Abstract
Purpose
Enhancer of zeste homolog 2 (EZH2), a chromatin remodeler, is implicated in the pathogenesis of clear cell renal cell carcinoma (ccRCC). However, the effect of EZH2 on outcomes in localized ccRCC is unclear, and molecular biomarkers are not currently integrated into prognostic models or adjuvant therapy trials.
Methods
We performed Cox regression to evaluate the association of tumor-based EZH2 gene and protein expression with survival in three independent cohorts: a cohort from The Cancer Genome Atlas (n = 532), a cohort from University of Texas Southwestern Medical Center (n = 122), and a cohort from Mayo Clinic (n = 1,338). Analyses were adjusted for the prognostic stage, size, grade, and necrosis (SSIGN) score as well as within low-, intermediate-, and high-risk SSIGN groups.
Results
Patients in The Cancer Genome Atlas cohort with EZH2-high gene expression were 1.5 times more likely to experience overall death than patients with EZH2-low expression (95% CI, 1.1 to 2.3; P = .028). Patients in the University of Texas Southwestern Medical Center cohort with EZH2-high protein expression were two times more likely to experience overall death than patients with EZH2-low expression (95% CI, 1.1 to 4.4; P = .034). Similarly, patients in the Mayo Clinic cohort with EZH2-high protein expression were 1.4 times more likely to experience overall death (95% CI, 1.2 to 1.7; P < .001). Patients in the Mayo Clinic cohort with EZH2-high protein expression were nearly two times more likely to experience RCC-specific death (95% CI, 1.5 to 2.6; P < .001); EZH2 protein expression was particularly prognostic among patients with low-risk SSIGN tumors (HR, 6.1; 95% CI, 3.4 to 11.1; P < .001).
Conclusion
EZH2 expression accurately predicts risk of RCC death beyond existing clinicopathologic models, particularly in low- and intermediate-risk SSIGN tumors. Further studies are required to incorporate molecular biomarkers into surveillance guidelines and adjuvant clinical trials.
INTRODUCTION
Clear cell renal cell carcinoma (ccRCC) is the most common RCC subtype, and alterations of chromatin remodelers (BAP1, PBRM1, and SETD2) on chromosome 3p occur in 50% of ccRCCs.1-4 Our group identified that expression of these chromatin remodelers defines subgroups with divergent clinical outcomes.3,4 Building on these observations, investigators have identified enhancer of zeste homolog 2 (EZH2) expression as a prognostic factor in ccRCC. Unfortunately, studies evaluating the association of EZH2 and RCC outcomes have reported conflicting results, with higher EZH2 expression being associated with a lower risk of recurrence in one study,5 whereas the other study indicates an association with higher risk of death.5,6 Improved understanding of this putative association has important clinical implications. Validated prognostic models (eg, the Mayo Clinic stage, size, grade, and necrosis [SSIGN] score) and adjuvant therapy trials do not incorporate any molecular biomarkers. Even patients with low-risk disease as defined by these clinical algorithms can experience cancer recurrence.7,8 Indeed, despite adherence to surveillance guidelines after surgery, roughly one-third of primary ccRCC recurrences experience disease progression, indicating a clinical need to better identify those patients with RCC with a high risk of death, particularly those mischaracterized as low risk by current prognostic algorithms or with small renal tumors (≤ 4 cm).9
Motivated by these clinical needs in ccRCC and the conflicting data from two studies, we evaluated the association in three independent cohorts. First, we evaluated EZH2 mRNA expression and overall survival in The Cancer Genome Atlas cohort (TCGA; n = 532). Second, we performed an immunohistochemistry (IHC) assay for EZH2 that is compatible with formalin-fixed, paraffin-embedded (FFPE) tissue to evaluate the association of EZH2 protein expression and overall death in a pilot cohort of patients (n = 122) at the University of Texas Southwestern Medical Center (UTSW). Finally, we validated our findings in an independent cohort of patients at Mayo Clinic (n = 1,338). From all of these analyses, we validated that increased tumor-based EZH2 expression is associated with a higher risk of overall death for patients undergoing surgery for ccRCC. We also provide the first evidence, to our knowledge, that this association remains for cancer-specific survival and among the clinically important subset of patients with ccRCC classified as low and intermediate risk by the SSIGN score.
METHODS
UTSW Patient Selection and Study Design (analytic cohort)
The analytic cohort included a previously described set of 122 patients who underwent resection for ccRCC at UTSW with clinical data for age, sex, tumor grade, histology, and clinical outcomes.10,11 Exclusion criteria included patients receiving neoadjuvant systemic therapies. Institutional review board approval was obtained at UTSW. Dates of death were obtained from the electronic medical records and Social Security Death Index. Twenty-four patients (19.7%) were deceased, and the follow-up was 5.8 years (range, 0.0 to 15.7 years). One patient was missing data.
To standardize the cases for clinicopathologic variables, a genitourinary pathologist (P.K.) reviewed all of the tumors. A tissue microarray12 was generated with two to five, 1.0-mm punches to represent different morphologic patterns from each tumor.
Mayo Clinic Patient Selection and Study Design (validation cohort)
Inclusion criteria included patients who underwent resection for ccRCC with data for age, sex, tumor grade, histology, and clinical outcomes. Exclusion criteria included patients receiving neoadjuvant systemic therapies. Institutional review board approval was obtained at Mayo Clinic. We identified 1,360 patients who underwent resection for ccRCC between 1990 and 2009. Overall and RCC-specific death data were obtained from the Mayo Clinic Nephrectomy Registry. For RCC-specific survival, if the death was in a prior year, the death certificate was reviewed to determine the cause of death. In the event that the certificate was uninformative, correspondence with the patient’s local physician and/or family occurred to determine cause of death. Two hundred sixty patients (19.4%) were deceased, and the median follow-up among patients who were alive was 8.8 years (range, 0.01 to 21.1 years).
To standardize the cases, a genitourinary pathologist (J.C.) reviewed all of the cases and selected an FFPE tumor block with the highest tumor content and Fuhrman grade. The median FFPE block age was 14.4 years (range, 8.1 to 26.3 years).
EZH2 Protein Expression
Antigen retrieval and IHC staining were performed using a Dako Autostainer Link 48 (Agilent, Santa Clara, CA) with EnVision FLEX Target Retrieval Solution. Appropriate controls (positive and negative) were used with each run of immunostaining. The EZH2 monoclonal antibody (Ventana SP129, Spring Bioscience, CA; 1:2,500) is directed against human EZH2 expressed in human cancer cells.13,14 Infiltrating reactive lymphocytes served as internal positive controls.
Evaluation of EZH2 IHC Intensity by Visual and Digital (Aperio) Scoring
For pathologist visual scoring, two different manual methods were compared. A genitourinary pathologist (P.K.) measured the average percentage of cells staining across the entire slide and the average percentage of cells staining in any single ×10 hot spot field. For digital image analysis, slides were scanned using an Aperio ScanScope AT Turbo and reviewed using the eSlide Manager (version 12.0.0.5040) and ImageScope (version 12.1.0.5029) systems (Leica Biosystems, Melbourne, Australia). The GENIE (version 11.2) histomorphology image analysis tool was trained to annotate tumor areas. Representative tumor regions were manually selected (tumor necrosis areas were avoided) to exclude regions where GENIE inadequately identified tumor cells. The GENIE Nuclear Algorithm (version 11.2) was used to select tumor areas for quantitative analysis, yielding a percentage of tumor nuclei positive for EZH2.
