Abstract
Purpose
After curative resection of gastric or gastroesophageal junction adenocarcinoma, Intergroup Trial 0116 (Phase III trial of postoperative adjuvant radiochemotherapy for high risk gastric and gastroesophageal junction adenocarcinoma: Demonstrated superior survival for patients who received postoperative chemoradiotherapy with bolus fluorouracil (FU) and leucovorin (LV) compared with surgery alone. CALGB 80101 (Alliance; Phase III Intergroup Trial of Adjuvant Chemoradiation After Resection of Gastric or Gastroesophageal Adenocarcinoma) assessed whether a postoperative chemoradiotherapy regimen that replaced FU plus LV with a potentially more active systemic therapy could further improve overall survival.
Patients and Methods
Between April 2002 and May 2009, 546 patients who had undergone a curative resection of stage IB through IV (M0) gastric or gastroesophageal junction adenocarcinoma were randomly assigned to receive either postoperative FU plus LV before and after combined FU and radiotherapy (FU plus LV arm) or postoperative epirubicin, cisplatin, and infusional FU (ECF) before and after combined FU and radiotherapy (ECF arm).
Results
With a median follow-up duration of 6.5 years, 5-year overall survival rates were 44% in the FU plus LV arm and 44% in the ECF arm (Plogrank = .69; multivariable hazard ratio, 0.98; 95% CI, 0.78 to 1.24 comparing ECF with FU plus LV). Five-year disease-free survival rates were 39% in the FU plus LV arm and 37% in the ECF arm (Plogrank = .94; multivariable hazard ratio, 0.96; 95% CI, 0.77 to 1.20). In post hoc analyses, the effect of treatment seemed to be similar across all examined patient subgroups.
Conclusion
After a curative resection of gastric or gastroesophageal junction adenocarcinoma, postoperative chemoradiotherapy using a multiagent regimen of ECF before and after radiotherapy does not improve survival compared with standard FU and LV before and after radiotherapy.
INTRODUCTION
Surgical resection is the primary treatment of nonmetastatic gastric cancer, and the 5-year survival rate diminishes rapidly with increasing stage of disease. Among Western patients with localized lesions, the 5-year survival rate is 65%; however, for those with regional spread, estimated 5-year survival drops to 30%.1-3 This poor prognosis among resected patients necessitates the evaluation of potentially promising adjuvant strategies.
In a US National Cancer Institute–sponsored randomized clinical trial of 556 patients who had undergone a curative resection of gastric or gastroesophageal junction adenocarcinoma— Intergroup Trial 0116 (Phase III trial of postoperative adjuvant radiochemotherapy for high risk gastric and gastroesophageal junction adenocarcinoma)—demonstrated a significant survival benefit with the use of adjuvant fluorouracil (FU)-based chemoradiotherapy compared with surgery alone.3,4 Of note, the benefit associated with the regimen seemed to be principally in the reduction of locoregional failure; differences in the rate of distant failure were less pronounced. As a consequence, subsequent trials have examined whether the use of multiagent chemotherapy regimens, potentially more systemically active than FU and leucovorin (LV), might further enhance the survival benefit associated with postoperative chemoradiotherapy.
In the absence of radiotherapy, the British MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial demonstrated the superiority of a combination of epirubicin, cisplatin, and FU (ECF) administered before and after surgical resection of gastric cancer compared with surgery alone.5 Despite the omission of radiotherapy, perioperative ECF conferred a statistically significant reduction in death and cancer recurrence, establishing perioperative chemotherapy, without radiation therapy, as an alternative, reasonable standard in the treatment of resectable gastric cancer.
On the basis of these aforementioned trials, we hypothesized that the survival benefit offered by postoperative FU-based chemoradiation may be enhanced by replacing bolus FU plus LV with a potentially more active multi-agent chemotherapy regimen (ECF). Cancer and Leukemia Group B (CALGB 80101, now Alliance; Phase III Intergroup Trial of Adjuvant Chemoradiation After Resection of Gastric or Gastroesophageal Adenocarcinoma) was designed to compare a postoperative regimen of FU plus LV before and after radiotherapy, as developed in Intergroup Trial 0116, with a regimen of ECF before and after radiotherapy in patients who had undergone a curative resection of stage IB through IV (M0) gastric or gastroesophageal junction adenocarcinoma. Here we report an analysis of overall survival (OS) and disease-free survival (DFS) from CALGB 80101 completed after a median follow-up of 6.5 years.
PATIENTS AND METHODS
Eligibility Criteria
Patients were recruited on the basis of the following eligibility criteria: histologically confirmed stage IB through IV (M0) adenocarcinoma of the stomach or gastroesophageal junction, according to the 2002 (6th edition) staging criteria of the American Joint Commission on Cancer6; en bloc surgical resection of tumor without residual disease; an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2; adequate function of major organs (serum creatinine ≤ 1.5 mg/dL and bilateral renal function; serum bilirubin ≤ 2.0 mg/dL; serum AST ≤ 3 times the upper limit of normal; absolute neutrophil count ≥ 1,500/μL; and platelet count ≥ 100,000/μL); a caloric intake sufficient to ensure a stable weight (< 2 pounds weight loss) for at least 1 week before registration; and random assignment and treatment initiation no earlier than 21 days and no later than 84 days after surgical resection. All patients provided written institutional review board–approved, protocol-specific informed consent according to institutional and federal guidelines. An evaluation by a radiation oncologist was required before enrollment to ensure appropriateness for radiation therapy.
Study Design and Treatment
Eligible patients were randomly assigned 1:1 to receive a postoperative adjuvant regimen of FU plus LV before and after combined FU and radiotherapy, or ECF before and after radiotherapy. Stratification factors were depth of tumor penetration (T1/T2; T3; T4), nodal status (no positive nodes; one to three positive nodes; four or more positive nodes), and total number of lymph nodes examined in the surgical resection specimen (< 7; seven to 14; or ≥ 15).
