Abstract
Purpose:
The aim is to present the incidence and determinants of neovascular glaucoma (NVG) in Saudi Arabia.
Methods:
A retrospective review of NVG cases (2002–2012) was included to estimate the incidence. The determinants included gender, age, comorbidities, lens status, type of NVG, and visual acuity on presentation. The impact of antiangiogenic therapy on NVG incidence was studied.
Results:
We studied 597 eyes with NVG. The incidence was 6.6/10,000. It declined from 13/10,000 in 2008–0.1/10,000 in 2012 (P < 0.001). The decline in 2008 coincided with the introduction of intravitreal injection bevacizumab in Saudi Arabia. Males had a significantly higher NVG risk (odds ratio [OR] = 2.2). Diabetes and hypertension were associated with NVG in 88% and 42.7% of cases, respectively. In 377 (72%) diabetic patients, the glycemic control was poor (HbA1C >7%). Visual acuity was 20/20–20/40 in 14 (2%), 20/50–20/200 in 79 (13%), 20/200–20/400 in 456 (76%), and <20/400 in 45 (7%) eyes. Intraocular pressure was higher than 30 mmHg in 438 (73%) eyes. The cup-to-disc (CD) ratio was >0.8 in 86 (14%) eyes. During the early period (2002–2007) and later period (2008–2012), CD ratio (χ2 = 4, P = 0.09) and anterior chamber angle (P = 0.8) were not different. The presence of NVG in contralateral eye (OR = 0.8, P = 0.3) in both periods was similar.
Conclusions:
NVG was associated with diabetes in a very large proportion of patients. It was significantly associated with males, and with poor glycemic control and poor vision at presentation. The incidence of NVG declined after the introduction of intravitreal bevacizumab.
Keywords: Diabetes, epidemiology, glaucoma, neovascular glaucoma, visual impairment
Neovascular glaucoma (NVG) is a potentially devastating sequel of serious underlying ocular and/or systemic diseases that is often refractory to pharmacological management and often requires surgical intervention.[1,2]
The clinical features, etiology and clinical management of NVG are described in the literature in detail.[3,4] However, the epidemiology of NVG should be periodically reviewed as it is linked to the epidemic of diabetes and introduction of newer modalities of treatment that control ocular complications of diabetes and the severity of diabetic retinopathy (DR). The information on the magnitude and determinants of NVG will enable the vitreoretinal surgeons to detect and manage NVG in the early stages with a hope of improving the impact of management and halting the progression of visual impairment.
With the introduction of intravitreal anti-vascular endothelial growth factor (anti-VEGF)-angiogenic therapy, the serious complications of DR, including retinal ischemia, have reduced considerably.[5,6] However, its effect on the incidence of NVG, to the best of our knowledge, has not been studied.
The reported prevalence of diabetes in the 18 years and older population in Saudi Arabia is 28%.[7] The prevalence of DR ranged from 37% to 47% among patients of 40 years and older.[8] Therefore, NVG, which seems to be a rare condition in other parts of the world, could potentially be a bigger blinding problem in such a population.[9]
The goal of this study was to fill this gap in knowledge and report the magnitude, determinants and presentation of NVG over 11 years, before and after the introduction of intravitreal anti-VEGF therapy, at a tertiary eye hospital in central Saudi Arabia.
Methods
The institutional research and ethical committee of our tertiary eye hospital (King Khaled Eye Specialist Hospital [KKESH], Riyadh, Saudi Arabia) approved this study. This hospital-based data review was undertaken in 2014. All cases of NVG diagnosed by retina and glaucoma subspecialist ophthalmologists between January 1985 and December 2013 were included in the study. Since the information on hospital/clinic attendance for male and female patients was available for 2002–2012, we calculated the annual incidence of NVG for these years only. The data of 11 years were grouped into “early period” (EP) if NVG patient had presented before the end of 2007. Those presenting after January 1, 2008 were grouped as “later period” (LP). This grouping was made to study the trends before and after the introduction of intravitreal anti-VEGF therapy in 2008. In case of unilateral NVG, if systemic risk factors such as diabetes or hypertension remained uncontrolled, the contralateral eye was considered to be at risk of developing NVG in the future.
The magnitude of NVG was calculated as the incidence of NVG per 10,000 cases and its 95% confidence interval (95% confidence interval [CI]). The incidence was also calculated for males and female patients separately. To compare the determinants of NVG in the early and late period, we used 2 × 2 table of Open Epi software and presented odds ratio (OR), 95% CI of OR and two-sided “P” value.[10] For a qualitative variable with more than two subgroups, we calculated Chi-square value, the degree of freedom and two-sided “P” value. For continuous variables such as age, we calculated the mean and the standard deviation and then calculated the difference of mean, its 95% CI and two-sided “P” value.
