Abstract
Purpose:
Only a few studies have analyzed the potential link between glaucoma and cognitive function impairment. They have found controversial results. This study aims to perform quick cognitive function assessment with clock drawing test (CDT) using two different scoring systems and compare between normal tension glaucoma (NTG) and cataract patients.
Methods:
Totally, 30 NTG and 30 patients with cataracts were included in a prospective, pilot study. The predrawn circle was given, and patients were asked to draw the clock showing a time of 11:10. The test was evaluated using two methods – Freund method using a 7-point scoring scale (optimal cutoff ≤4) and Rakusa using a 4-point scoring scale (optimal cutoff ≤3). The level of significance was set at P < 0.05.
Results:
CDT result was significantly better in cataract group than in NTG group: 3.5 (2) versus 2 (2) by Freund, (P = 0.003) and 6.5 (1) versus 4.5 (2.75) by Rakusa, respectively (P = 0.004). Sixty percent (n = 18) of NTG group and 10% (n = 3) of cataract group patients completed the CDT in the specific picture manner (the short hand on 11 and the long hand between 11 and 12), (P = 0.001).
Conclusions:
Lower CDT results were seen in NTG patients according to two different scoring systems. NTG patients showed a specific manner of drawing. Further prospective studies are needed to investigate the CDT reliability as fast screening test of cognitive function impairment in glaucoma patients.
Keywords: Cataract, clock drawing test, cognitive function, normal tension glaucoma
Glaucoma is the second leading cause of blindness worldwide. It is predicted to reach a number of 79.6 million, of whom 5.9 million will be bilaterally blind, by 2020.[1] With a growing elderly population, more people are at the risk of visual impairment due to chronic eye diseases caused by aging processes as well as dementia.[2]
Dementia affects many older people in different countries every year. According to the World Health Organization, there are 35.6 million people suffering from dementia world over. It is predicted that by 2030 this number will increase to 65.7 million.[3] Alzheimer's disease (AD) is the most common form of dementia in the elderly.[4]
There is some evidence that AD may be more frequent among glaucoma patients. Common genetic risk factors and similar pathological changes in the optic nerves have been demonstrated.[5,6] It has been hypothesized that these two neurodegenerative disorders may have common pathogenetic pathways.[7] A causal relationship might be explained by decreased cerebrospinal fluid pressure (CSFP) in patients with AD. The results of a study by Wostyn et al. supported idea that elevated intraocular pressure (IOP), reduced CSFP, or both determinate an abnormally high translaminar pressure difference (TPD), which plays an important role in glaucomatous optic nerve damage. Recently, researchers have emphasized that an abnormally high TPD could be the reason for developing glaucoma in AD patients.[8]
Associations between age-related degenerative eye diseases including cataract, glaucoma, age-related macular degeneration (AMD), and dementia have been suggested. Several studies found an association between AMD and decreased cognitive function,[9,10,11,12] while no such relation was found between cataract and cognitive function impairment.[13,14,15,16] Only a few studies analyzed the potential link between glaucoma and cognitive function impairment and found controversial results.[17,18,19,20]
A lot of screening tests are applied for cognitive function assessment. One of them is Clock Drawing Test (CDT), which matches all requirements of an ideal cognitive screening test. Basic cognitive skills are evaluated while drawing a simple clock.[21] There are many CDT scoring systems. We used two different methods: method of Freund, which uses a 7-point scoring scale[22] and method of Rakusa, which uses a 4-point scoring scale.[23]
Expecting that glaucoma and AD may have common pathogenetic pathways, the aim of our study was to compare cognitive function levels in two different eye diseases: NTG and cataract. We hypothesized that NTG patients as neurodegenerative disease representative will have poorer CDT scores compared to cataract patients.
Methods
Thirty NTG and 30 patients with uncomplicated cataracts were included in a prospective, pilot study. The research followed the tenets of the Declaration of Helsinki and consent of the Ethical Committee was obtained for the study protocol. All patients provided written informed consent before participation.