Patients assigned to FU plus LV received bolus FU (425 mg/m2 per day) and LV (20 mg/m2 per day) for 5 days starting on day 1, followed by chemoradiotherapy, which began 28 days after the start of the initial cycle of chemotherapy. Four weeks after the completion of radiotherapy, two additional 5-day cycles of bolus FU (425 mg/m2 per day) plus LV (20 mg/m2 per day) were given 1 month apart.
Patients assigned to ECF received epirubicin (50 mg/m2 on day 1), cisplatin (60 mg/m2 on day 1), and FU (200 mg/m2 per day continuous infusion for 21 days starting on day 1), followed by chemoradiotherapy beginning 28 days after the start of the initial cycle of chemotherapy. Four weeks after the completion of radiotherapy, two additional cycles of epirubicin (40 mg/m2 on day 1), cisplatin (50 mg/m2 on day 1), and FU 200 mg/m2 per day continuous infusion for 21 days were given starting on day 1; each cycle was given 21 days apart.
In both arms, radiotherapy consisted of 45 Gy of radiation at 1.8 Gy per day, 5 days per week, for 5 weeks with continuous infusion FU (200 mg/m2 per day, 7 days per week throughout radiation). Target radiotherapy volume was based on preoperative imaging, surgical findings, including the size and location of the primary lesion and pathologically involved lymph nodes, and location of surgical margins. Doses were limited to ensure that < 30% of the liver was exposed to > 30 Gy, two thirds or more of one functioning kidney was not irradiated, < 50% of the combined volume of the right and left ventricles of the heart received > 25 Gy, and the spinal cord received < 45 Gy. In general, for patients with node-positive gastric tumors, radiation covered the tumor bed, residual stomach, resection margins, and nodal drainage regions. For node-negative gastric tumors with pathology evaluation of ≥ 10 nodes and surgical margins ≥ 5 cm, treatment of the nodal beds was not required. For tumors of the gastroesophageal junction, radiation fields extended into the chest approximately 5 cm proximal to the area of known esophageal involvement with an intent to fully treat periesophageal tissues at the level of the tumor.
Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 2.0), and doses of chemotherapy were reduced according to toxicity assessments as prescribed in the study protocol (Data Supplement).
Radiotherapy Quality Assurance
Prior approval of the radiotherapy treatment plan was required before treatment initiation by the Quality Assurance Review Center (now the Imaging and Radiation Oncology Core Rhode Island), and the radiation oncology study co-chairs (J.E.T. and H.J.M.). Treatment fields, dosimetry, surgery and pathology reports, and preoperative tumor imaging were submitted for review. Plans that were not approved were principally based on toxicity risks to critical organs or the failure to treat appropriate target volumes. At pretreatment review, 15% of treatment plans were found to contain a potential major protocol deviation, most of which were corrected before radiotherapy initiation. A final quality assurance review of radiotherapy (conducted after the delivery of radiation) revealed major deviations in 4% of the treatment plans.
Statistical Considerations
The primary end point of CALGB 80101 was OS, with DFS and adverse events as key secondary end points. Patients were only followed for first disease recurrence. Time to first disease recurrence was measured from study entry until documented recurrence of disease or last known follow-up. Assessment of treatment efficacy was performed on an intent-to-treat basis. Safety analyses included all patients who received at least one dose of study therapy.
The trial was originally designed for a planned enrollment of 824 patients with 80% power to detect a 25% improvement in OS among patients treated with ECF compared with those treated with FU plus LV, with a one-sided alpha at a significance level of .05. In August 2005, with all data still masked to the investigators and with 171 patients randomly assigned, the protocol was amended to address slower than expected enrollment; total enrollment was changed to 540 patients, which provided 80% power to detect a 30% improvement in OS among patients treated with ECF compared with those treated with FU plus LV (an improvement in median survival from 3.0 to 3.9 years), on the basis of a one-sided, stratified (by the stratification factors used at random assignment) log-rank test at a significance level of .05. Formal interim analyses for superiority and futility were conducted after 20% of the expected deaths were observed and submitted for review by the CALGB Data and Safety Monitoring Board. Interim analyses were repeated approximately every 6 months thereafter. An adjusted test of significance for OS on the basis of the stratified log-rank test was used to test superiority of ECF versus FU; an adjusted, CI approach was used to exclude a log hazard ratio (HR) of 0.2624, corresponding to an HR of 1.3. The one-sided Lan-DeMets analog of the O’Brien-Fleming boundaries was used to adjust for multiplicity in both cases.7
Comparisons of OS and DFS between arms were performed using a one-sided, stratified log-rank test. OS was measured from study enrollment until death from any cause. DFS was measured from the date of study enrollment until death or documented second primary tumor, or cancer recurrence. HRs with 95% CIs were calculated using the Cox proportional hazards model. Multivariable analyses adjusted by patient and disease characteristics were also performed post hoc using the Cox regression model. Models were adjusted for age (years as a continuous variable); sex; race (white v black v other); ECOG performance status (0 v 1or 2); depth of tumor penetration (T1 or T2 v T3 or T4); histologic grade (well v moderately v poorly differentiated v undifferentiated); number of positive lymph nodes (0 v 1 to 3 v ≥ 4); number of nodes examined (< 7 v 7 to 14 v ≥ 15); site of tumor location (gastroesophageal junction v proximal stomach v distal stomach v multicentric v stomach not otherwise specified). Fifty patients with missing data on at least one of these variables were excluded from the analysis. Survival curves were estimated using the Kaplan-Meier method. In addition, analyses within patient subgroups were conducted post hoc using Cox regression models. Statistical interactions were represented by the cross-products of the corresponding variables. The statistical methods proposed by Gray8 for the analysis of the cumulative incidence of competing risks were used to estimate the rates of locoregional versus distal first recurrence and investigate the effect of treatment regimen on locoregional first recurrence versus distant metastases.9
Patient registration and data collection were managed by the Alliance (formerly CALGB) Statistics and Data Center. Data quality was ensured by careful review of data by Alliance Statistics and Data Center staff and by the study chairperson. All analyses were based on the study database frozen on February 19, 2016, and were performed by Alliance statisticians using SAS 9.2 (SAS Institute, Cary, NC) or R.
