter the eye directly, and the management is a challenge to the surgeon. The type and nature of the injury, the presence of intraocular foreign body (IOFB), and the pathogenic organism can all influence the occurrence of endophthalmitis.
Culture is positive in 28% and 33% of the eyes of open globe injury from the anterior chamber and vitreous tap, respectively, at the time of primary repair.[1,2] In an analysis of endophthalmitis, Sharma et al. found gram-positive bacteria in postcataract (90%) and posttraumatic (55%) groups, whereas hyphate fungus was common in endogenous endophthalmitis (50%) (P < 0.001).[3]
Delay in primary repair, ruptured lens capsule, and dirty wound was each independently associated with the development of posttraumatic endophthalmitis.[4] Since prevention is better than cure, efforts should be made to prevent the occurrence of endophthalmitis.
There are numerous controversies regarding the management of the posterior segment in open globe injuries. The use of prophylactic intravenous antibiotics is empirical.[5,6] Most eye surgeons fear complications associated with intravitreal injection and their use has therefore been advocated only in high-risk cases of open globe injuries.[7]
The commonly used intravitreal antibiotics are vancomycin, ceftazidime, amikacin, amphotericin, ciprofloxacin, moxifloxacin, and imepenam.[8] Mehta et al. reported that vancomycin, ceftazidime and moxifloxacin prepared in single-use polypropylene syringes retain potency, sterility, and stability up to 24 weeks when stored at −20°C or −80°C.[9]
The antibiotics have to be prepared with adequate sterility by trained personnel and injected with a 30 gauge needle directed towards the mid-vitreous cavity through the pars plana in phakic and pseudophakic individuals. In aphakic individuals entry is from the limbus through the anterior chamber into the vitreous.[8]
How the intervention might work
Static and dynamic barriers of the ocular structures limit the penetration of systemic and topical antibiotics, and satisfactory levels can only reach the vitreous through intravitreal injections. Topically instilled medicines are diluted by the tear film, causing loss of significant drug in the lacrimal flow.[10] Further low molecular weight antibiotics also undergo systemic absorption from the conjunctival capillaries and the nasolacrimal mucosal surfaces, leading to further drop in bioavailability.[11] The corneal epithelium also has tight junctions, leading to poor paracellular drug penetration, especially for ionic drugs.[12] Systemically administered drugs easily gain access to the choroidal extravascular space, but thereafter, distribution into the intraocular space via the retina is limited by the RPE and the retinal endothelium.[13] The drug diffuses freely in the vitreous cavity and reaches the retinal surface, facilitated by extraocular movements.[13] Diffusion from plasma to vitreous cavity is not high enough to assure clinical efficacy for hydrophilic antibiotics such as glycosamides and beta-lactams systemically so that intravitreal administration would be the preferred choice.[14]
Why is it important to do this review?
The globe with an open injury is susceptible to developing infection. The practice of administering intravitreal antibiotics varies from center to center. Out of 153 (30.6%) participants who responded at a conference, 20.9% were routinely administered with a prophylactic intraocular injection of antibiotics which included intracameral (47.9%) and intravitreal (42.0%) injections.[15] More respondents from referral hospitals used intraocular injections (92.7%) compared to primary hospitals (69.4%) (P = 0.001).[15] All eyes with posterior segment IOFBs received intravitreal antibiotics, and there were no cases of endophthalmitis after initial management in a study of IOFBs between 1999 and 2008 in Bascom Palmer Eye Institute.[16] To the best of our knowledge, there has been no review anywhere in the literature on the effects of intravitreal antibiotics in the prevention of endophthalmitis in open globe injuries.
Objective
To assess the effects of intravitreal antibiotics in preventing endophthalmitis in open globe injuries.
Materials and Methods
Criteria for including studies for this review:
Types of studies
Randomized control trials and controlled clinical trials.
Types of participants
Patients of all ages who had open globe injuries.
Types of interventions
Intravitreal antibiotics regardless of the dose and type of antibiotic used.