The inclusion criteria for patients in both groups were an age over 65 years, and best-corrected visual acuity (BCVA) of better eye ≥0.6 by Snellen chart. Patients were included in the NTG group if they were diagnosed with NTG by a glaucoma specialist and had characteristic OND changes, visual field loss consistent with glaucoma, and IOP <21 mmHg before treatment. NTG group consisted of early and moderate glaucoma stages. Patients with uncomplicated cataract without evidence of glaucoma, IOP lower than 21 mmHg were included in the cataract group.
Patients with ocular conditions such as AMD and high degree myopia, who had a history of previous intraocular surgery, retinal or vitreous pathology, traumatic cataract, steroid or laser treatment, hypothyroidism or hyperthyroidism, uncompensated cardiovascular and pulmonary disorders, neurological and other diseases that could skew results were excluded from the study. Anamnesis was collected from patients and their medical records retrieved from their general practitioners. No neurological evaluation was performed.
Patients were examined under the same conditions: comfortably seated in a silent room after good instructions. All study procedures were conducted by one examiner AD. The predrawn circle of 10 cm diameter was given to patients, and they were asked to draw a clock showing the time 11:10: “This circle represents a clock face. Please put in numbers so that it looks like a clock and then set the time showing 10 min past 11.” Many other times could be used like: 3:00, 3:40, 8:40, 2:45, and so on. There were no recommendations which time it was better to use; however, the time 11:10 was suggested because it appeared to be the most sensitive to neurocognitive dysfunction. This time included both visual fields and patients who had a tendency to be “pulled” (frontal pull due to executive dysfunction) while setting this time. Patients were allowed to self-correct. No clues were given. In general, there is no time limit to perform the test, and it usually takes about 2 min. The time was evaluated by the same examiner using chronometer. The timer was started after instructions were given and stopped on completion of drawing.
The test was evaluated by two different methods: method of Freund[22] and the Rakusa system.[23] The Freund scoring system uses 7-point evaluation scale: from 0 to 7 indicating poor to excellent cognitive status, respectively. Table 1 shows the scoring system's distribution into three categories and terms. For identification of cognitive problems, an optimal cutoff ≤4 was used. Freund scoring system provides excellent sensitivity (94.3%) and high specificity (87.4%).[24]
Table 1.
Freund scoring system
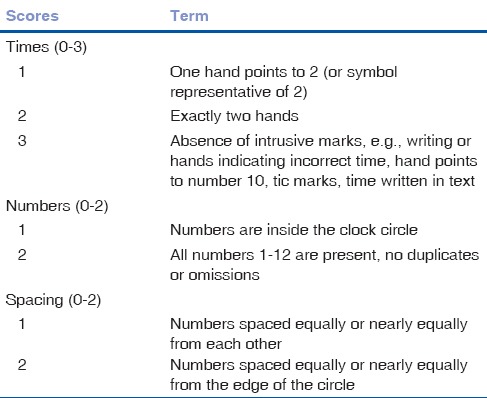
Evaluation method of Rakusa uses a 4-point scoring scale, with 0–4 indicating from poor to excellent cognitive status [Table 2]. The optimal cutoff score for identification of cognitive problems is ≤3. Method of Rakusa suggests that the cutoff 3 out of 4 points sensitivity for cognitive impairment is 87% and specificity is 93%.[23]
Table 2.
Rakusa scoring system

The statistical data analysis was performed using software SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). All variables were defined by methods of descriptive statistics. The analysis of the quantitative variables included calculation of the mean and standard deviation (× [SD]). Student's t- and Mann–Whitney U-tests were used to compare two independent groups. Chi-square test was used to compare the frequencies of qualitative variables. The nonparametric Spearman correlation coefficient was used. The level of significance was P < 0.05.
Results
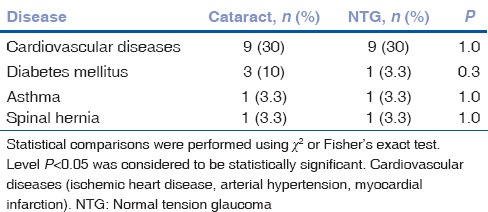
Sixty patients (18; 30% men) were examined. Subject distribution by age, sex, and BCVA in NTG and cataract groups is shown in Table 3. The rates of systemic diseases did not differ significantly between groups [Table 4].
Table 3.