RESULTS
Baseline Characteristics
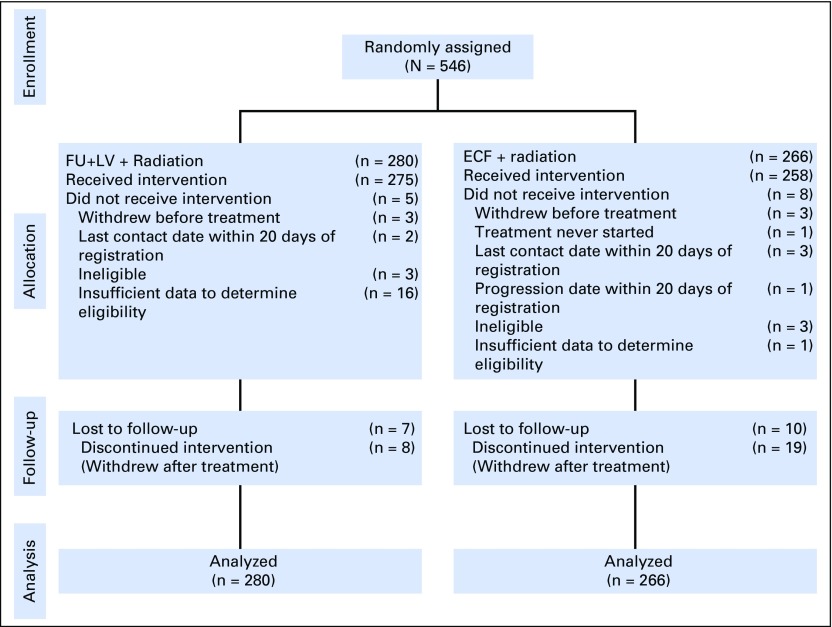
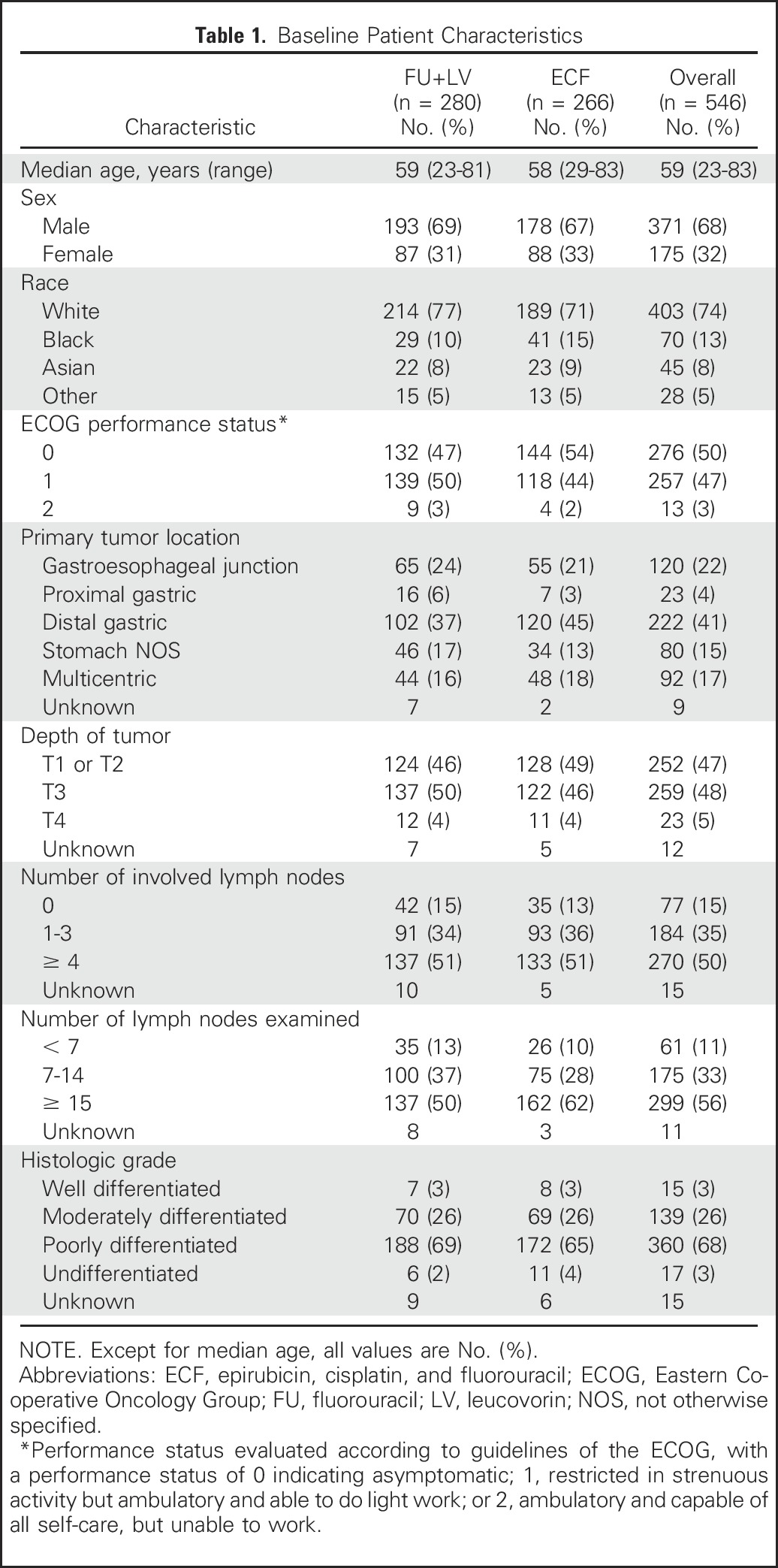
Between April 30, 2002, and May 29, 2009, 546 patients were randomly assigned to receive either FU plus LV before and after combined FU and radiotherapy (n = 280) or ECF before and after combined FU and radiotherapy (n = 266). Enrollment, treatment allocation, eligibility, follow-up, and the number of patients included in the final data analysis are shown in Figure 1. The Alliance Data and Safety Monitoring Board released the trial data after the eighth interim analysis, in which the futility boundary was nearly crossed and the conditional probability of observing a significant difference between treatment regimens was low. Baseline patient, disease, and prior treatment characteristics were largely similar between treatment arms (Table 1). A slightly higher proportion of patients in the ECF arm (62%) had ≥ 15 lymph nodes examined in the surgical resection specimen compared with the FU plus LV arm (50%).