Results
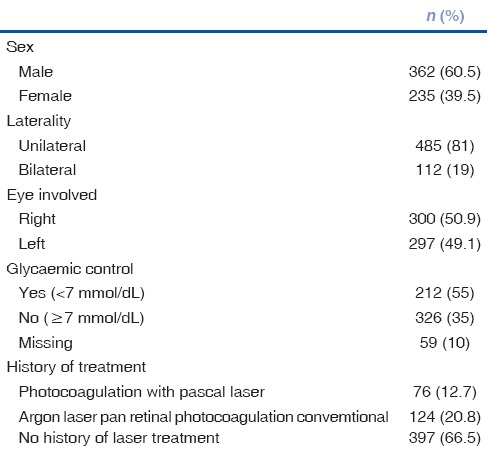
There were 597 patients with NVG in this study. There were 362 (61%) males and 235 (39%) females. The mean age was 60.3 ± 13.4 years (range from 10 to 97 years). The profile of patients with NVG is presented in Table 1. NVG was bilateral in 112 persons (19%) and unilateral in 485 (81%) cases.
Table 1.
Profile of study participants with neovascular glaucoma reported at a tertiary eye hospital of Saudi Arabia between 2002 and 2012
During the study, 597 NVG cases were detected among 898,885 new eye cases. The overall annual incidence of NVG was 6.6/100,000. In the year 2007, there were 92 NVG cases among 75,000 new eye patients with an incidence of 12/100,000 cases. In the year 2012, there were 12 NVG cases in 117,000 eye patients with an incidence of 1/100,000 cases. The trend of NVG by year is presented in Fig. 1. The incidence of NVG declined significantly (P < 0.001) following the introduction of anti-VEGF treatment of NVG in 2008 at the hospital.
Figure 1.
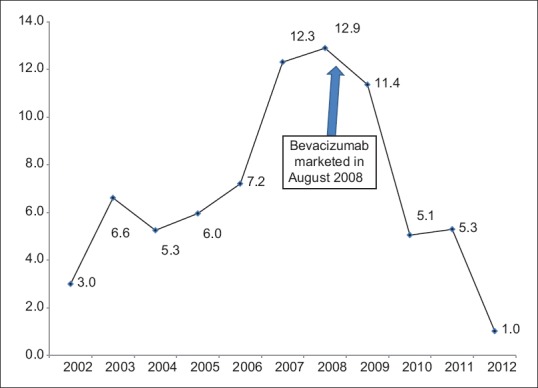
Incidence of neo-vascular glaucoma between 2002 and 2012 at tertiary eye hospital of Saudi Arabia. X - Axis denote year of presentation. Y - Axis denotes incidence per 10,000 eye patients
The distribution of study participants according to the etiologic cause of NVG is shown in Fig. 2. DR was the main cause in 81% of NVG cases. Ischemic vascular causes without DR contributed 15% of NVG. The proportion of NVG due to DR increased in LP compared to EP.
Figure 2.
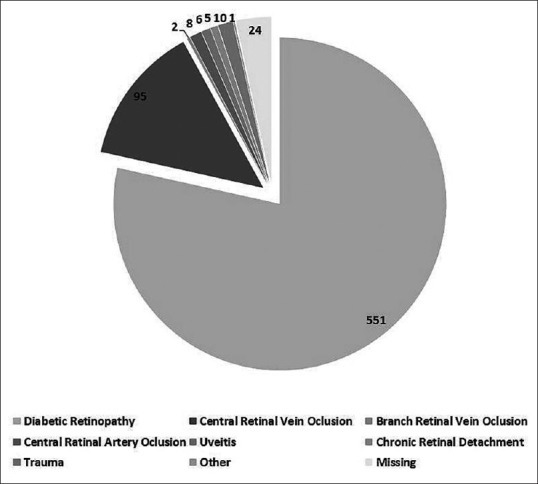
Distribution of principal causes of neo-vascular glaucoma during two time period
The ocular characteristics of eyes with NVG at presentation are shown in Table 2. Hemorrhage in the anterior and posterior chamber was noted at presentation in one-third of eyes with NVG.
Table 2.