Normal tension glaucoma and cataract patients characteristics
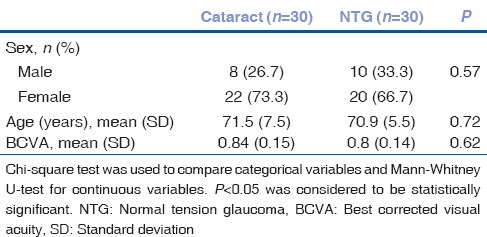
Table 4.
The rates of systemic diseases and combinations of cardiovascular diseases rates in normal tension glaucoma and cataract groups
Mean CDT result was significantly better in the cataract group than the NTG group: 3.5 (2) and 2 (2) by Freund, (P = 0.003) and 6.5 (1) 4.5 and (2.75) by Rakusa, respectively (P = 0.004). We found probable cognitive dysfunction in 50% (n = 15) of patients by Freund and 76.7% (n = 23) by Rakusa in NTG group while in the cataract group, there were 16.7% (n = 5) and 50% (n = 15) patients, respectively. The most common CDT scores by Freund evaluation system were 4 in NTG and 7 in cataract group. According to Rakusa evaluation system, the most common CDT scores were 1 in NTG and 4 in cataract group. The most common CDT scores by Freund and Rakusa are presented in Tables 5 and 6.
Table 5.
The estimates of clock drawing test by Freund in normal tension glaucoma and cataract groups
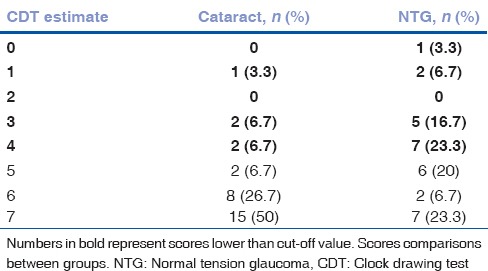
Table 6.
The estimates of clock drawing test by Rakusa in normal tension glaucoma and cataract groups
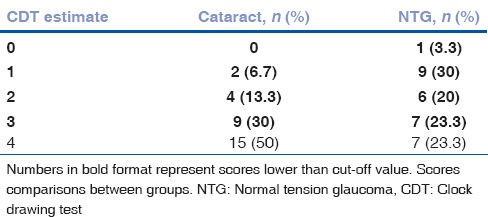
Time for CDT completion did not differ statistically significantly between groups (cataract [96.03 (39.6)] vs. glaucoma [103.83 (39.2) sec], (P = 0.446)). CDT estimates by Freund correlated with those by Rakusa [Fig. 1].
Figure 1.
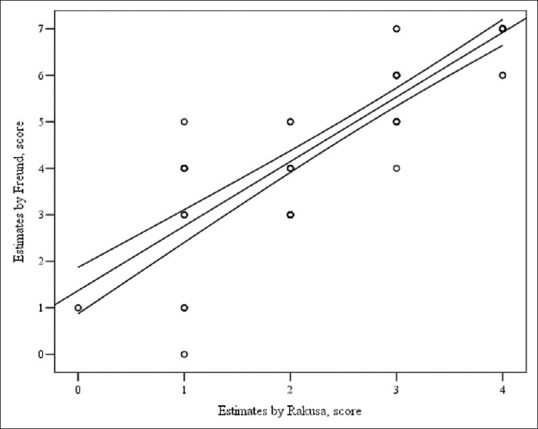
Correlation of estimates by Rakusa and Freund. Spearman's correlation coefficient, r = 0.9, P < 0.001. Y = 1.371 + 1.388x, were y: Estimates by Freung, x: Estimates by Rakusa
Eighteen (60%) NTG patients had completed the CDT by the specific picture drawing the short hand on 11 and the long hand between 11 and 12 [Fig. 2]. Such a specific drawing manner was seen only in 3 (10%) cataract patients. This difference was statistically significant (P = 0.001).
Figure 2.