Types of outcome measures
Endophthalmitis
Visual acuity
Search methods for identification of studies
Relevant studies were identified on PubMed, Google Scholar, Science Direct, and Cochrane Library using the words open globe injuries, trauma penetrating/perforating injuries, endophthalmitis. The last search was done on May 5, 2017. We obtained full texts through the Ministry of Health Virtual Library and the Library of Melaka Manipal Medical College Malaysia.
Data Collection and Analysis
Selection of studies
The review authors (TT and ALA) independently assessed the trial eligibility of the studies and screened the studies to be entered. We resolved disagreements through discussion. We reviewed the full texts of all the articles.
Data extraction and management
The review authors independently selected the studies. Any dispute was settled by discussion among the authors.
Assessment of risk of bias in included studies
We assessed random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). We assessed the risks of bias as low risk, moderate risk, and high risk.
Data synthesis
We carried out meta-analysis using Review Manager Software (RevMan 2014) for the trials that were eligible. We utilized fixed-effect meta-analysis model for trials that were sufficiently similar with no significant heterogeneity. We utilized risk ratio or mean differences as summary measures where applicable.
Assessment of heterogeneity
We used the Chi-square test for heterogeneity (significance level P < 0.1) and quantify the degree of heterogeneity by means of the I2 statistic. We regard an I2 value of 30% or more as having moderate heterogeneity.
Assessment of reporting bias
Comprehensive searches were made in an attempt to minimize publication and reporting biases. We considered and assessed selective outcome reporting within studies as part of the risk of bias assessment. We aimed to utilize funnel plot analysis to assess for publication bias; however, there were insufficient trials with similar outcome measures to perform these funnel plot analysis.
Sensitivity analysis
We planned to carry out a sensitivity analysis to explore the effects of the risk of bias of the trials (assessed by concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting) and thereafter by excluding trials with a high risk of bias for this domain. However, there were insufficient trials with similar outcome measure to permit us do this analysis in this review.
Results