Statistical Analyses
To assess categorical variables across groups, the χ2 or Fisher’s exact test was used, and continuous variables were analyzed via Wilcoxon rank-sum or Kruskal-Wallis test. To assess the association of EZH2 expression with outcome, Cox proportional hazards models were used. Cox model assumptions were evaluated by assessing the parallel alignment of the log-log survival curves; no violation was observed. Overall survival was calculated from time of surgery to death as a result of any cause. Cancer-specific survival was calculated from time of surgery to RCC-related death; patients were censored if they were lost to follow-up or died of other causes. Time-dependent receiver operating characteristic curves were used to evaluate prognostic performance; the area under the curve (AUC) was calculated at 1, 2, 5, 10, and 15 years of follow-up.
To dichotomize mRNA and protein expression, a cut point was chosen that maximized the concordance index (C-index). In doing so, for EZH2 mRNA expression in the TCGA cohort, tumors with > 5.36 and ≤ 5.36 (expectation-maximization normalized counts) were categorized as having high and low expression, respectively. For the UTSW cohort, tumors with > 8.0% and ≤ 8.0% EZH2-positive nuclei were categorized as having high and low expression, respectively. For the Mayo cohort, tumors with > 8.7% and ≤ 8.7% EZH2-positive nuclei were categorized as having high and low expression, respectively. To assess predictive ability, bootstrap methodology was used to obtain optimism-corrected estimates of the C-index.15 Statistical analyses were performed using R (version 3.2.3). P values < .05 were considered statistically significant.
RESULTS
Association of EZH2 mRNA Expression With Overall Survival in TCGA ccRCC Data Set
In the TCGA cohort, 65% of patients were male, the median age was 61 years, and the median tumor size was 5.5 cm (Table 1). Continuous EZH2 expression was significantly associated with overall survival after adjusting for age (hazard ratio [HR], 2.26; 95% CI, 1.69 to 3.02; P < .001; Table 2). When modeled as a dichotomized variable, patients with EZH2-high mRNA expression were 2.56 times more likely to experience death than patients with EZH2-low expression (95% CI, 1.85 to 3.54; P < .001). After adjustment for age and SSIGN, patients with EZH2-high mRNA expression were 1.54 times more likely to experience death than patients with EZH2-low expression (95% CI, 1.05 to 2.27; P = .028). The association of age and SSIGN with survival is provided in Appendix Table A1 (online only).
Table 1.
Clinical Characteristics of TCGA, UTSW, and Mayo Clinic Cohorts
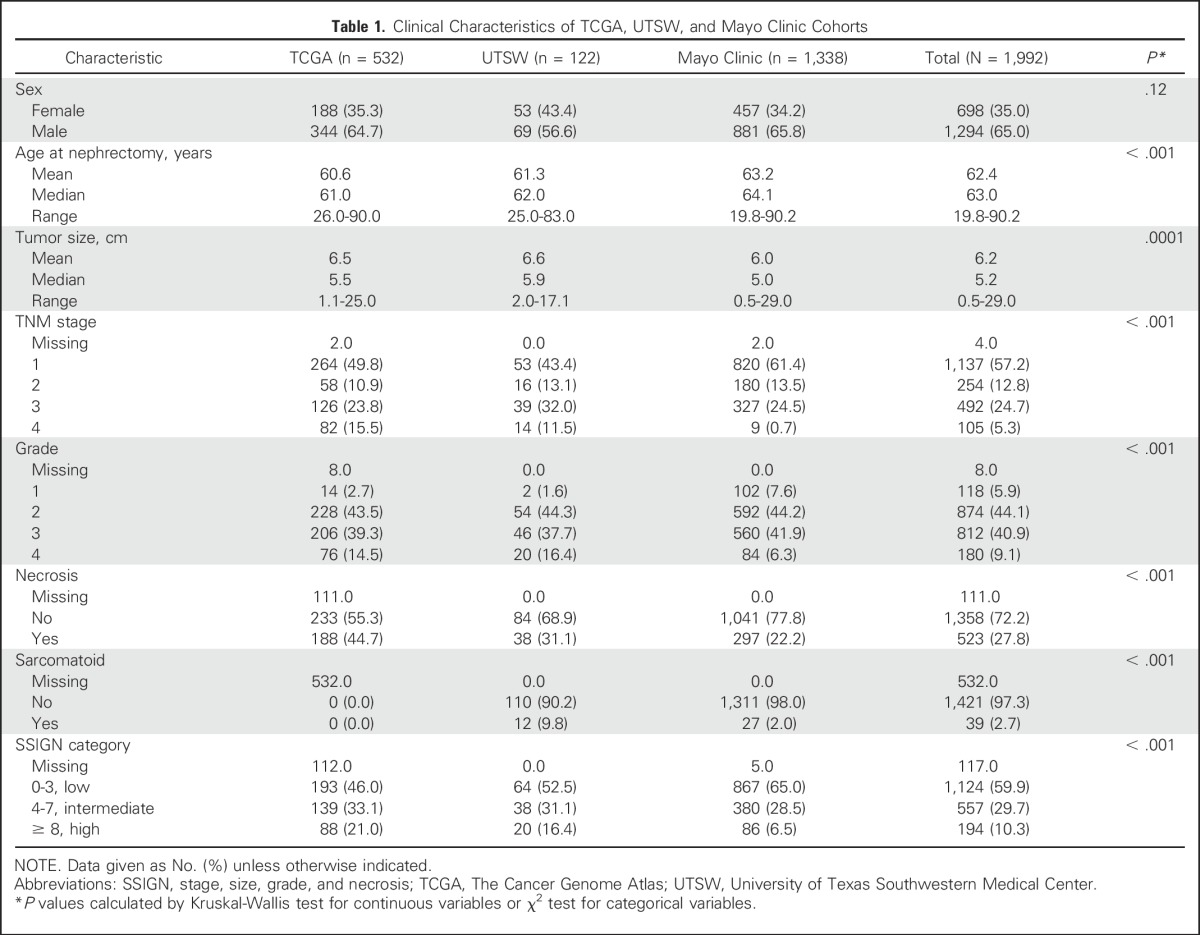
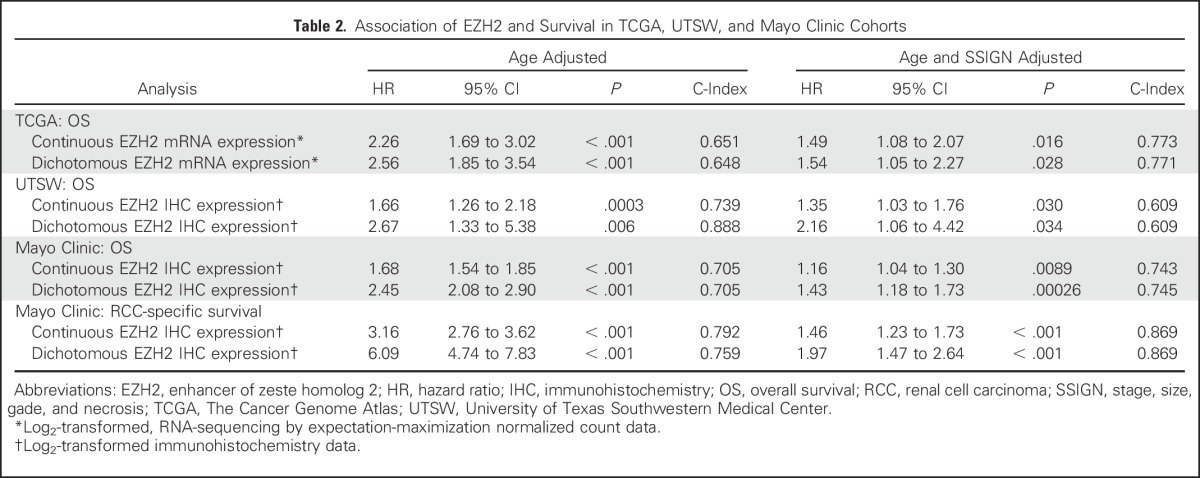
Table 2.