Fig 1.
Flow diagram of all registered patients. ECF, epirubicin, cisplatin, and fluorouracil; FU, fluorouracil; LV, leucovorin.
Table 1.
Baseline Patient Characteristics
Treatment Delivery
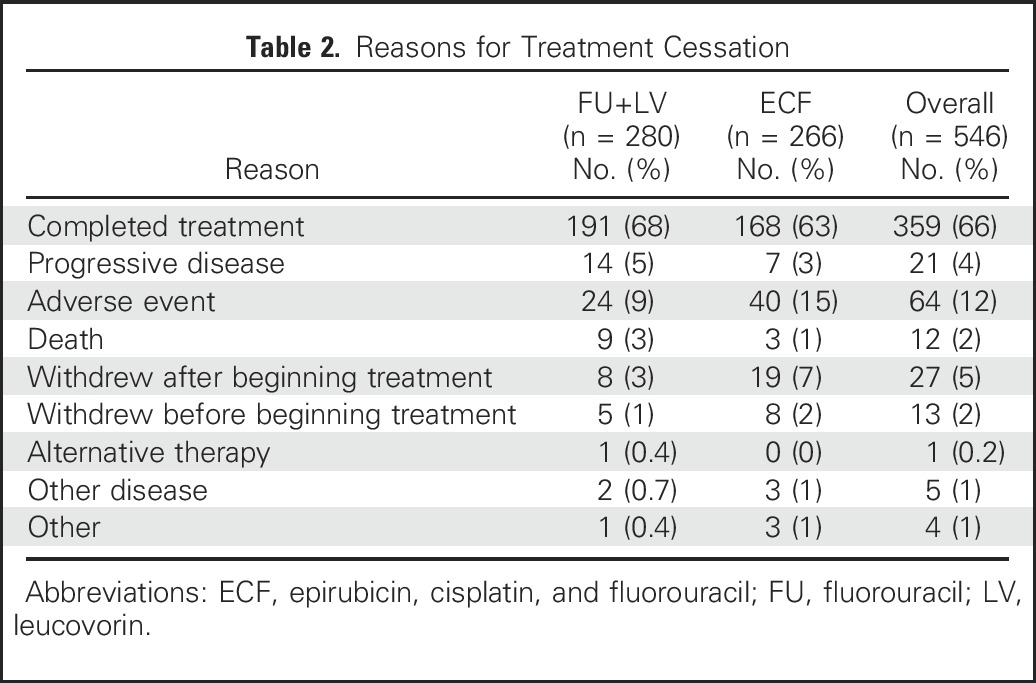
The median time to treatment initiation after surgical resection was 54 days for FU plus LV and 53 days for ECF. All planned postoperative adjuvant therapy was completed by 68% of patients (191 of 280) in the FU plus LV arm and 63% (168 of 266) in the ECF arm (Table 2). A lower proportion of patients in the FU plus LV arm discontinued treatment because of an adverse event or treatment withdrawal than in the ECF arm (13% v 24%, respectively; P = .001).
Table 2.
Reasons for Treatment Cessation
Safety
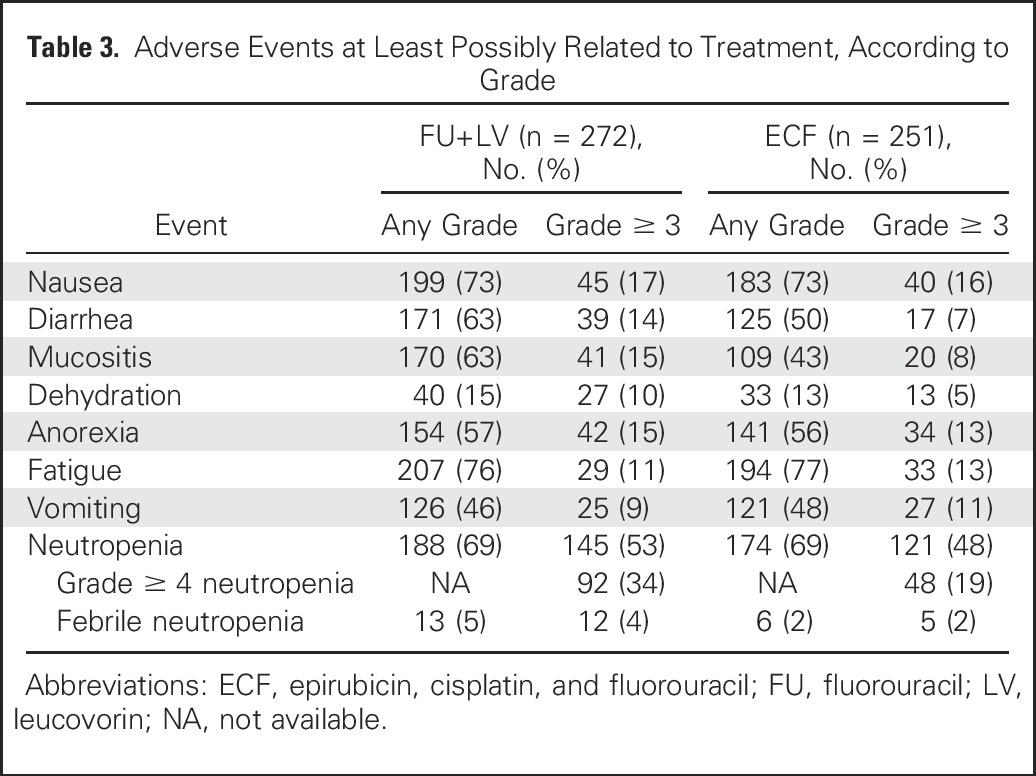
The overall incidence of adverse events (any grade) was 98% in the FU plus LV arm and 94% in the ECF arm. Grade 3 or higher diarrhea, mucositis, and dehydration were each more common in the FU plus LV arm compared with the ECF arm (Table 3). Grade 3 or higher neutropenia rates were comparable between treatment arms, although rates of grade 4 or higher neutropenia was higher in the FU plus LV arm (34% v 19%, respectively). Adverse events that led to treatment modifications (delays or dose reductions) occurred in 57% of patients in the FU plus LV arm and 54% in the ECF arm.