Search results
We established seven records of which all seven were identified through database searching via the Cochrane Library, MEDLINE, PubMed and Google Scholar [Fig. 1]. No other record was identified through other sources. From this list, we removed four records that did not fully fulfill the inclusion criteria, leaving us a total of three trials. We proceeded to obtain the full texts of all three trials. Following the assessment of these full-text articles, we considered three trials (published in three papers) for inclusion in this review and excluded four trials. Of the four trials excluded, we classified one of these[17] as an on-going trial [Fig. 1].
Figure 1.
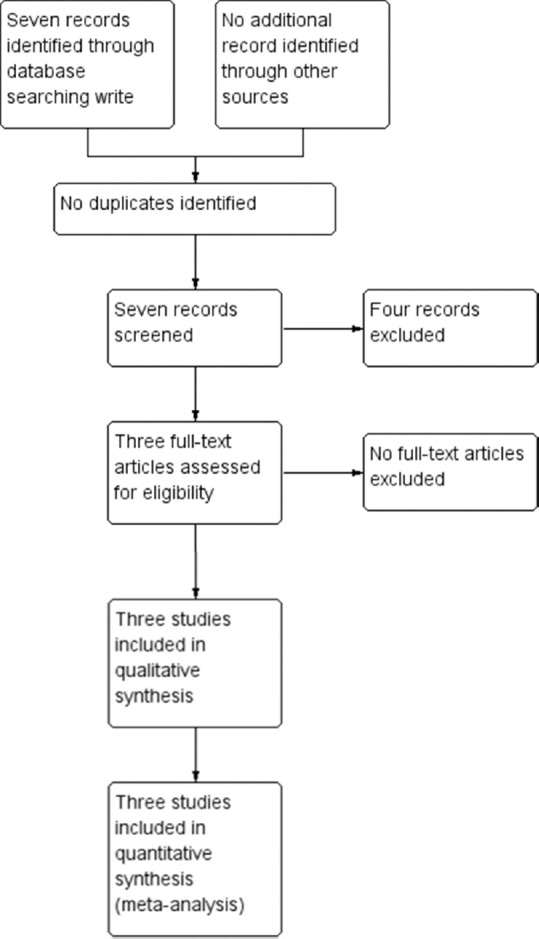
Flow chart - selection of studies for inclusion
Included studies
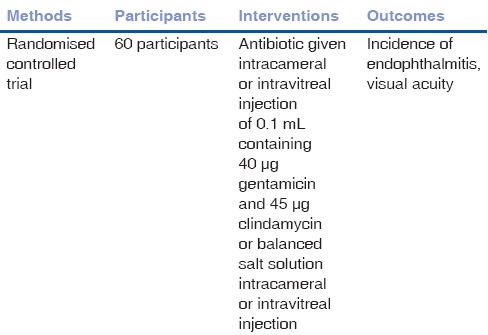
We included three trials that met our inclusion criteria. There were altogether a total of 309 participants involved in these studies [Tables 1–3]. In Narang et al.[18] 2003, 70 participants comprising of 57 males and 13 females received either an intravitreal injection of vancomycin 1 mg and ceftazidime 2.25 mg or placebo with expected outcomes of endophthalmitis and visual acuity. In another trial,[19] a total of 60 participants were exposed to either intracameral or intravitreal injection of 0.1 mL containing 40 μg gentamicin and 45 μg clindamycin or balanced salt solution with expected outcomes of endophthalmitis and visual acuity. A third trial[20] saw a total of 179 participants in the antibiotic group receiving a combination of 40 μg gentamicin and 45 μg clindamycin and 167 in the placebo group receiving a balanced salt solution. The outcomes analysed in this trial included incidence of endophthalmitis, rate, and types of additional procedures required.
Table 1.
Characteristics of included study - Narang 2003

Table 3.
Characteristics of included study - Soleihian 2007
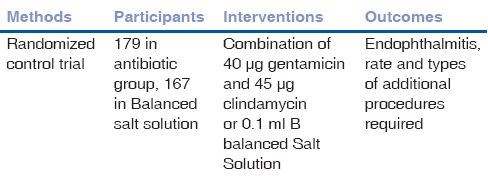
Table 2.
Characteristics of included study - Soleihian 2001
Excluded studies
We excluded four studies from the review. Three trials did not fully fulfill the inclusion criteria (Wang[21] 2007; Siqueira[22] 2009; Tabatabaei[23] 2016). Another trial was excluded as ongoing.[17]
Risk of bias in included studies
The overall risks of bias are presented graphically in Tables 4–6.
Table 4.
Risk of bias - Narang 2003
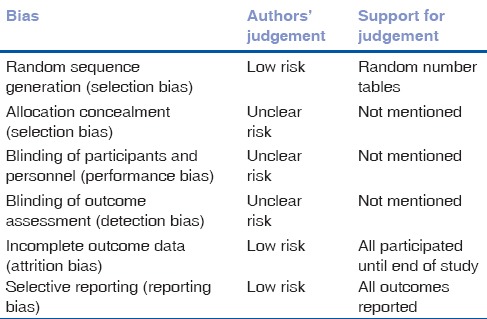
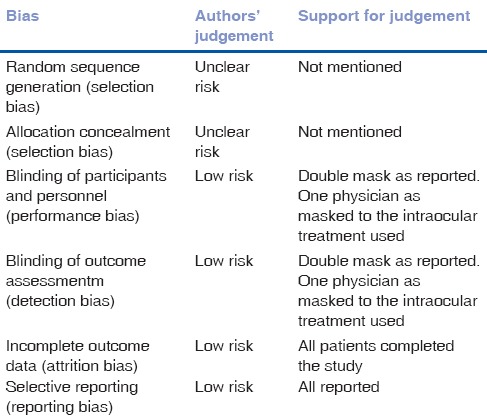
Table 6.