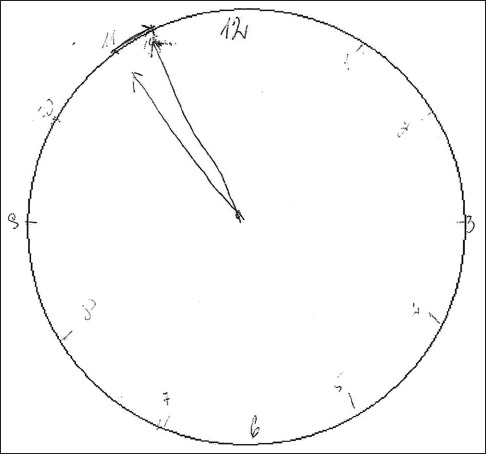
The specific drawing picture. Specific drawing character statistically significantly correlated with normal tension glaucoma diagnosis (Spearman's correlation coefficient 0.524, P = 0.001)
Discussion
According to the hypothesis that NTG and AD share common risk factors and pathogenesis,[5,6,7,8] we have performed quick cognitive function assessment with CDT and found lower CDT scores and higher probable cognitive dysfunction rate in NTG compared to cataract patients.
Other studies found similar results using different cognitive function assessment methods.[17,19,20] Yochim and colleagues examined 41 glaucoma patients (aged 70 years) and found memory impairment in 20% (measured by the California Verbal Learning Test) and executive functioning impairment in 22% (verbal fluency subtest of the Delis-Kaplan Executive Functioning System).[17] As 16% of American older adults have cognitive impairment in general, they have concluded that cognitive impairment may be common in older patients with glaucoma.[17] Jefferis et al. in the systemic meta-analysis determined that glaucoma patients were more likely to have lower standardized Mini-Mental State Examination (sMMSE) scores.[19] However, a significant relationship was lost when ordinal logistic regression was performed using the Mini-Mental State Exam Blind Version (MMblind test) scores. In MMblind test, items requiring vision are removed to give the blind version of the sMMSE. While Harabi et al. included 420 patients (aged 65) in their study and assessed that patients with glaucoma, AMD, Fuch's corneal dystrophy had statistically significantly lower cognitive scores than healthy controls (MMblind test).[20] However, Ong et al. found no significant associations between cognitive dysfunction and cataract, AMD, or glaucoma (Abbreviated Mental Test was applied for 1179 participants aged 60–80 years).[18]
We found that NTG patients drew CDT worse and their estimates were lower than those of the cataract group. Specific drawing seen in 60% of NTG patients may be associated with the loss of inhibition of the tendency to be pulled by perceptual features of the stimulus, or so called “frontal pull” (inhibition to stop after drawing first hand before starting another one). Accurate neuropsychological assessment should be suggested for patients with a suspicion of cognitive function decline.
Several studies showed correlation between cataract and cognitive impairment.[13,14,15,16] In our study, 16.7% of cataract patients using Freund scoring system and 50% using the more strict Rakusa system were scored lower than cutoff values and were recommended for more detailed neurological examination. Cataract group consisted of various manifestations of cataract: clinically insignificant cataract in both or in one eye, clinically significant cataract in one eye with other eye already treated. A more detailed grouping of these patients could influence CDT results. However, the subgrouping of cataract group might not be significant due to small sample size in our study. Jefferis et al. divided cataract patients into three groups: those with no recorded diagnosis of cataract, those with a history of previous cataract surgery, and those with a diagnosis of cataract but who had not had previous surgery, and compared these groups to each other in various relations.[19] They found that patients with cataract (treated and untreated) had higher sMMSE and MMblind scores compared to the no cataract group. These results disagree with the suggested theory of common cataract and cognitive impairment pathogenesis. It is supposed that better visual acuity after cataract surgery or corrective glasses does not improve cognition. It might be that patients with a diagnosis of cataract or those who had undergone cataract surgery were more likely to be socially active and seeking better vision.
To the best of our knowledge, we performed the first study that used CDT for cognitive function evaluation in NTG patients. An ideal cognitive screening test has some requirements, and CDT matches them all. Simple clock drawing is capable of evaluating several cognitive skills: (a) comprehension, (b) planning, (c) visual memory and reconstruction, (d) ability to concentrate, (e) motor programming, execution, (f) numbering, (g) semantic instruction, (h) inhibition of the tendency to be pulled by perceptual features of the stimulus, and (i) visuospatial abilities.