Table 3.
Adverse Events at Least Possibly Related to Treatment, According to Grade
Seven deaths (3%) were considered related to treatment in the FU plus LV group. The reported causes of death included colitis (n = 1), infection associated with neutropenia (n = 1), infection without neutropenia (n = 1), pneumonitis (n = 2), acute respiratory distress syndrome (n = 1), and cause unknown (n = 1). One death (< 1%) was considered related to treatment in the ECF group (gastrointestinal obstruction).
Efficacy
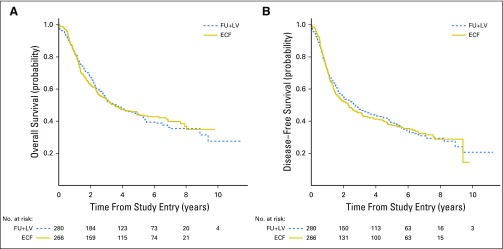
After a median follow-up duration of 6.5 years, 322 deaths were documented (170 in the FU plus LV arm and 152 in the ECF arm). The estimated 5-year OS rates were 44% in the FU plus LV arm and 44% in the ECF arm (stratified Plogrank = .69; Fig 2A). With 358 DFS events observed (186 in the FU plus LV arm and 172 in the ECF arm), the estimated 5-year DFS rates were 39% in the FU plus LV arm and 37% in the ECF arm (stratified Plogrank = .94; Fig 2B). Adjusting for other known or suspected predictors of patient outcome, the multivariable HR for mortality of 0.98 (95% CI, 0.78 to 1.24) for patients in the ECF arm, compared with those treated in the FU plus LV arm. The multivariable HR for cancer recurrence or mortality (DFS) was 0.96 (95% CI, 0.77 to 1.20) comparing treatment arms.
Fig 2.
(A) Overall survival by treatment arm. (B) Disease-free survival by treatment arm. ECF, epirubicin, cisplatin, and fluorouracil; FU, fluorouracil; LV, leucovorin.
Subgroup Analyses
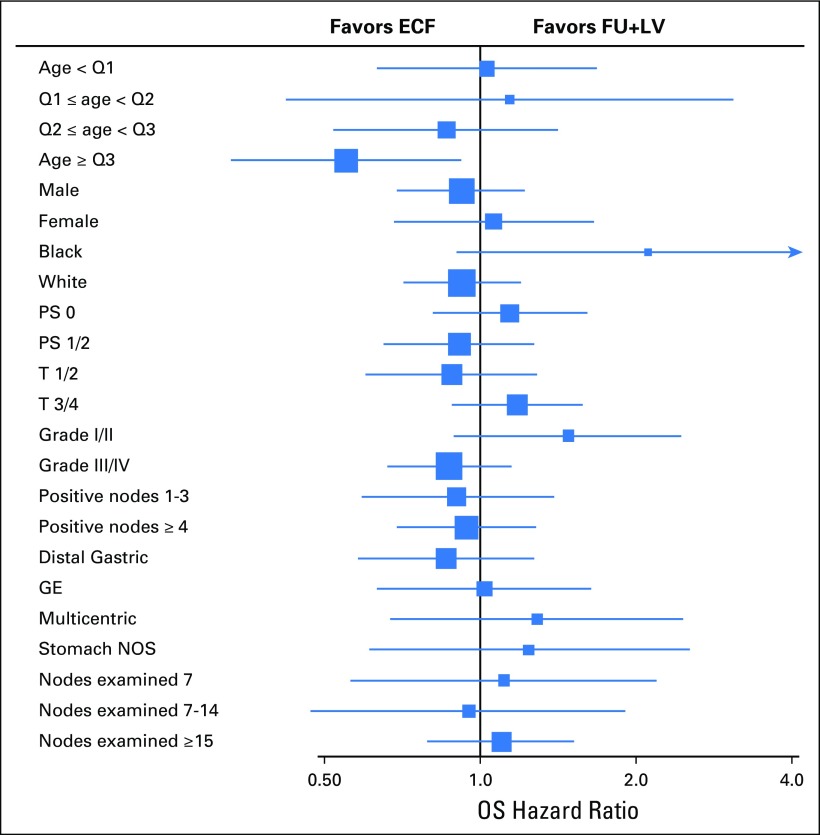
We further examined post hoc the effect of treatment according to strata of known or suspected predictors of cancer recurrence (Fig 3). Across each of these strata, there continued to be no significant survival benefit for patients treated in the ECF arm compared with those in the FU plus LV arm (Pinteraction > .005 on the basis of a Bonferroni-adjusted P value for 10 tests of interaction).
Fig 3.
Forest plot of hazard ratios and 95% CIs for overall survival by variable stratum. Numbers of events were too small to estimate the hazard ratios for other race, 0 positive nodes, and proximal gastric primary site. Box size reflects the precision of the estimate, with a larger box size indicating greater precision. ECF, epirubicin, cisplatin, and fluorouracil; FU, fluorouracil; GE, gastroesophageal; LV, leucovorin; NOS, not otherwise specified ; PS, performance status; Q, Quartile.