Risk of bias - Soleihian 2007
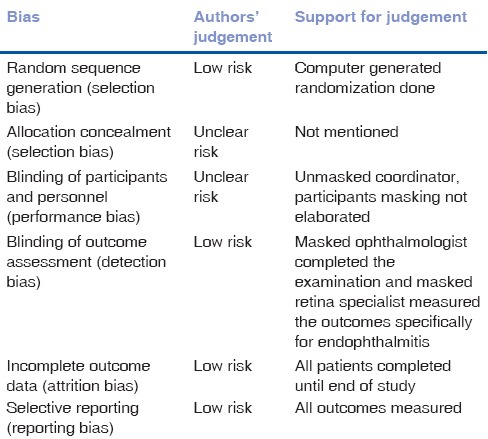
Table 5.
Risk of bias - Soleihian 2001
Allocation (selection bias)
All three trials reported the use of randomization as techniques to divide into intervention and placebo groups. Two provided further details of these and was therefore assessed as having a low risk of bias.[18,20] We have assessed the remaining one trial as having an unclear risk.[19] Method of allocation concealment was not mentioned in any of the included trials and we therefore assessed all as having an unclear risk of bias.
Blinding (performance bias and detection bias)
Two of the three trials included in the analysis reported the use of a double-blinding procedure during the trial.[19,20] The remaining one trial[18] did not provide details of the blinding procedure and was therefore categorized as having an unclear risk of bias.
Incomplete outcome data (attrition bias)
All three included trials[18,19,20] had reported complete outcome data. We had therefore classified all these trials as having low risk bias in this domain.
Selective reporting (reporting bias)
We categorized all included trials[18,19,20] as having low risk of reporting bias as all outcomes objectives of these trials were analyzed and presented in the manuscripts.
Effects of interventions
Incidence of endophthalmitis
Overall, additions of intravitreal or intracameral antibiotics were noted to significantly reduce the occurrence of endophthalmitis (relative risk [RR] 0.19, 95% confidence interval [CI] 0.06–0.57; Table 7 and Fig. 2). Perusing at the trials individually, we noted two trials did not reduce development of endophthalmitis[18,19] while another trial[20] observed a significant reduction of incidence of endophthalmitis among those receiving intravitreal antibiotics (RR 0.12, 95% CI 0.10–0.92).
Table 7.
Incidence of endophthalmitis among those receiving antibiotics and placebo
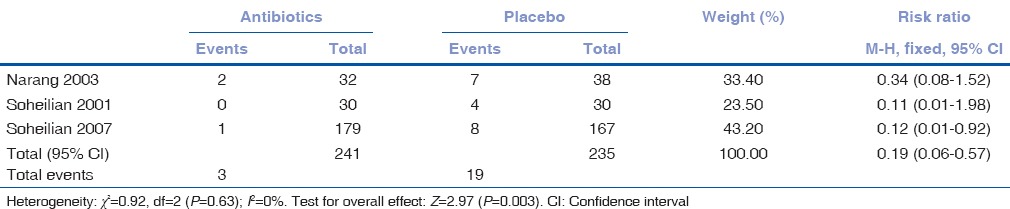
Figure 2.
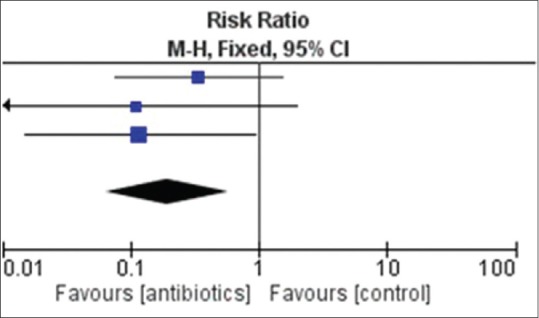
Forest plot-incidence of endophthalmitis among those receiving antibiotics and placebo
Visual acuity
There was no overall effect of addition of intravitreal or intracameral antibiotics in enhancing visual acuity (RR 1.17, 95% CI 0.61–2.23; [Table 8 and Fig. 3]). Two trials[18,19] were observed to have no significant effect on visual acuity while another trial[20] did not list visual acuity as part of its objectives and hence returned no outcome data.