Association of EZH2 and Survival in TCGA, UTSW, and Mayo Clinic Cohorts
Correlation Between EZH2 mRNA Expression and EZH2 IHC Staining in RCC Tumor Grafts
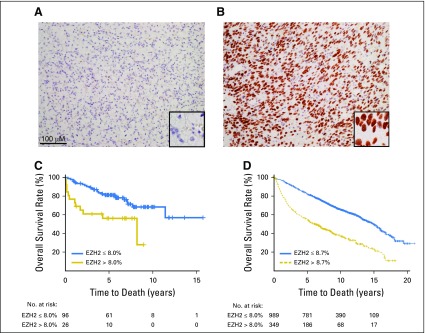
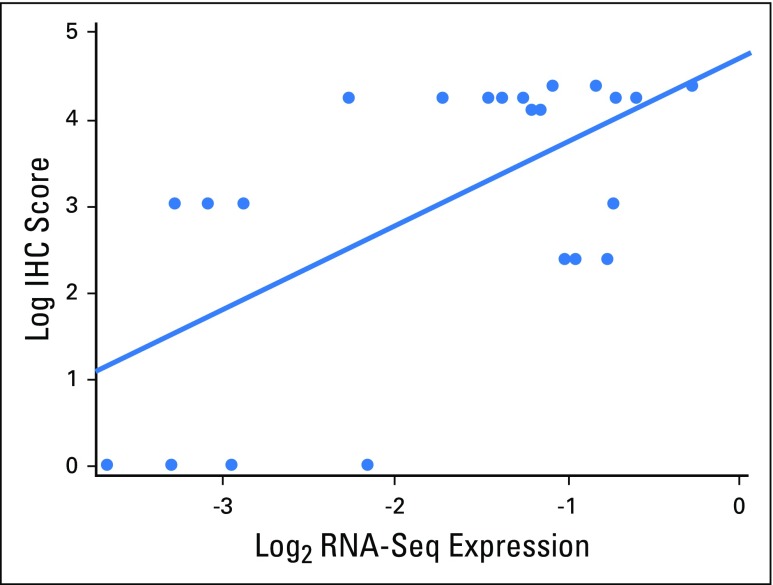
Given the availability of IHC platforms in clinical laboratories, we next evaluated the correlation between EZH2 mRNA and protein expression, leveraging 23 RCC tumorgraft samples for which we published RNA-seq results (n = 9).16 We optimized an EZH2 IHC assay in FFPE using an antibody validated for in vitro diagnostic use.13 A genitourinary pathologist blinded to the clinical results and RNA sequencing data recorded the percentages of positive tumor nuclei in the entire section. As expected, EZH2 had a nuclear expression pattern (Figs 1A and 1B). Benign kidney was negative. We observed a correlation between EZH2 mRNA expression and IHC intensity (Pearson correlation, 0.60; Appendix Fig A1, online only) in 23 samples from published tumorgrafts.16
Fig 1.
Enhancer of zeste homolog 2 (EZH2) immunohistochemical staining and Kaplan-Meier estimate of overall survival in independent analytic and validation cohorts. Representative images for EZH2 staining: (A) negative (≤ 8.7%) and (B) positive (> 8.7%) in renal cell carcinoma. Scale bar, 100 μM. (Original magnification, ×200; inset of representative nuclei, ×400.) Overall survival by EZH2 expression in (C) analytic and (D) validation cohorts.
Clinicopathologic Characteristics of Analytic (UTSW) and Validation (Mayo Clinic) Cohorts
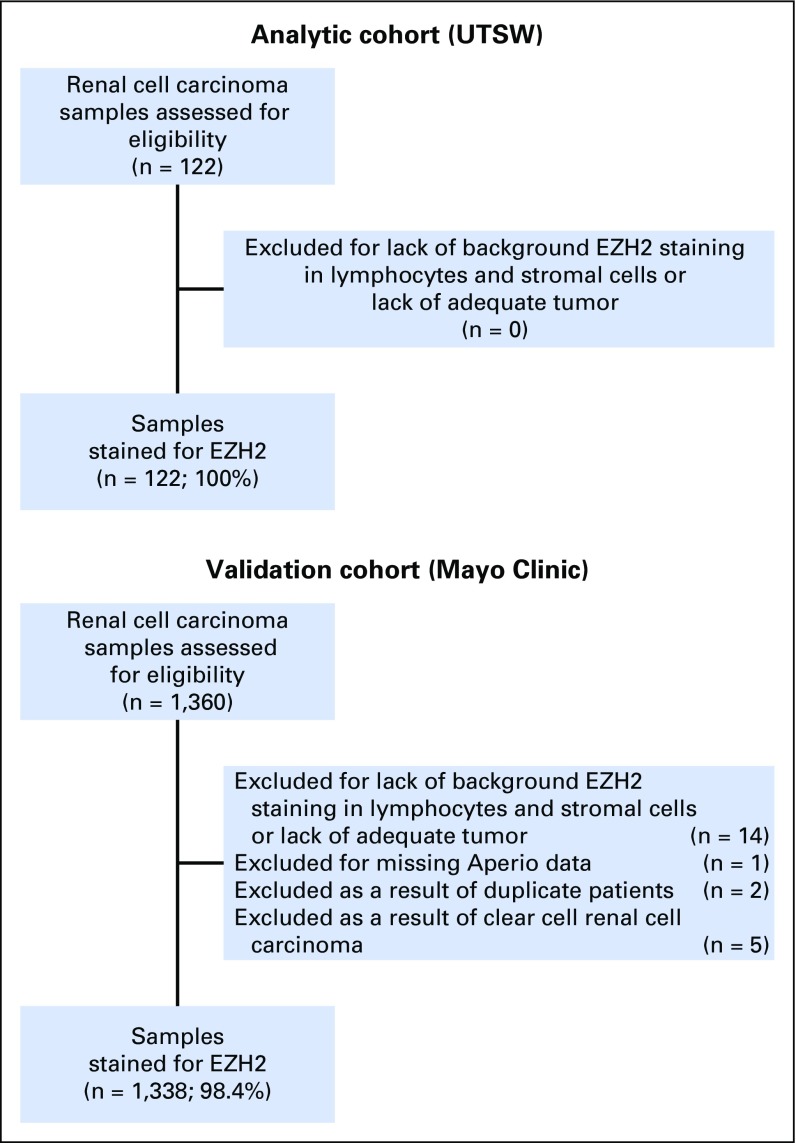
We next evaluated the association of EZH2 protein expression and survival using two cohorts. In the UTSW cohort, 57% of patients were male, the median age was 62 years, the median tumor size was 5.9 cm, and 53% of the tumors had low-risk SSIGN scores (Table 1). Of the 1,360 patients evaluated in the Mayo cohort, 1,338 (98.4%) were analyzed by EZH2 IHC (Appendix Fig A2, online only). In the Mayo Clinic cohort, 66% of patients were male, the median age was 64 years, the median tumor size was 5.0 cm, and 65% of the tumors had low-risk SSIGN scores (Table 1). In both cohorts, EZH2-high expression was associated with increased tumor size, higher TNM stage, higher tumor grade, presence of necrosis, and sarcomatoid de-differentiation (Appendix Tables A2 and A3, online only).