Ocular characteristics of eyes with neovascular glaucoma at presentation
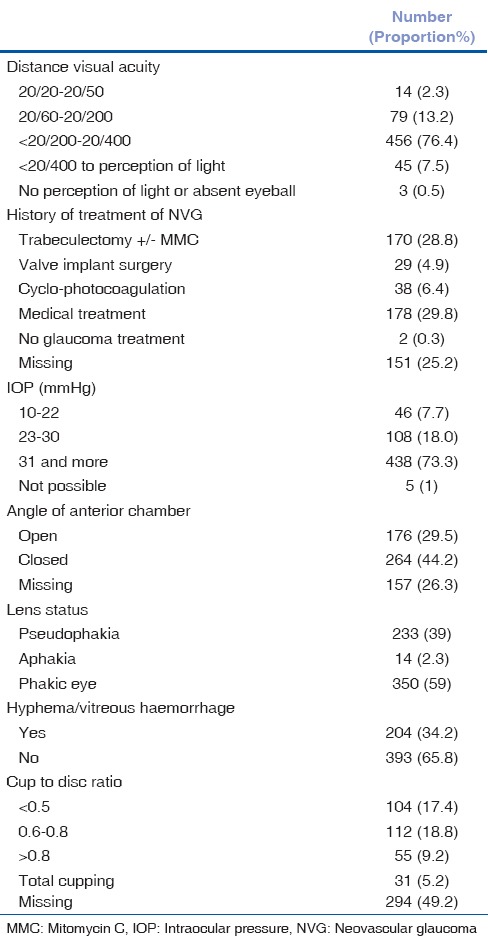
In 220 (72%) diabetic patients, glycemic control was poor (HbA1C >7%). As many as 340 (57%) eyes with NVG were phakic. The cup-to-disc (CD) ratio was >0.8 in 86 (14%) eyes.
We compared the trends of epidemiological variables in EP and LP [Table 3]. Glaucoma presentation, CD ratio (χ2 = 4, P = 0.1) and configuration if the anterior chamber angle (open or closed) (P = 0.8) were not different between early and late period. The proportion of NVG cases with DR (P = 0.07) and poor glycemic control (P = 0.07) were significantly higher in LP than EP.
Table 3.
Comparison of epidemiology of neovascular glaucoma in early (2002-2007) and late (2008-2012) period at King Khaled Eye Specialist Hospital, Saudi Arabia (n=597)
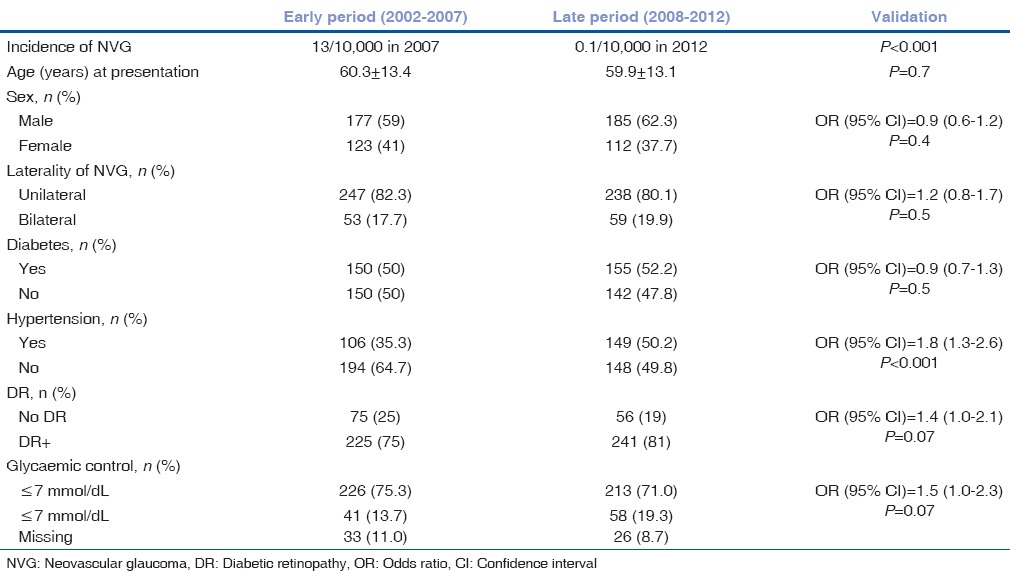
Among 485 unilateral NVG cases during the entire study, glycemic control was poor in 262 (54%) and hypertension control was poor in 114 (24%) of NVG patients, posing a risk of developing NVG in the fellow eye. The NVG in contralateral eye at presentation (OR = 0.8, P = 0.3) in both periods was not different.