Table 8.
Improvement on visual acuity among those receiving antibiotics and placebo
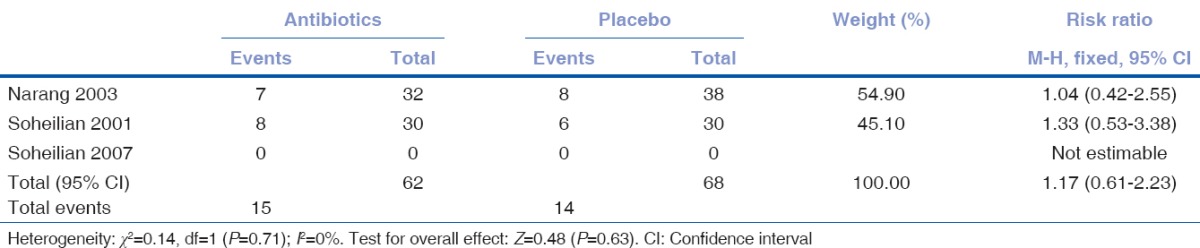
Figure 3.
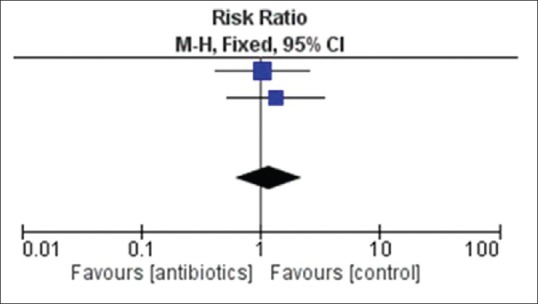
Forest plot-enhancement of visual acuity among those receiving antibiotics and placebo
Discussion
Summary of the main results
We identified seven records but removed four that did not fulfill our criteria, thereby leaving us with a total of three trials. Overall, we found that the addition of intravitreal or intracameral antibiotics reduced the incidence of endophthalmitis. Individually, two trials did not reduce the development of endophthalmitis,[18,19] whereas only one trial[20] observed a reduction of endophthalmitis with the use of intravitreal antibiotics (RR 0.12, 95% CI 0.10–0.92). We observed that visual acuity did not improve with intracameral or intrabitreal antibiotics in 2 trials[18,19] while the third trial[20] did not study visual acuity.
Overall completeness and applicability of evidence
Although the total number of trials is small, the overall conclusion is that the addition of intravitreal or intracameral antibiotics was beneficial in reducing the risk of endophthalmitis in open globe injuries although inconclusive outcome of improved visual acuity. Endophthalmitis occurs in 3%–10% of cases after penetrating trauma to the eye, although early surgical repair and prophylactic systemic antibiotics may reduce this incidence to <1%.[24] The role of intravitreal antibiotics in posterior segment trauma in the absence of infection is still debated.[25] In these high-risk cases (history of soil contamination, retinal periphlebitis, and exudation around a retained IOFB), it is important to achieve high drug concentration in the vitreous, which only intravitreal injections can provide.[26]
Quality of evidence
The trial evidence is generally of good quality, with a low risk of bias. Two of the three reported the use of blinding procedures.[19,20] One trial[18] did not provide the details of blinding. All three trials reported complete outcome and hence; had low risk of attrition bias. All three also reported all outcomes and therefore had low risk of reporting bias.
All three trials reported using randomization technique though only two[18,20] provided the details and hence are a low risk of bias. Method of allocation concealment was not mentioned in any of the trials.
Potential biases in the review process
Although intracameral/intravitreal antibiotics were useful in reducing the incidences of endophthalmitis, there were limitations in noted in the trials. Narang et al.[18] excluded patients with intraocular foreign bodies and those in whom a foreign body was removed beyond 1 week of open globe injury-since these are established factors for endophthalmitis. Soheilian et al.[19] 2001 and Soheilian et al.[20] 2007 did not exclude patients with IOFB and did not take into consideration the time of removal of the IOFB. Due to the limited number of trials, we could not analyse the use of intravitreal antibiotics alone in preventing endophthalmitis.