There are a lot of different CDT scoring systems.[22,25,26,27] Some of them are more precise and evaluate clock drawing in detail. In our opinion, the most essential feature of CDT is saving the time, so it is better to choose easier and quicker estimation systems as this is only a screening and not a diagnostic test. CDT is quick for subjects to perform, has high sensitivity and specificity and also correlates with MMSE – the gold standard for cognitive function screening.[28] Some authors suggest that users be cautious when patients with low education take the CDT, but giving them a predrawn circle precludes the influence by education level.[21] The weak part of CDT is that vision disorders may worsen the results because performance depends partly on the patient's vision. On the other hand, visual deterioration and limiting interaction should be considered as a distinct factor for cognitive dysfunction. We included patients with BCVA higher than 0.6 in the better eye. Mean BCVA of better eye was similar between the groups in our study (0.84 [0.15] and 0.8 [0.14] in cataract and glaucoma groups, respectively). Visual-field defects might have impact on CDT results, but they were not analyzed in our study.
CDT results, as well as results of other neuropsychological tests, can be influenced by poor patient motivation and understanding of the instructions arising from tiredness, self-doubt, physical, or mental disorders.[28]
A remaining, intriguing question is the quality of glaucoma treatment. It is important to know if patients have enough knowledge of their disease, whether they take prescribed medications in a correct way if they have cognitive dysfunction. Poor adherence to treatment may adversely affect preventable vision loss. The recommended treatment for glaucoma may be disrupted not only by cognitive function impairment but also by mental health complications. Depression was mentioned as one of the negative factors seen in patients who used lower doses of medicine than recommended.[29]
Performing detailed neuropsychological examination may help us find patients during preclinical AD stages. According to our results, cognitive function tests are easy to perform and reliable in detecting early cognitive impairment in NTG patients.
Conclusions
Normal tension glaucoma patients showed lower cognitive function scores and performed their test using a specific drawing style more often than those with cataracts. Further studies are needed to investigate the benefit of CDT as a fast screening test for cognitive function assessment in normal tension glaucoma patients and evaluating possible connections between normal tension glaucoma and other neurodegenerative diseases as AD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Factsheet N 282. [Last accessed on 2015 Jul 01]. Available from: http://www.who.int/mediacentre/factsheets/fs282/en .
- 3.Update on 2004 Background Paper, BP 6.11. Alzheimer Disease. [Last accessed on 2015 Jul 01]. Available from: http://www.who.int/medicines/areas/priority_medicines/BP6_11Alzheimer.pdf .
- 4.Khan TK, Alkon DL. Peripheral biomarkers of Alzheimer's disease. J Alzheimers Dis. 2015;44:729–44. doi: 10.3233/JAD-142262. [DOI] [PubMed] [Google Scholar]
- 5.Tsilis AG, Tsilidis KK, Pelidou SH, Kitsos G. Systematic review of the association between Alzheimer's disease and chronic glaucoma. Clin Ophthalmol. 2014;8:2095–104. doi: 10.2147/OPTH.S69534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer's disease. Ophthalmology. 1990;97:9–17. doi: 10.1016/s0161-6420(90)32621-0. [DOI] [PubMed] [Google Scholar]
- 7.Heaton GR, Davis BM, Turner LA, Cordeiro MF. Ocular biomarkers of Alzheimer's disease. Cent Nerv Syst Agents Med Chem. 2015;15:117–25. doi: 10.2174/1871524915666150319123015. [DOI] [PubMed] [Google Scholar]
- 8.Wostyn P, Audenaert K, De Deyn PP. Alzheimer's disease and glaucoma: Is there a causal relationship? Br J Ophthalmol. 2009;93:1557–9. doi: 10.1136/bjo.2008.148064. [DOI] [PubMed] [Google Scholar]