Discussion
This large series of NVG emphasizes the changing trends in NVG following the addition of anti-VEGF therapy to conventional laser treatment for DR. The study shows that NVG in the Saudi population was more common in males and associated with the poor glycemic control. Most patients had poor visual acuity at presentation. Due to the presence of risk factors that are known to cause NVG (poor glycemic and hypertension control), there was a risk of the contralateral eye developing glaucoma. A dramatic decrease in the incidence of NVG was noted following the introduction of anti-VEGF therapy (intravitreal bevacizumab) in 2008.
The number of NVG cases in both the early and late period was similar. However, the rate of retinopathy and poor glycemic control among patients with NVG was higher in the LP. This could be a result of rising prevalence of diabetes and hypertension and poor health-related behavior for controlling risk factors, leading to ocular complications such as DR, and ischemic retinopathies.[11,12] While the impact of anti-VEGF therapy on NVG reduction seems obvious, in the absence of primary prevention of NVG (control of diabetes and hypertension), the decline in incidence could give a false sense of security. Use of anti-VEGF treatment, based on its pharmacokinetics, serves as a temporary measure in reducing pathologic neovascularization. The risk of further progress and development of NVG in the fellow eye in patients with poor glycemic control cannot be ruled out.
The annual incidence of NVG in our study was 0.07%. Based on the incidence rate, one can calculate the prevalence of a chronic disease. If we assume that the life expectancy of a Saudi national (75 years) is also applicable to a Saudi with NVG, a person diagnosed with NVG for the first time and included in the present study at an average age of 59 years would suffer from NVG for at least another 16 years.[13] The prevalence of NVG, therefore, would be 0.07 × 16 = 1.1%. In a study conducted in the western province of Saudi Arabia, the prevalence of NVG was 1.4%.[14] In that study, however, the prevalence was calculated based on a group of patients seen in the glaucoma clinic, and the denominator was cases attending glaucoma clinic, which is different from our study. The total cases coming to KKESH had increased in LP. That could result in a decline in overall incidence in LP. The prevalence is far less compared to the that in China where researchers noted NVG prevalence of 5.8%.[15] The risk factors like poor glycemic control in diabetics and hypertension in existing NVG cases suggest a higher risk of recurrence of NVG and development of NVG in the contralateral eye. Thus, increase in the prevalence of NVG (old + new) cases in the coming years could not be ruled out. Eye care services, especially those in the peripheral areas of Saudi Arabia, should be further strengthened to address NVG.
We found a statistically significant higher incidence of NVG in males than the females. NVG was also noted more in males than females in a study conducted in Nigeria.[16] The rate of hypertension was noted to be significantly higher in Saudi males than females.[12] In the web-based national diabetes registry of Saudi Arabia, the males with diabetes were higher in number compared to females with diabetes.[11] Even the trend of judicious medical control of diabetes and hypertension was poor in males when compared to females in Saudi Arabia. We, therefore, assume that the risk of NVG is likely to be higher in males compared to females in Saudi Arabia. Further studies may be necessary to understand the gender risk of NVG in the Saudi population. Poor glycemic control was an independent risk factor for NVG in our study. A separate epidemiologic survey has shown that all registered diabetics from 22 randomly selected health institutes had the mean HbA1c levels of more than 7%, reflecting poor glycemic control.[12] Thus, the incidence of NVG due to DR is not likely to reduce in the country unless proactive intervention strategies are applied to reduce retinal complications of diabetes.
NVG is largely a complication of ischemic retinopathy. Sight threatening DR (STDR) was the leading cause of NVG in our study. Preda et al.[17] and Liao et al.[15] also noted that proliferative DR, followed by ischemic vascular diseases were the main underlying causes of NVG. Hamard and Baudouin[18] noted that retinal ischemia in elderly patients and DR in younger patients were the underlying causes of NVG. A study in 2009 from our Institute revealed that 56% of NVG were due to DR and 26% following retinal venous occlusion (RVO) – both are ischemic conditions.[19] Other, less common causes of anterior segment neovascularization and possible NVG include chronic posterior uveitis and long-standing retinal detachments, but these were not analyzed in this study.
The role of intravitreal anti-VEGF injections in preventing and reducing progression of neovascularization has been established.[6] Unfortunately, these injections in patients that lack proper primary prevention are temporary measures, and even the number of intravitreal injections needed for a reasonable outcome is not known. Hence, anti-VEGF treatment may not be as cost-effective to prevent as well as treat NVG. The management of STDR includes laser treatment as well. Previous reports have shown that among diabetics with STDR in need of laser therapy in Saudi Arabia, only 71% had undergone laser therapy.[18] Thus, increasing awareness of patients for timely management of STDR should further reduce the incidence of NVG.