Agreements and disagreements with other studies or reviews
We are unaware of similar reviews covering this topic.
Authors’ Conclusions
Implications for practice
There is evidence that the use of intracameral/intravitreal antibiotics reduces the risk of endophthalmitis in open globe injuries although there was no improvement in the visual acuity. We, therefore, recommend the use of intravitreal/intracameral injections in open globe injuries to prevent this devastating complication.
Implications for research
Initial results favor the use of intracameral/intravitreal antibiotics in preventing the occurrence of endophthalmitis in penetrating eye injuries. However, further multicenter randomized control trials need to be done to investigate whether the previous findings were consistent and sustained.
Recommended guidelines
Based on our meta-analysis, until more evidence can be deduced from future multicenter randomized trials, we recommend the use of intracameral/intravitreal antibiotics to reduce the risk of endophthalmitis following open globe injuries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the Director General of Health for granting permission to publish the study.
References
- 1.Ariyasu RG, Kumar S, LaBree LD, Wagner DG, Smith RE. Microorganisms cultured from the anterior chamber of ruptured globes at the time of repair. Am J Ophthalmol. 1995;119:181–8. doi: 10.1016/s0002-9394(14)73871-1. [DOI] [PubMed] [Google Scholar]
- 2.Mieler WF, Ellis MK, Williams DF, Han DP. Retained intraocular foreign bodies and endophthalmitis. Ophthalmology. 1990;97:1532–8. doi: 10.1016/s0161-6420(90)32381-3. [DOI] [PubMed] [Google Scholar]
- 3.Sharma YR, Gaur N, Chandra P, Takkar B. Predictors of visual outcomes and microbial profile in endophthalmitis. Ophthalmic Surg Lasers Imaging Retina. 2016;47:991–8. doi: 10.3928/23258160-20161031-02. [DOI] [PubMed] [Google Scholar]
- 4.Essex RW, Yi Q, Charles PG, Allen PJ. Post-traumatic endophthalmitis. Ophthalmology. 2004;111:2015–22. doi: 10.1016/j.ophtha.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JT, Parver LM, Enger CL, Mieler WF, Liggett PE. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. National eye trauma system. Ophthalmology. 1993;100:1468–74. doi: 10.1016/s0161-6420(93)31454-5. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds DS, Flynn HW., Jr Endophthalmitis after penetrating ocular trauma. Curr Opin Ophthalmol. 1997;8:32–8. doi: 10.1097/00055735-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mittra RA, Mieler WF. Controversies in the management of open-globe injuries involving the posterior segment. Surv Ophthalmol. 1999;44:215–25. doi: 10.1016/s0039-6257(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 8.Verma A, Muralidharan V, Nigam E. Endophthalmitis: Current trends, drugs and protocols. Sci J Med Vis Res Foun. 2015;33:61–70. [Google Scholar]
- 9.Mehta S, Armstrong BK, Kim SJ, Toma H, West JN, Yin H, et al. Long-term potency, sterility, and stability of vancomycin, ceftazidime, and moxifloxacin for treatment of bacterial endophthalmitis. Retina. 2011;31:1316–22. doi: 10.1097/IAE.0b013e31820039af. [DOI] [PubMed] [Google Scholar]
- 10.Urtti A, Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol. 1993;37:435–56. doi: 10.1016/0039-6257(93)90141-s. [DOI] [PubMed] [Google Scholar]
- 11.Urtti A, Pipkin JD, Rork GS, Sendo T, Finne U, Repta AJ. Controlled drug delivery devices for experimental ocular studies with timolol. 2. Ocular and systemic absorption in rabbits. Int J Pharm. 1990;61:241–9. [Google Scholar]