- 9.Baker ML, Wang JJ, Rogers S, Klein R, Kuller LH, Larsen EK, et al. Early age-related macular degeneration, cognitive function, and dementia: The Cardiovascular Health Study. Arch Ophthalmol. 2009;127:667–73. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT, et al. Is age-related maculopathy associated with Alzheimer's disease? The Rotterdam study. Am J Epidemiol. 1999;150:963–8. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- 11.Clemons TE, Rankin MW, McBee WL Age-Related Eye Disease Study Research Group. Cognitive impairment in the age-related eye disease study: AREDS report no 16. Arch Ophthalmol. 2006;124:537–43. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Nieto FJ, Moraes SA, Mosley TH, Couper DJ, et al. Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;134:828–35. doi: 10.1016/s0002-9394(02)01672-0. [DOI] [PubMed] [Google Scholar]
- 13.Tamura H, Tsukamoto H, Mukai S, Kato T, Minamoto A, Ohno Y, et al. Improvement in cognitive impairment after cataract surgery in elderly patients. J Cataract Refract Surg. 2004;30:598–602. doi: 10.1016/j.jcrs.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Gray CS, Karimova G, Hildreth AJ, Crabtree L, Allen D, O’connell JE, et al. Recovery of visual and functional disability following cataract surgery in older people: Sunderland cataract study. J Cataract Refract Surg. 2006;32:60–6. doi: 10.1016/j.jcrs.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Anstey KJ, Lord SR, Hennessy M, Mitchell P, Mill K, von Sanden C, et al. The effect of cataract surgery on neuropsychological test performance: A randomized controlled trial. J Int Neuropsychol Soc. 2006;12:632–9. doi: 10.1017/S1355617706060954. [DOI] [PubMed] [Google Scholar]
- 16.Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: A review of the literature. Br J Ophthalmol. 2011;95:17–23. doi: 10.1136/bjo.2009.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yochim BP, Mueller AE, Kane KD, Kahook MY. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J Glaucoma. 2012;21:250–4. doi: 10.1097/IJG.0b013e3182071b7e. [DOI] [PubMed] [Google Scholar]
- 18.Ong SY, Cheung CY, Li X, Lamoureux EL, Ikram MK, Ding J, et al. Visual impairment, age-related eye diseases, and cognitive function: The Singapore Malay Eye study. Arch Ophthalmol. 2012;130:895–900. doi: 10.1001/archophthalmol.2012.152. [DOI] [PubMed] [Google Scholar]
- 19.Jefferis JM, Taylor JP, Collerton J, Jagger C, Kingston A, Davies K, et al. The association between diagnosed glaucoma and cataract and cognitive performance in very old people: Cross-sectional findings from the newcastle 85+study. Ophthalmic Epidemiol. 2013;20:82–8. doi: 10.3109/09286586.2012.757626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrabi H, Kergoat MJ, Rousseau J, Boisjoly H, Schmaltz H, Moghadaszadeh S, et al. Age-related eye disease and cognitive function. Invest Ophthalmol Vis Sci. 2015;56:1217–21. doi: 10.1167/iovs.14-15370. [DOI] [PubMed] [Google Scholar]
- 21.Shulman KI. Clock-drawing: Is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–61. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Freund B, Gravenstein S, Ferris R, Burke BL, Shaheen E. Drawing clocks and driving cars. Use of brief tests of cognition to screen driving competency in older adults. J Gen Intern Med. 2005;20:240–4. doi: 10.1111/j.1525-1497.2005.40069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakusa M, Kogoj A. A clock drawing test-new scoring system. Eur J Neurol. 2007;14:66. [Google Scholar]
- 24.Lycke M, Ketelaars L, Boterberg T, Pottel L, Pottel H, Vergauwe P, et al. Validation of the Freund clock drawing test as a screening tool to detect cognitive dysfunction in elderly cancer patients undergoing comprehensive geriatric assessment. Psychooncology. 2014;23:1172–7. doi: 10.1002/pon.3540. [DOI] [PubMed] [Google Scholar]
- 25.Death J, Douglas A, Kenny RA. Comparison of clock drawing with mini mental state examination as a screening test in elderly acute hospital admissions. Postgrad Med J. 1993;69:696–700. doi: 10.1136/pgmj.69.815.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2nd ed. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 27.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer's disease by clock drawing. J Am Geriatr Soc. 1989;37:730–4. doi: 10.1111/j.1532-5415.1989.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 28.Assessment of Effort in Clinical Testing of Cognitive Functioning for Adults. The British Psychological Society. 2009. [Last accessed on 2017 Jun 01]. Available from: http://www.bps.org.uk/sites/default/files/documents/assessment_of_effort_in_clinical_testing_of_cognitive_functioning_for_adults.pdf .
- 29.Friedman DS, Okeke CO, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116:1097–105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]