In the beginning of 2008, intravitreal antiangiogenic agents became available in addition to laser treatment. Following their introduction, there was a marked decline in the incidence of NVG. One might associate this decline to the revised NVG management protocol at our Institute. Availability of such treatment modalities in other institutes of Saudi Arabia, availability of trained personnel to undertake management and ease of providing such treatment even by general ophthalmologists could also have resulted in reduced incidence of NVG in our study after 2008.[20] There was no standard protocol for anti-VEGF use, but injections were indicated in cases where laser photocoagulation was not sufficient to control NVG or if the view to retina was too poor to allow laser photocoagulation. A comparison of anti-VEGF therapy with combination therapy of laser and injections would require a separate study.
As many as 83% of eyes with NVG had a severe visual impairment (SVI) at a presentation in our study. Three eyes had no vision while 8% had vision <20/400. A previous study reported that around 58% of eyes with NVG had SVI in Japan.[21] A large number of cases with late stages of visual impairment in this study could have been due to a combination of glaucomatous and retinal damage resulting from underlying causes like STDR and RVO. Early detection of NVI and monitoring the intraocular pressure in cases of DR and ischemic retinopathy is recommended to avoid/delay presentation of NVG cases in late visual disabilities.[22,23]
In the pesent study, NVG was unilateral in 82% of participants. Nakatake et al.[24] noted that 75% of NVG cases had a unilateral presentation that was comparable to what was seen in our study. In view of the poor control of underlying causes of NVG among Saudi patients, the risk of developing NVG in the fellow eye might increase in the future. A periodic assessment of both eyes; the eye treated for NVG and the fellow eye with a high risk of developing NVG should be undertaken by a skilled ophthalmologist.
More than half of NVG cases in our study were phakic, which was comparable to that reported by Saito et al.[21] In this study, the risk of recurrence of anterior segment neovascularization 6 months after retinal management was also higher in phakic eyes. The management of NVG in eyes with lens opacities could inhibit proper viewing of the retina and therefore in addition to a number of glaucoma surgeries, pars plana vitrectomy, and lensectomy are recommended before retinal treatment of NVG.[2]
The present study, which is a retrospective review of health records, has a few inherent limitations. Incomplete data on different variables associated with NVG could have introduced a bias. The information on NVG cases at presentation were mainly used for the present review. Our institute adopted standard preferred pattern of practice to manage blinding eye diseases. Accordingly, detailed information of managing blinding eye disease like NVG is usually maintained in case files. As these were the source of information for the present study, the effect of such bias on the study outcomes was likely to be limited. The history of systemic comorbidities could be influenced by recall bias in our study. Therefore, the subjective information obtained from patients was confirmed from the notes of the referring doctor and by laboratory test results documented in the medical records.
Conclusions
This study updates epidemiologic data on NVG at the beginning of the new century. It demonstrates that introducing new therapies for NVG, such as anti-angiogenic intravitreal therapy has resulted in a sharp decrease in the incidence of NVG. However, the risk factors for underlying causes, including DR and hypertension, continue to rise. The resources to manage NVG should be increased in the coming years to manage this condition promptly in different parts of Saudi Arabia and other countries with high prevalence of lifestyles-related metabolic diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sivak-Callcott JA, O’Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108:1767–76. doi: 10.1016/s0161-6420(01)00775-8. [DOI] [PubMed] [Google Scholar]
- 2.Sarwat S, Scott IU, Fekrat S. Ophthalmic Pearls: Glaucoma Eye Net American Academy of Ophthalmology. [Last accessed on 2017 Jan 11]. Available from: http://www.aao.org/publications/eyenet/200607/pearls.cfm .