Association of EZH2 and Clinical Outcomes (UTSW cohort)
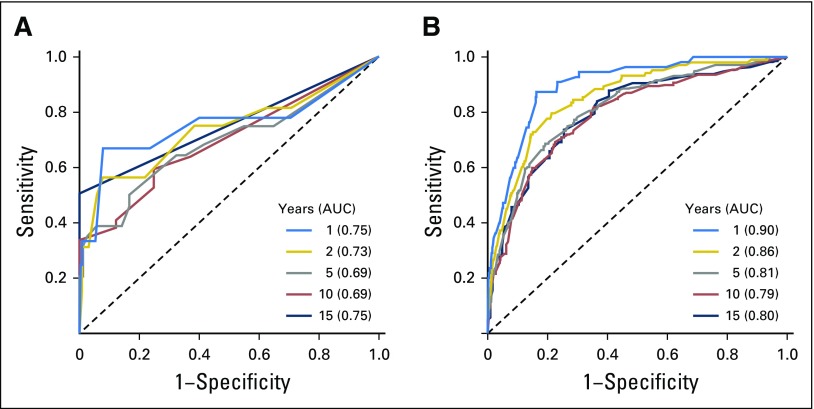
To examine the association between EZH2 protein expression by IHC (average percentage of cells staining across the entire slide) and overall survival, we modeled EZH2 expression as a continuous and dichotomous variable. Continuous EZH2 protein expression was significantly associated with overall survival after adjusting for age (HR, 1.66; 95% CI, 1.26 to 2.18; P = .0003; Table 2). When modeled as a dichotomized variable, patients with EZH2-high protein expression were 2.67 times more likely to experience death than patients with EZH2-low expression (95% CI, 1.33 to 5.38; P = .006) after adjusting for age (Fig 1C). After adjustment for age and SSIGN, patients with EZH2-high protein expression were 2.16 times more likely to experience death than patients with EZH2-low expression (95% CI, 1.06 to 4.42; P = .034). The prognostic performance of EZH2 was evaluated at 1, 2, 5, 10, and 15 years of follow-up using time-dependent receiver operating characteristic curves (Appendix Fig A3A, online only); the AUC ranged from 0.69 to 0.75.
Association of EZH2 and Clinical Outcomes (Mayo cohort)
To validate the association between EZH2 protein expression and overall survival, we evaluated EZH2 expression in an independent cohort. After adjusting for age, continuous EZH2 protein expression was significantly associated with overall survival (HR, 1.68; 95% CI, 1.54 to 1.85; P < .001; Table 2). When modeled as a dichotomized variable, patients with EZH2-high protein expression were 2.45 times more likely to experience death than patients with EZH2-low expression (95% CI, 2.08 to 2.90; P < .001) after adjusting for age (Fig 1D). After adjusting for age and SSIGN, patients with EZH2-high protein expression were 1.43 times more likely to experience death than patients with EZH2-low expression (95% CI, 1.18 to 1.73; P = .00026). The addition of EZH2 expression to a model with SSIGN and age improved the C-index to 0.745, from 0.689 for SSIGN alone and 0.740 for SSIGN and age (Table 2 and Appendix Table A1). The AUC at 1, 2, 5, 10, and 15 years of follow-up ranged from 0.79 to 0.90 (Appendix Fig A3B).
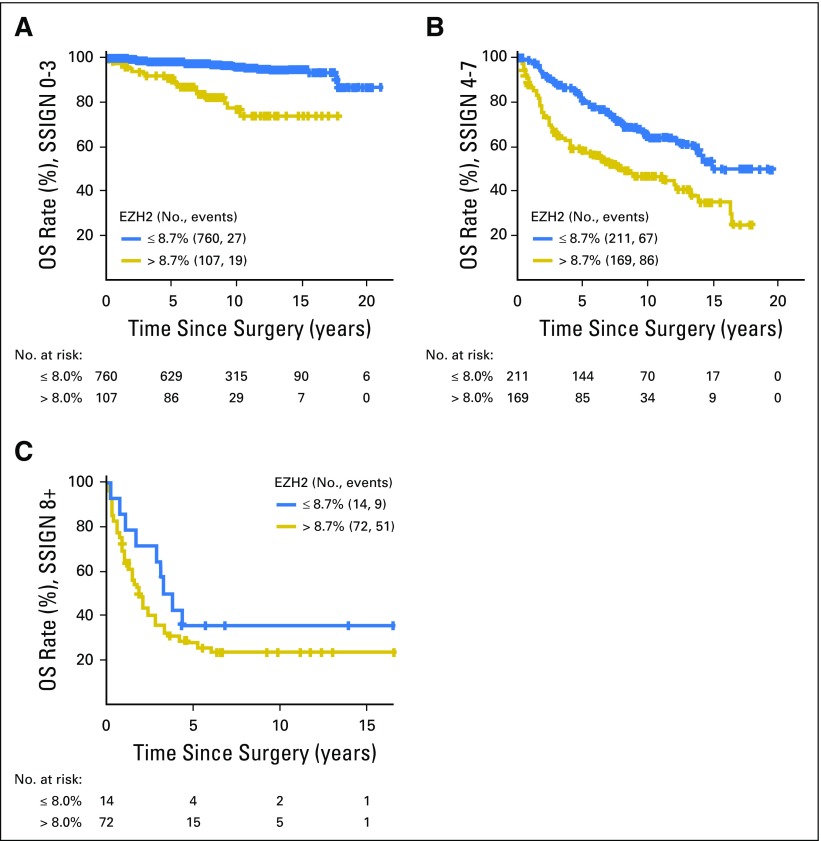
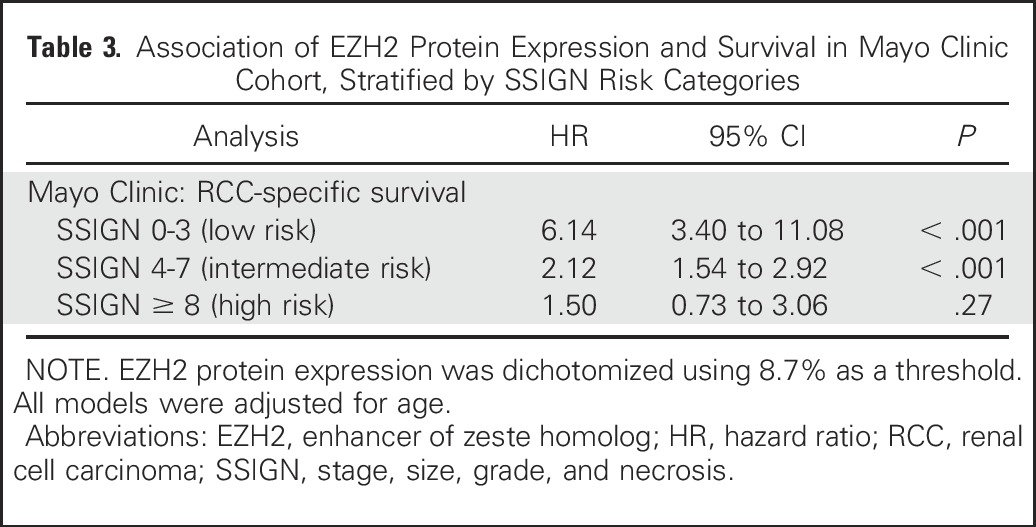
To follow Reporting Recommendations for Tumor Marker Prognostic Studies guidelines,17 we next examined the effect of dichotomized EZH2 protein expression in the context of RCC-specific survival and standard clinicopathologic variables. After adjusting for age, SSIGN score–classified low-risk patients with high EZH2 protein expression were 6.14 times more likely to experience RCC-specific death than patients with EZH2-low tumors (HR, 6.14; 95% CI, 3.40 to 11.08; P < .001; Fig 2A, Table 3). Similarly, among patients with intermediate-risk SSIGN tumors, patients with high EZH2 protein expression were 2.12 times more likely to experience RCC-specific death (95% CI, 1.54 to 2.92; P < .001). We did not observe a significant association among high-risk SSIGN tumors (P = .27).