Pattern of Recurrence
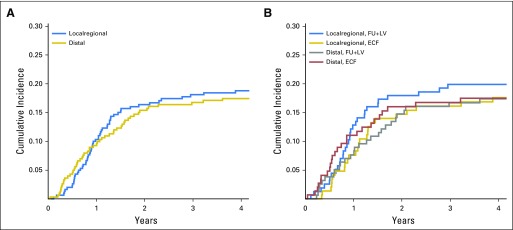
Locoregional recurrence was defined as recurrence at the anastomotic site, duodenal stump, tumor bed, or remnant stomach or at the regional lymph nodes within the radiation field. Distant metastasis was defined as lymph node recurrence outside the radiation field, peritoneal seeding, liver metastasis, or metastasis to other extra-abdominal sites. To date, 257 patients experienced cancer recurrence (129 who received FU plus LV and 128 who received ECF). Data on location of cancer recurrence were available for 112 patients (61 who received FU plus LV, with 32 locoregional and 29 distal; 51 who received ECF, with 26 locoregional and 25 distal). No differences in the cumulative incidence of locoregional recurrences versus distant metastases were apparent overall (Appendix Fig A1A, online only). No significant differences were observed for either locoregional recurrences (P = .56) or distant metastases (P = .88) between treatment arms (Appendix Fig A1B). Two patients who died without evidence of recurrence were omitted from this analysis.
DISCUSSION
In this randomized clinical trial of patients who had undergone a curative resection of gastric or gastroesophageal junction adenocarcinoma, we sought to build on the survival benefit associated with adjuvant chemoradiotherapy in Intergroup Trial 01163,4 by replacing standard FU plus LV with the multiagent regimen of ECF, delivered before and after concomitant FU and radiotherapy. However, postoperative chemoradiotherapy with ECF before and after radiotherapy did not improve OS or DFS compared with a regimen of bolus FU plus LV before and after radiotherapy. We failed to observe any significant benefit associated with the ECF regimen within any specific patient subgroup, although the power to examine such interactions was limited.
Intergroup Trial 0116 was widely criticized for the limited lymph node dissections performed on patients enrolled in the study. Although several large randomized trials comparing D1 and D2 lymph node dissections in gastric cancer failed to demonstrate a significant OS benefit associated with the D2 dissection,10-13 D2 lymphadenectomy has become a standard surgical approach in many centers.14 In the current trial, only 55% of patients had ≥ 15 lymph nodes examined in the surgical resection specimen, and 11% of patients had fewer than seven lymph nodes examined. Nonetheless, there is no evidence that the extent of lymph node dissection influenced the relative benefit of ECF compared with FU plus LV. Our findings did not change when we examined relative treatment effects across different strata of number of lymph nodes examined.
Treatment-related toxicity could influence the efficacy of the individual adjuvant chemoradiotherapy regimens. However, treatment completions rates and rates of grade 3 or higher adverse events were comparable between the arms. Rates of grade 3 or higher gastrointestinal toxicities and grade 4 or higher neutropenia were higher in the FU plus LV arm, reflecting the toxicity profile of the Mayo Clinic bolus FU plus LV regimen.15
We recognized that compliance with protocol-designed radiotherapy planning could also have confounded our results. Our trial included detailed instructions for radiotherapy planning; moreover, the radiation oncology study leaders reviewed all treatment plans before radiotherapy initiation. At central review, 15% of treatment plans were found to contain a major protocol deviation, which were corrected before the start of radiotherapy.
Recent trials have provided additional insight into the role of anthracyclines and radiotherapy in the management of resectable gastroesophageal cancer. In the neoadjuvant therapy of gastroesophageal or esophageal cancer, Combination Chemotherapy in Treating Patients With Esophageal Cancer (MRC OE05) found no survival benefit for the regimen of epirubicin, cisplatin, and capecitabine compared with FU and cisplatin without an anthracycline.16 In the postoperative management of resected gastric cancer, the ARTIST (Adjuvant Chemoradiation Therapy in Stomach Cancer) trial observed no significant benefit for the addition of radiotherapy to adjuvant capecitabine and cisplatin compared with capecitabine and cisplatin alone.17 More recently, after neoadjuvant chemotherapy for resectable gastric cancer, the CRITICS (Randomized Phase III Trial of Adjuvant Chemotherapy or Chemoradiotherapy in Resectable Gastric Cancer) trial found that the addition of radiation to postoperative chemotherapy did not improve survival compared with postoperative chemotherapy alone.18
Ongoing randomized clinical trials are examining other adjuvant and neoadjuvant treatments in patients with gastric cancer, and we await the results of those studies.19-21 Beyond those approaches, recent molecular analyses document unique subtypes of gastric cancer that possess distinctive, salient genomic features.22 Future adjuvant trials will likely leverage these genomic findings to guide targeted approaches for these distinct populations of patients with gastric cancer.
Appendix
Fig A1.
(A) Cumulative incidence plot for locoregional versus distal recurrence among all patients. Recurrence is measured from study entry until documented recurrence of disease or last follow-up. (B) Cumulative incidence plot for locoregional versus distal recurrence by treatment assignment. Recurrence was measured from study entry until documented recurrence of disease or last follow-up. ECF, epirubicin, cisplatin, and fluorouracil; FU, fluorouracil; LV, leucovorin.
The following institutions participated in this study:
Carle Cancer Center National Cancer Institute Community Oncology Research Program (NCORP), Urbana, IL; Kendrith Rowland, grant: UG1CA189861.
Carolinas Medical Center/Levine Cancer Institute, Charlotte, NC; Richard White.
Coborn Cancer Center at Saint Cloud Hospital, Saint Cloud, MN, Donald Jurgens.
Dana-Farber/Partners CancerCare Lead Academic Participating Site (LAPS), Boston, MA; Harold Burstein, grants: U10CA180867 and U10CA032291.
Dayton NCORP, Dayton, OH; Howard Gross, grant: UG1CA189957.
Delaware/Christiana Care NCORP, Newark, DE; Gregory Masters, grant: UG1CA189819.
Duke University - Duke Cancer Institute LAPS, Durham, NC; Jeffrey Crawford, grants: U10CA180857 and U10CA047577.
Essentia Health NCORP, Duluth, MN; Bret Friday, grant: UG1CA189812.
Flower Hospital, Sylvania, OH; Rex Mowat.
Geisinger Cancer Institute NCORP, Danville, PA; Srilatha Hosur, grant: UG1CA189847.
Grand Rapids Clinical Oncology Program, Grand Rapids, MI; Martin J. Bury.
Heartland Cancer Research NCORP, Decatur, IL; James Wade, (Alan P. Lyss), grants: UG1CA189830 and (U10CA114558).