- 3.Shazly TA, Latina MA. Neovascular glaucoma: Etiology, diagnosis and prognosis. Semin Ophthalmol. 2009;24:113–21. doi: 10.1080/08820530902800801. [DOI] [PubMed] [Google Scholar]
- 4.SooHoo JR, Seibold LK, Kahook MY. Recent advances in the management of neovascular glaucoma. Semin Ophthalmol. 2013;28:165–72. doi: 10.3109/08820538.2012.730103. [DOI] [PubMed] [Google Scholar]
- 5.Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra GM. Intravitreal bevacizumab (Avastin®) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142:1054–6. doi: 10.1016/j.ajo.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Simha A, Braganza A, Abraham L, Samuel P, Lindsley K. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev. 2013;10:CD007920. doi: 10.1002/14651858.CD007920.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011;9:76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Ghamdi AH, Rabiu M, Hajar S, Yorston D, Kuper H, Polack S. Rapid assessment of avoidable blindness and diabetic retinopathy in Taif, Saudi Arabia. Br J Ophthalmol. 2012;96:1168–72. doi: 10.1136/bjophthalmol-2012-301874. [DOI] [PubMed] [Google Scholar]
- 9.Klemm M, Gesser C. The relevance of diabetes for patients with glaucoma. Klin Monbl Augenheilkd. 2014;231:116–20. doi: 10.1055/s-0033-1360143. [DOI] [PubMed] [Google Scholar]
- 10.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. [Last updated on 2014 Sep 22; Last accessed on 2017 Jan 11]. Available from: http://www.OpenEpi.com .
- 11.Al-Rubeaan KA, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Ibrahim HM. A Web-based interactive diabetes registry for health care management and planning in Saudi Arabia. J Med Internet Res. 2013;15:e202. doi: 10.2196/jmir.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeed AA, Al-Hamdan NA, Bahnassy AA, Abdalla AM, Abbas MA, Abuzaid LZ. Prevalence, Awareness, Treatment, and Control of Hypertension among Saudi Adult Population: A National Survey. Int J Hypertens. 2011;2011:174135. doi: 10.4061/2011/174135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health Saudi Arabia. Demography in the Annual Statistical Book for the Hijri Year 1433. 2012. [Last accessed on 2017 Jan 11]. Available from: http://www.moh.gov.sa/en/Ministry/Statistics/book/Documents/1433.pdf .
- 14.Eid TM, el-Hawary I, el-Menawy W. Prevalence of glaucoma types and legal blindness from glaucoma in the western region of Saudi Arabia: A hospital-based study. Int Ophthalmol. 2009;29:477–83. doi: 10.1007/s10792-008-9269-4. [DOI] [PubMed] [Google Scholar]
- 15.Liao N, Li C, Jiang H, Fang A, Zhou S, Wang Q. Neovascular glaucoma: A retrospective review from a tertiary center in China. BMC Ophthalmol. 2016;16:14. doi: 10.1186/s12886-016-0190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashaye AO, Adeoti CO. Neovascular glaucoma in a Nigerian African population. East Afr Med J. 2006;83:559–64. doi: 10.4314/eamj.v83i10.9469. [DOI] [PubMed] [Google Scholar]
- 17.Preda M, Davidescu L, Damian C, Irimia A, Sollosy M. Neovascular glaucoma – Prevention. Oftalmologia. 2006;50:108–14. [PubMed] [Google Scholar]
- 18.Hamard P, Baudouin C. Consensus on neovascular glaucoma] J Fr Ophtalmol. 2000;23:289–94. [PubMed] [Google Scholar]
- 19.Al-Shamsi HN, Dueker DK, Nowilaty SR, Al-Shahwan SA. Neovascular glaucoma at King Khaled Eye Specialist Hospital-Etiologic considerations. Middle East Afr J Ophthalmol. 2009;16:15–9. doi: 10.4103/0974-9233.48860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Motowa S, Khandekar R, Al-Towerki A. Resources for eye care at secondary and tertiary level government institutions in Saudi Arabia. Middle East Afr J Ophthalmol. 2014;21:142–6. doi: 10.4103/0974-9233.129761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito Y, Higashide T, Takeda H, Murotani E, Ohkubo S, Sugiyama K. Clinical factors related to recurrence of anterior segment neovascularization after treatment including intravitreal bevacizumab. Am J Ophthalmol. 2010;149:964–72.e1. doi: 10.1016/j.ajo.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson-Berka JL, Miller AG. Update on the treatment of diabetic retinopathy. ScientificWorldJournal. 2008;6(8):98–120. doi: 10.1100/tsw.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion. N Engl J Med. 2010;363:2135–44. doi: 10.1056/NEJMcp1003934. [DOI] [PubMed] [Google Scholar]
- 24.Nakatake S, Yoshida S, Nakao S, Arita R, Yasuda M, Kita T, et al. Hyphema is a risk factor for failure of trabeculectomy in neovascular glaucoma: A retrospective analysis. BMC Ophthalmol. 2014;14:55. doi: 10.1186/1471-2415-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]