Fig 2.
Kaplan-Meier estimate of overall survival (OS) by dichotomized enhancer of zeste homolog 2 (EZH2) expression in patients stratified by Mayo stage, size, grade, and necrosis (SSIGN) scores. (A) low-risk, (B) intermediate-risk, and (C) high-risk scores.
Table 3.
Association of EZH2 Protein Expression and Survival in Mayo Clinic Cohort, Stratified by SSIGN Risk Categories
Comparison of EZH2 IHC Staining Quantitation Between Two Visual Scoring Methods As Well As With a Computer-Assisted Digital Image Analysis
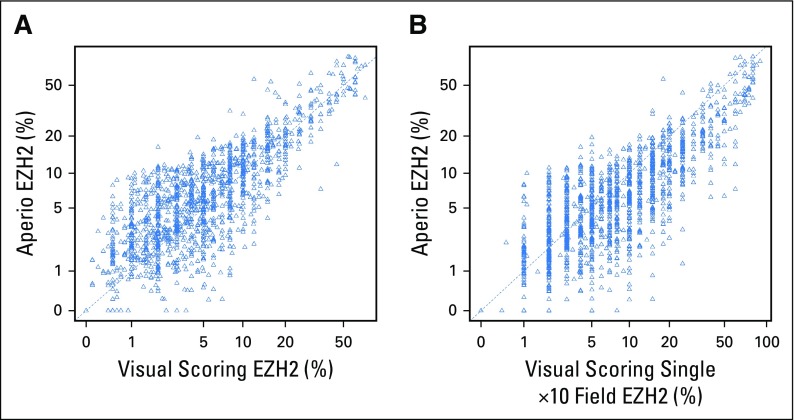
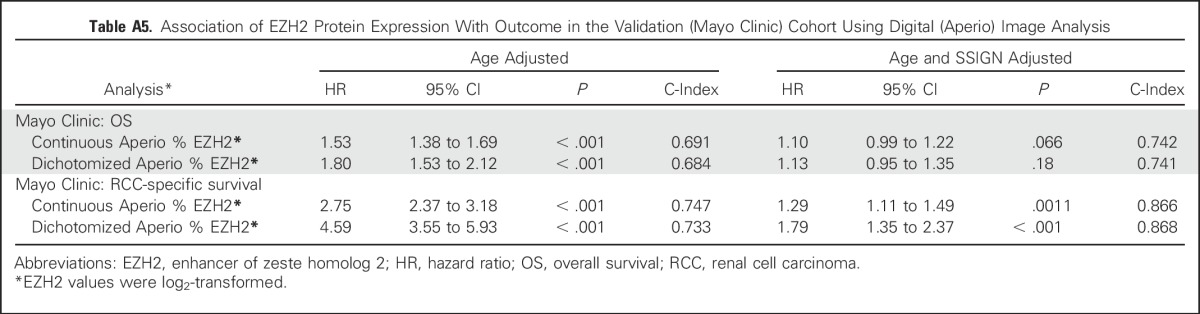
Using the Mayo cohort, we first compared the Cox model results using the average visual percentage of cells staining across the entire slide and the average visual percentage of staining in any single ×10 field. As shown in Appendix Table A4 (online only), the hazard rates are similar across the two visual scoring methods. Similarly, we ran the Cox models using the Aperio digital quantification. The quantification was highly correlated with the average visual percentage of cells staining across the entire slide (Pearson correlation = 0.88; Appendix Fig A4A, online only). Similarly, the quantification was highly correlated with the highest visual percentage of staining in a single 10× field (Pearson correlation = 0.86; Appendix Fig A4B). The Cox model hazard rates for the Aperio quantification are provided in Appendix Table A5 (online only).
DISCUSSION
Although preclinical models implicate EZH2 in tumorigenesis, there are conflicting results in the literature regarding the prognostic effect of EZH2 protein expression on the outcomes of patients with RCC.5,6,18-22 Indeed, some authors report that increased EZH2 expression is associated with a lower risk of recurrence, whereas others report an association with a higher risk of death following surgery for RCC.5,6 One explanation for these contrasting observations is that differences in EZH2 antibody epitopes may influence EZH2 detection and quantitation. To improve on this, we used an antibody that is already validated for in vitro diagnostics. Furthermore, the prior studies were limited by a small sample size (ie, < 200 patients) and failed to adjust for clinicopathologic factors associated with RCC outcome. In our study, we included three independent cohorts (total of 1,992 tumors) with centralized pathology review to report that EZH2 expression is associated with higher risk of poor outcome following surgery, in a univariate setting, and after adjusting for clinicopathologic prognostic factors. Our observations using a single-slide IHC assay in ccRCC include the following: (1) concordance of EZH2 IHC expression data between a digital Aperio and pathologist visual scoring; (2) an association between increased EZH2 expression and poor outcomes (overall survival) in three independent cohorts, even after adjusting for the Mayo SSIGN score; and (3) EZH2 protein expression can further stratify patients into prognostic groups within both low- and intermediate-risk patients (as defined by the SSIGN score).
The association of higher tumor-based EZH2 protein expression with a greater risk of RCC-specific death suggests a link between EZH2 and tumor progression. Related to this, increased EZH2 expression is mechanistically associated with loss of tumor suppressors (p27KIP1) and tumor invasion.19,20,22 Of clinical interest, in contrast to the 3p chromatin remodelers that are challenging to therapeutically target given the loss of function, an increase in EZH2 enzymatic activity could be directly targeted with EZH2 inhibitors such as tazemetostat23 or CPI-1205.24
Although our study was designed to address limitations of previous investigations, there are limitations of our own work that should be considered. First, we evaluated EZH2 expression in tissue microarrays (analytic cohort) and whole slides (validation cohort). Related to this, intratumor heterogeneity occurs in RCC, and prognostic biomarkers may be influenced by tumor sampling or inter-rater variability.25,26 We used the Aperio platform to systematically assess EZH2 staining and found that the results had a high correlation with pathologist scoring. Furthermore, EZH2 protein expression in both the whole slide and a single ×10 field was associated with a higher risk of death. Second, the median duration of follow-up in the analytic cohort was relatively short, at 5.8 years (range, 0.0 to 15.7 years). By comparison, for the validation cohort, the median duration of follow-up was 8.8 years (range, 0.01 to 21.1 years). Finally, from a purely prognostic standpoint, the positive association of higher EZH2 expression with risk of poor outcome was attenuated among high-risk SSIGN score ≥ 8 tumors, suggesting that adverse pathologic variables are already a strong predictor of poor outcomes. This is logical, given that the pathology-based SSIGN score is a surrogate measure for the underlying molecular mechanisms that drive tumor progression. Therefore, the importance of our findings is underscored by the fact that they support EZH2 as a target (and potentially a companion biomarker) for novel therapeutic approaches to improve outcomes of patients with RCC.