Hematology Oncology Associates of Central New York-East Syracuse, East Syracuse, NY; Jeffrey Kirshner, grant: U10CA045389.
Illinois Oncology Research Association Community Clinical Oncology Program (CCOP), Peoria, IL; John W. Kugler, grant: U10CA035113.
Joliet Oncology-Hematology Associates Limited, Joliet, IL.
Kansas City CCOP, Kansas City, MO; Rakesh Gaur.
Lima Memorial Hospital, Lima, OH; Rex Mowat.
Mayo Clinic LAPS, Rochester, MN; Steven Alberts, grant: U10CA180790.
MedStar Georgetown University Hospital, Washington, DC; Chaitra Ujjani and (Bruce Cheson), grant: (U10CA077597).
Memorial Sloan Kettering Cancer Center LAPS, New York, NY; Michael Morris and (Clifford A. Hudis), grants: U10CA180791 and (U10CA077651).
Metro Minnesota Community Oncology Research Consortium, Saint Louis Park, MN; Daniel Anderson, grant: UG1CA189863.
Michiana Hematology Oncology, PC, South Bend, IN.
Michigan Cancer Research Consortium NCORP, Ann Arbor, MI; Philip Stella, grant: UG1CA189971.
Missouri Valley Cancer Consortium, Omaha, NE; Gamini Soori.
Mount Sinai Hospital, New York, NY; Lewis Silverman, grant: U10CA004457.
Mount Sinai Medical Center, Miami Beach, FL; Michael Schwartz, grant: U10CA045564.
NCORP of the Carolinas (Greenville Health System NCORP), Greenville, SC; Jeffrey Giguere, grants: UG1CA189972 and U10CA029165.
Nevada Cancer Research Foundation CCOP, Las Vegas, NV; John A. Ellerton, grant: U10CA035421.
New Hampshire Oncology Hematology PA-Hooksett, Hooksett, NH; Douglas Weckstein.
Northern Indiana Cancer Research Consortium, South Bend, IN; Rafat Ansari, grant: U10CA086726.
Northwell Health NCORP, Lake Success, NY; Daniel Budman, grant: UG1CA189850.
The Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH; Clara Bloomfield, grants: U10CA180850 and U10CA077658.
Princeton Community Hospital, Princeton, WV; Rowena Gonzales-Chambers.
Roswell Park Cancer Institute LAPS, Buffalo, NY; Ellis Levine, grants: U10CA180866 and U10CA059518.
Saint Vincent's Medical Center, Bridgeport, CT.
Sanford NCI Community Oncology Research Program of the North Central Plains, Sioux Falls, SD; Preston Steen, grant: UG1CA189825.
Southeast Clinical Oncology Research (SCOR) Consortium NCORP, Winston-Salem, NC; James Atkins, grants: UG1CA189858 and U10CA045808.
State University of New York Upstate Medical University, Syracuse, NY; Stephen Graziano, grant: U10CA021060.
Toledo Clinic Cancer Centers-Toledo, Toledo, OH; Rex Mowat.
University of North Carolina Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC; Thomas Shea, grants: U10CA180838 and U10CA047559.
University of California San Diego, San Diego, CA; Barbara A. Parker, grant: U10CA011789.
University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL; Hedy Kindler, grants: U10CA180836 and U10CA041287.
University of Illinois, Chicago, IL; John Quigley and (David J. Peace), grant: (U10CA074811).
University of Iowa/Holden Comprehensive Cancer Center, Iowa City, IA; Laith Abushahin and (Daniel A. Vaena), grant: (U10CA047642).
University of Maryland/Greenebaum Cancer Center, Baltimore, MD; Martin Edelman, grant: U10CA031983.
University of Minnesota/Masonic Cancer Center, Minneapolis, MN; Bruce Peterson, grant: U10CA016450.
University of Nebraska Medical Center, Omaha, NE; Apar Ganti, grant: U10CA077298.
University of Oklahoma Health Sciences Center LAPS, Oklahoma City, OK; Adam Asch and (Shubham Pant), grant: U10CA180798.
Upstate Carolina CCOP, Spartanburg, SC.
Wake Forest University Health Sciences, Winston-Salem, NC; Heidi Klepin and (David D. Hurd), grant: (U10CA003927).
Walter Reed Army Medical Center, Washington, DC; David C. Van Echo, grant: U10CA026806.
Washington University - Siteman Cancer Center LAPS, Saint Louis, MO; Nancy Bartlett, grants: U10CA180833 and U10CA077440.
West Penn Hospital, Pittsburgh, PA; Gene Finley and John Lister.
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health Grants No. U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology); U10CA031946 and U10CA033601 (to the legacy CALGB); and U10CA032291, U10CA047577, U10CA047642, U10CA077651, U10CA077658, U10CA138561, U10CA180790, U10CA180791, U10CA180838, U10CA180850, U10CA180857, U10CA180867, and U10CA180820 (to the ECOG-ACRIN). C.S.F. is supported in part by grants from the National Cancer Institute (R01 CA118553, R01 CA149222, R01 CA169141, and P50 CA127003).
Presented at the 2011 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, January 3-7, 2011.