- 12.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60:207–25. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Maurice DM, Mishima S. Ocular pharmacokinetics. In: Sears ML, editor. Pharmacology of Theeye. New York: Springer, Heidelberg; 1984. [Google Scholar]
- 14.López-Cabezas C, Muner DS, Massa MR, Mensa Pueyo JM. Antibiotics in endophthalmitis: Microbiological and pharmacokinetic considerations. Curr Clin Pharmacol. 2010;5:47–54. doi: 10.2174/157488410790410597. [DOI] [PubMed] [Google Scholar]
- 15.Lou B, Lin L, Tan J, Yang Y, Yuan Z, Lin X, et al. Survey of intraocular antibiotics prophylaxis practice after open globe injury in China. PLoS One. 2016;11:e0156856. doi: 10.1371/journal.pone.0156856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parke DW, 3rd, Pathengay A, Flynn HW, Jr, Albini T, Schwartz SG. Risk factors for endophthalmitis and retinal detachment with retained intraocular foreign bodies. J Ophthalmol. 2012;2012:758526. doi: 10.1155/2012/758526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viestenz A, Schrader W, Behrens-Baumann W. Traumatic endophthalmitis prevention trial (TEPT) Klin Monbl Augenheilkd. 2008;225:941–6. doi: 10.1055/s-2008-1027840. [DOI] [PubMed] [Google Scholar]
- 18.Narang S, Gupta V, Gupta A, Dogra MR, Pandav SS, Das S, et al. Role of prophylactic intravitreal antibiotics in open globe injuries. Indian J Ophthalmol. 2003;51:39–44. [PubMed] [Google Scholar]
- 19.Soheilian M, Rafati N, Peyman GA. Prophylaxis of acute posttraumatic bacterial endophthalmitis with or without combined intraocular antibiotics: A prospective, double-masked randomized pilot study. Int Ophthalmol. 2001;24:323–30. doi: 10.1023/b:inte.0000006768.66170.c1. [DOI] [PubMed] [Google Scholar]
- 20.Soheilian M, Rafati N, Mohebbi MR, Yazdani S, Habibabadi HF, Feghhi M, et al. Prophylaxis of acute posttraumatic bacterial endophthalmitis: A multicenter, randomized clinical trial of intraocular antibiotic injection, report 2. Arch Ophthalmol. 2007;125:460–5. doi: 10.1001/archopht.125.4.460. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Dong N, Kang Y, et al. Clinical study of pars plana vitrectomy with silicone oil endotamponade in posttraumatic endophthalmitis on eyes without retinal detachment. Yan Ke Xue Bao Eye Sci Yan Ke Xue bao Bian Ji Bu. 2007;23:48–52. [PubMed] [Google Scholar]
- 22.Siqueira RC, Gil AD, Canamary F, Minari M, Jorge R. Pars plana vitrectomy and silicone oil tamponade for acute endophthalmitis treatment. Arquivos brasileiros de oftalmologia. 2009;72:28–32. doi: 10.1590/s0004-27492009000100006. [DOI] [PubMed] [Google Scholar]
- 23.Tabatabaei SA, Soleimani M, Behrooz MJ, Sheibani K. Systemic oral antibiotics as a prophylactic measure to prevent endophthalmitis in patients with open globe injuries in comparison with intravenous antibiotics. Retina. 2016;36:360–5. doi: 10.1097/IAE.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 24.Andreoli CM, Andreoli MT, Kloek CE, Ahuero AE, Vavvas D, Durand ML. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol. 2009;147:601–8. doi: 10.1016/j.ajo.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal R, Shah M, Mireskandari K, Yong GK. Controversies in ocular trauma classification and management: Review. Int Ophthalmol. 2013;33:435–45. doi: 10.1007/s10792-012-9698-y. [DOI] [PubMed] [Google Scholar]
- 26.Cebulla CM, Flynn HW., Jr Endophthalmitis after open globe injuries. Am J Ophthalmol. 2009;147:567–8. doi: 10.1016/j.ajo.2008.12.016. [DOI] [PubMed] [Google Scholar]