Authors of two prior studies, A 16-Gene Assay to Predict Recurrence After Surgery in Localised Renal Cell Carcinoma27 (Recurrence Score), and A Prognostic Risk Predictor for Localized Clear Cell Renal Cell Carcinoma28 (ClearCode34), have evaluated prognostic RNA expression signatures. The Recurrence Score is associated with a three-fold increase in risk of recurrence. Similarly, ClearCode34 subtypes are associated with a two-fold increase in risk of recurrence. The importance of these pioneering observations notwithstanding, RNA-based assays may be affected by selection bias because larger amounts of RNA are required for the assay, necessitating access to larger tumors and potentially biasing results away from smaller ones. Indeed, a 20.2% failure rate for adequate RNA yield/assay performance was reported in the ClearCode34 study. In contrast, the EZH2 IHC assay uses a monoclonal antibody validated for in vitro diagnostics, requires only a single slide (1.6% assay failure rate), and is compatible with an imaging platform that is available in clinical laboratories.
The rise in RCC incidence over the past three decades, particularly of small (≤ 4 cm) tumors, can be partially attributed to the widespread use of imaging.29 Despite adherence to surveillance guidelines, one-third of patients with RCC experience disease progression following surgery, underscoring the need to identify patients with a higher risk of recurrence, particularly those with smaller tumors.9 Of note, the design of two adjuvant RCC trials, A Clinical Trial Comparing Efficacy and Safety of Sunitinib Versus Placebo for the Treatment of Patients at High Risk of Recurrent Renal Cell Cancer30 (S-TRAC), and ASSURE: Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma31 (ECOG-ACRIN E2805) incorporate clinicopathologic models to identify high-risk patients, but do not incorporate molecular alterations. Moreover, although subsets of patients with RCC are excluded from adjuvant studies because their tumors would be stratified as low risk, we show that patients classified as low risk by the SSIGN score who have EZH2-high expression are six times more likely to die of RCC compared with patients with EZH2-low expression. Thus, there is an opportunity to leverage this biomarker to design more robust clinical trials that are focused on those patients with aggressive disease.
In conclusion, in this study, we validated the association between increased EZH2 expression and a higher risk of death in three independent cohorts after adjusting for the SSIGN score. The association is particularly significant in patients classified as low or intermediate risk by the SSIGN score. With the increasing incidence of small RCC tumors detected by cross-sectional imaging, our study emphasizes the clinical utility of a biomarker that is compatible with a single FFPE slide that accurately predicts risk of RCC death beyond existing clinicopathologic models. Further studies are required to determine how to better incorporate molecular biomarkers with prognostic information into surveillance guidelines and adjuvant clinical trials.
ACKNOWLEDGMENT
We acknowledge the Mayo Clinic Cancer Center Biospecimens Accessioning and Processing Core and Pathology Research Core. We thank all patients and their families for their contributions to this study.
Appendix
METHODS
Analysis of Enhancer of Zeste Homolog 2 mRNA Expression in The Cancer Genome Atlas Data Set
The Cancer Genome Atlas kidney renal clear cell carcinoma RNA sequencing level 3 data (expectation-maximization normalized data) were downloaded from Broad GDAC Firehose (https://confluence.broadinstitute.org/display/GDAC/Home). The clinical data were downloaded from The Cancer Genome Atlas on May 19, 2017 (https://tcga-data.nci.nih.gov/tcga/).
Analysis of Enhancer of Zeste Homolog 2 mRNA in Tumorgrafts
To determine if enhancer of zeste homolog 2 (EZH2) RNA levels identified from RNA-seq analysis translate to protein changes, we conducted a pilot EZH2 immunohistochemistry analysis on 23 University of Texas Southwestern Medical Center samples from renal cell carcinoma tumorgrafts. RNA sequencing data are available at the Sequence Read Archive (SRP073253).
Fig A1.
Correlation between enhancer of zeste homolog 2 immunohistochemistry (IHC) protein analysis and enhancer of zeste homolog 2 mRNA expression by RNA sequencing (RNA-Seq).
Fig A2.
Flow diagram of enhancer of zeste homolog 2 (EZH2) immunohistochemistry analysis in the analytic and validation cohorts. UTSW, University of Texas Southwestern Medical Center.
Fig A3.
Time-dependent receiver operating characteristic curve with regard to enhancer of zeste homolog 2 expression for (A) analytic and (B) validation cohorts. AUC, area under the curve.
Fig A4.
Correlation of enhancer of zeste homolog 2 (EZH2) immunohistochemical staining quantitation between digital image analysis and pathologist visual scoring in the validation cohort. (A) Analysis of average percentage of cells staining positive for EZH2 across an entire slide. (B) Analysis of average percentage of cells staining positive for EZH2 in a single ×10 field. The dotted line represents equality.
Table A1.
Association of Age and SSIGN With Survival
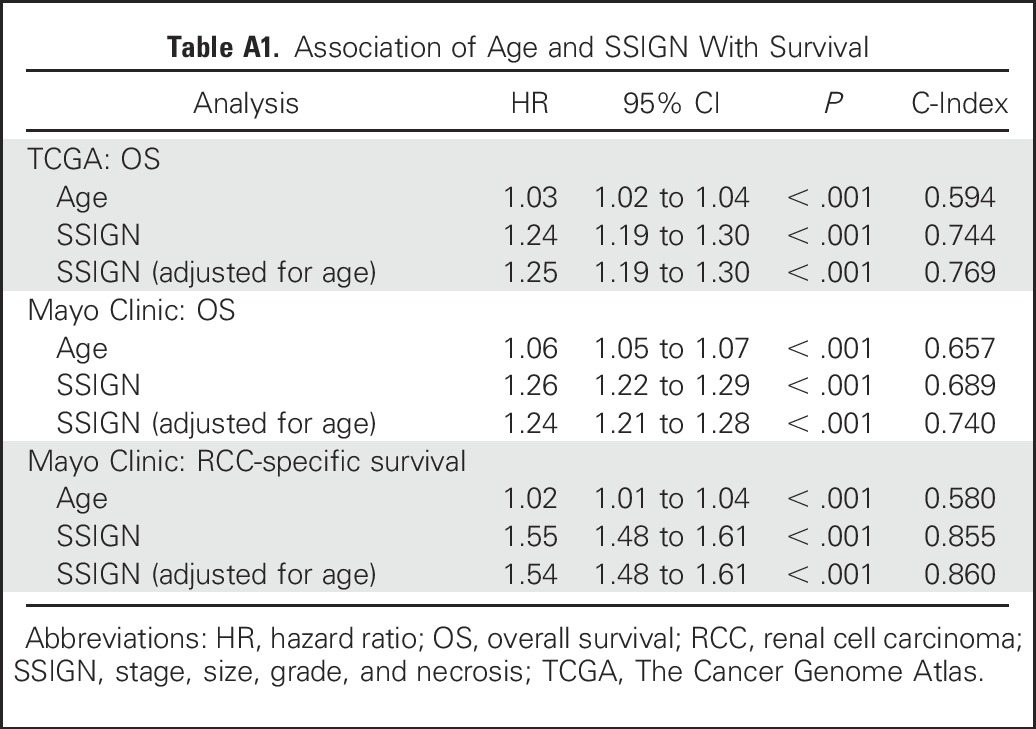
Table A2.
Clinical Characteristics of University of Texas Southwestern Medical Center Cohort by Dichotomized EZH2 Expression
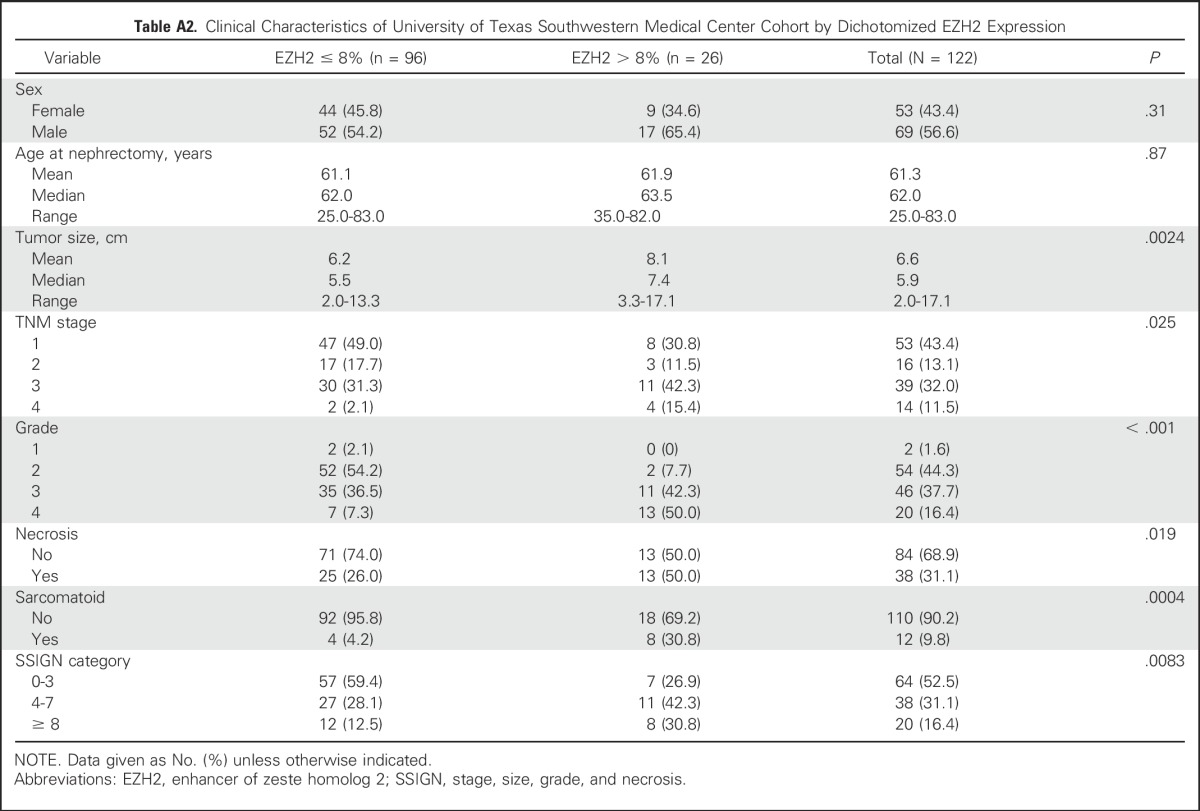
Table A3.
Clinical Characteristics of Mayo Clinic Cohort by Dichotomized EZH2 Expression
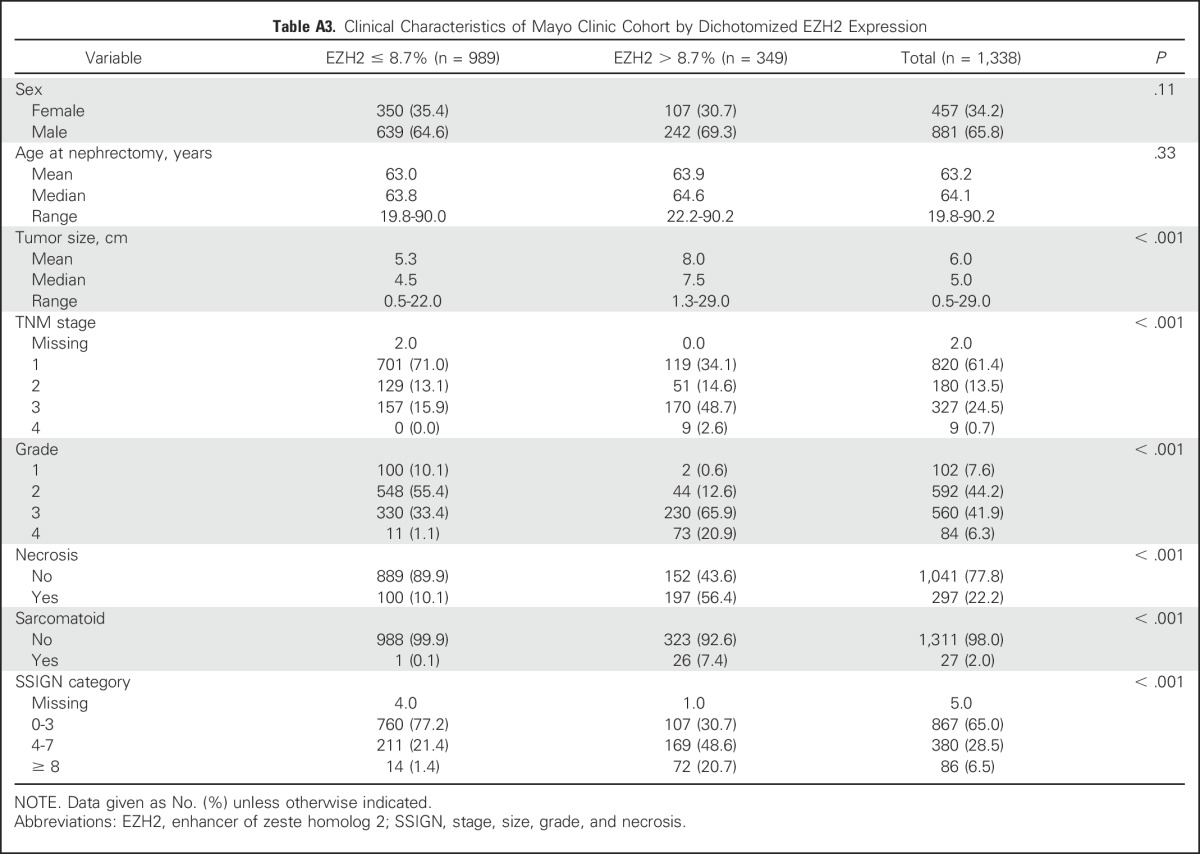
Table A4.
Association of Continuous EZH2 Protein Expression With Outcome in the Validation (Mayo Clinic) Cohort for Two Different Visual Scoring Methods
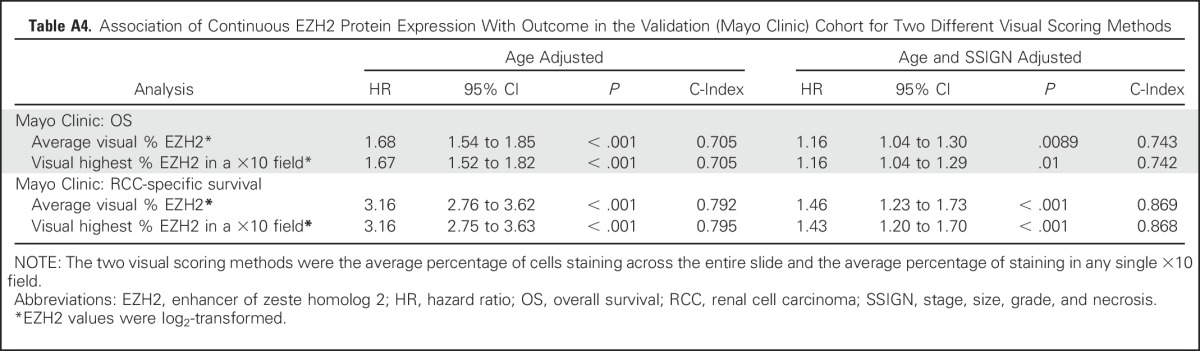
Table A5.
Association of EZH2 Protein Expression With Outcome in the Validation (Mayo Clinic) Cohort Using Digital (Aperio) Image Analysis
Footnotes
Supported by the Mayo Clinic Center for Individualized Medicine Clinomics Program (T.H.H.); a Kathryn H. and Roger Penske Career Development Award to Support Medical Research (T.H.H.); Gerstner Family Career Development Award (T.H.H.); the National Cancer Institute (K12CA90628 to T.H.H., R01CA134466 to A.S.P., P50CA196516 to P.K., P50CA196516 and R01CA175754 to J.B., and R21CA176422 to J.E.E.); Cancer Prevention Research Institute of Texas (RP160440 to J.B.). The funding agencies had no role in the study design. Support was also provided by the Gloria A. and Thomas J. Dutson Jr Kidney Research Endowment, and by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Cancer Research Program, under Award No. W81XWH-17-1-0546. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
AUTHOR CONTRIBUTIONS
Conception and design: Thai Huu Ho, Payal Kapur, Jeanette E. Eckel-Passow, Richard W. Joseph, Daniel J. Serie, R. Houston Thompson, James Brugarolas, Alexander S. Parker
Collection and assembly of data: Thai Huu Ho, Payal Kapur, Jeanette E. Eckel-Passow, Daniel J. Serie, John C. Cheville, R. Houston Thompson, Alexander S. Parker
Data analysis and interpretation: Thai Huu Ho, Payal Kapur, Jeanette E. Eckel-Passow, Alana Christie, Daniel J. Serie, Farrah Homayoun, Vandana Panwar, James Brugarolas, Alexander S. Parker
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter Validation of Enhancer of Zeste Homolog 2 Expression as an Independent Prognostic Marker in Localized Clear Cell Renal Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Thai Huu Ho
No relationship to disclose
Payal Kapur
No relationship to disclose
Jeanette E. Eckel-Passow
Patents, Royalties, Other Intellectual Property: Breast cancer PDX models
Alana Christie
No relationship to disclose
Richard W. Joseph
Consulting or Advisory Role: Bristol-Myers Squibb, Nektar, Genoptix, Eisai, Novartis, Merck (Inst), Exelixis, Incyte
Research Funding: Merck, Bristol-Myers Squibb, Genentech (Inst), X4P (Inst), Amgen (Inst)
Daniel J. Serie
No relationship to disclose
John C. Cheville
No relationship to disclose
R. Houston Thompson
Patents, Royalties, Other Intellectual Property: Patent for B7-H1 and survivin as prognostic markers in renal cell carcinoma.
Farrah Homayoun
No relationship to disclose
Vandana Panwar
No relationship to disclose
James Brugarolas
Consulting or Advisory Role: Nektar
Research Funding: Genentech, Peloton Therapeutics
Patents, Royalties, Other Intellectual Property: Patent on biomarkers of HIF-2 dependency.
Alexander S. Parker
No relationship to disclose
REFERENCES
- 1.Ho TH, Park IY, Zhao H, et al. : High-resolution profiling of histone h3 lysine 36 trimethylation in metastatic renal cell carcinoma. Oncogene 35:1565-1574, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph RW, Kapur P, Serie DJ, et al. : Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer 120:1059-1067, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph RW, Kapur P, Serie DJ, et al. : Clear cell renal cell carcinoma subtypes identified by BAP1 and PBRM1 expression. J Urol 195:180-187, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho TH, Kapur P, Joseph RW, et al. : Loss of histone H3 lysine 36 trimethylation is associated with an increased risk of renal cell carcinoma-specific death. Mod Pathol 29:34-42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz S, Weikert S, Magheli A, et al. : Expression profile of the polycomb group protein enhancer of Zeste homologue 2 and its prognostic relevance in renal cell carcinoma. J Urol 182:2920-2925, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Wagener N, Macher-Goeppinger S, Pritsch M, et al. : Enhancer of zeste homolog 2 (EZH2) expression is an independent prognostic factor in renal cell carcinoma. BMC Cancer 10:524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zigeuner R, Hutterer G, Chromecki T, et al. : External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. Eur Urol 57:102-109, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Frank I, Blute ML, Cheville JC, et al. : An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J Urol 168:2395-2400, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Stewart SB, Thompson RH, Psutka SP, et al. : Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol 32:4059-4065, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapur P, Peña-Llopis S, Christie A, et al. : Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: A retrospective analysis with independent validation. Lancet Oncol 14:159-167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. : BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 44:751-759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwish OM, Kapur P, Youssef RF, et al. : Cumulative number of altered biomarkers in mammalian target of rapamycin pathway is an independent predictor of outcome in patients with clear cell renal cell carcinoma. Urology 81:581-586, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Jacobs C, Tumati V, Kapur P, et al. : DOC-2/DAB2 interacting protein status in high-risk prostate cancer correlates with outcome for patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 89:729-735, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs C, Tumati V, Kapur P, et al. : Pretreatment biopsy analysis of DAB2IP identifies subpopulation of high-risk prostate cancer patients with worse survival following radiation therapy. Cancer Med 4:1844-1852, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361-387, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Hill H, Christie A, et al. : Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539:112-117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McShane LM, Altman DG, Sauerbrei W, et al. : Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23:9067-9072, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Xu Z, Zhong L, et al. : Prognostic value of EZH2 expression and activity in renal cell carcinoma: A prospective study. PLoS One 8:e81484, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Xu Z, Zhong L, et al. : Enhancer of zeste homolog 2 (EZH2) promotes tumour cell migration and invasion via epigenetic repression of E-cadherin in renal cell carcinoma. BJU Int 117:351-362, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Adelaiye R, Ciamporcero E, Miles KM, et al. : Sunitinib dose escalation overcomes transient resistance in clear cell renal cell carcinoma and is associated with epigenetic modifications. Mol Cancer Ther 14:513-522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang T, Wang Y, Zhou F, et al. : Prognostic value of high EZH2 expression in patients with different types of cancer: A systematic review with meta-analysis. Oncotarget 7:4584-4597, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai T, Bilim VN, Ugolkov AV, et al. : The enhancer of zeste homolog 2 (EZH2), a potential therapeutic target, is regulated by miR-101 in renal cancer cells. Biochem Biophys Res Commun 422:607-614, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Knutson SK, Kawano S, Minoshima Y, et al. : Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther 13:842-854, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Garapaty-Rao S, Nasveschuk C, Gagnon A, et al. : Identification of EZH2 and EZH1 small molecule inhibitors with selective impact on diffuse large B cell lymphoma cell growth. Chem Biol 20:1329-1339, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Gerlinger M, Horswell S, Larkin J, et al. : Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet 46:225-233, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlinger M, Rowan AJ, Horswell S, et al. : Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883-892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rini B, Goddard A, Knezevic D, et al. : A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: Development and validation studies. Lancet Oncol 16:676-685, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Brooks SA, Brannon AR, Parker JS, et al. : ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol 66:77-84, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollingsworth JM, Miller DC, Daignault S, et al. : Rising incidence of small renal masses: A need to reassess treatment effect. J Natl Cancer Inst 98:1331-1334, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ravaud A, Motzer RJ, Pandha HS, et al. : Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 375:2246-2254, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Haas NB, Manola J, Uzzo RG, et al. : Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387:2008-2016, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]