Clinical trial information: NCT00052910.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Charles S. Fuchs, Donna Niedzwiecki, Harvey J. Mamon, Joel E. Tepper, Leonard L. Gunderson, Robert J. Mayer
Provision of study materials or patients: Charles S. Fuchs, Harvey J. Mamon, Joel E. Tepper, Richard S. Swanson, Peter C. Enzinger, Daniel G. Haller, Tomislav Dragovich, Steven R. Alberts, Georg A. Bjarnason, Christopher G. Willett, Leonard L. Gunderson, Richard M. Goldberg, Alan P. Venook, David Ilson, Eileen O’Reilly, Kristen Ciombor, David J. Berg, Jeffrey Meyerhardt, Robert J. Mayer
Collection and assembly of data: Charles S. Fuchs, Donna Niedzwiecki, Harvey J. Mamon, Joel E. Tepper, Richard S. Swanson, Peter C. Enzinger, Daniel G. Haller, Tomislav Dragovich, Steven R. Alberts, Georg A. Bjarnason, Christopher G. Willett, Richard M. Goldberg, Alan P. Venook, David Ilson, Eileen O’Reilly, Kristen Ciombor, David J. Berg, Jeffrey Meyerhardt, Robert J. Mayer
Data analysis and interpretation: Charles S. Fuchs, Donna Niedzwiecki, Harvey J. Mamon, Joel E. Tepper, Xing Ye, Robert J. Mayer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy with Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results from CALGB 80101 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Charles S. Fuchs
Consulting or Advisory Role: Eli Lilly, Sanofi, Bayer, Merck, Entrinsic Health, Five Prime Therapeutics, Agios, Gilead Sciences, Dicerna, Merrimack, Taiho Pharmaceutical, KEW Group, Genentech/Roche
Donna Niedzwiecki
No relationship to disclose
Harvey J. Mamon
No relationship to disclose
Joel E. Tepper
Consulting or Advisory Role: EMD Serono
Xing Ye
Employment: Gilead Sciences (I)
Richard S. Swanson
No relationship to disclose
Peter C. Enzinger
Consulting or Advisory Role: Merck, Five Prime Therapeutics, Astellas Pharma
Daniel G. Haller
Consulting or Advisory Role: Genentech, Eli Lilly
Speakers' Bureau: Taiho Pharmaceutical, Amgen, Genentech/Roche
Expert Testimony: Celgene
Tomislav Dragovich
No relationship to disclose
Steven R. Alberts
No relationship to disclose
Georg A. Bjarnason
Honoraria: Pfizer, Novartis, Bristol-Myers Squibb
Consulting or Advisory Role: Pfizer, Novartis, Bristol-Myers Squibb, Eisai
Research Funding: Pfizer (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis
Christopher G. Willett
Honoraria: Extrinsic Health Solutions
Leonard L. Gunderson
No relationship to disclose
Richard M. Goldberg
Honoraria: Merck
Consulting or Advisory Role: Merck Serano, Merck, Merck KGaA, Taiho Pharmaceutical
Research Funding: Sanofi (Inst), Merck (Inst)
Alan P. Venook
Honoraria: Gilead Sciences
Consulting or Advisory Role: Gilead Sciences, Merrimack
Research Funding: Genentech/Roche (Inst), Bristol-Myers Squibb (Inst), Merck Serono
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Travel, Accommodations, Expenses: Genentech, Roche, Bristol-Myers Squibb, Merck Serono, Taiho Pharmaceutical, Merrimack, Halozyme Therapeutics
David Ilson
Consulting or Advisory Role: Eli Lilly/ImClone, Roche/Genentech, Bristol-Myers Squibb, Pieris Pharmaceuticals, Merck, Bayer, AstraZeneca
Eileen O’Reilly
Consulting or Advisory Role: Celgene, Aduro Biotech, Astellas Pharma (I), Celsion (I), IntegraGen (I), Sanofi, Silenseed, Vicus Therapeutics, Gilead Sciences, Merrimack, Bristol-Myers Squibb (I), Bristol-Myers Squibb, Agios (I), ASLAN Pharmaceuticals (I), Bayer (I), Blueprint Medicines (I), Boston Scientific (I), Casi (I), Delcath Systems (I), Eisai (I), Halozyme (I), Ipsen (I), Janssen, Merck (I), New B Innovation (I), Onxeo (I), Servier (I), Sillajen (I), Sirtex Medical (I), Westhaven (I), AstraZeneca
Research Funding: Momenta Pharmaceuticals (Inst), Incyte (Inst), OncoMed (Inst), Celgene (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Mabvax (Inst)
Kristen Ciombor
Research Funding: Pfizer, Boston Biomedical, MedImmune, Onyx, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Novartis, Incyte, Amgen, Sanofi
David J. Berg
No relationship to disclose
Jeffrey Meyerhardt
Honoraria: Chugai Pharma
Consulting or Advisory Role: Genentech
Robert J. Mayer
Honoraria: Taiho Pharmaceutical
Consulting or Advisory Role: CASI Pharmaceuticals
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 [DOI] [PubMed] [Google Scholar]
- 2. http://seer.cancer.gov/csr/1975_2012/ National Cancer Institute: Previous Version: SEER Cancer Statistics Review, 1975-2012.
- 3.Macdonald JS, Smalley SR, Benedetti J, et al. : Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725-730, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Smalley SR, Benedetti JK, Haller DG, et al. : Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 30:2327-2333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham D, Allum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11-20, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Greene FL, Page DL, Fleming ID, et al (eds): AJCC Cancer Staging Manual, Sixth Edition. New York, NY, Springer, 2002. [Google Scholar]
- 7.Lan KKG, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70:659-663, 1983 [Google Scholar]
- 8.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 9.Gray R: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 10.Bonenkamp JJ, Hermans J, Sasako M, et al. : Extended lymph-node dissection for gastric cancer. N Engl J Med 340:908-914, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri A, Weeden S, Fielding J, et al. : Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Br J Cancer 79:1522-1530, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartgrink HH, van de Velde CJ, Putter H, et al. : Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22:2069-2077, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Sasako M, Sano T, Yamamoto S, et al. : D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359:453-462, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Goodman KA: Refining the role for adjuvant radiotherapy in gastric cancer: Risk stratification is key. J Clin Oncol 33:3082-3084, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Buroker TR, O’Connell MJ, Wieand HS, et al. : Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 12:14-20, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Cunningham D, Alderson D, Nankivell M, et al: Toxicity, surgical complications, and short-term mortality in a randomized trial of neoadjuvant cisplatin/5FU versus epirubicin/cisplatin and capecitabine prior to resection of lower esophageal/gastroesophageal junction (GOJ) adenocarcinoma (MRC OEO5, ISRCTN01852072, CRUK 02/010). J Clin Oncol 32, 2014 (suppl; abstr 4014) [Google Scholar]
- 17.Park SH, Sohn TS, Lee J, et al. : Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 33:3130-3136, 2015 [DOI] [PubMed] [Google Scholar]
- 18. Verheij M, Jansen E, Cats A, et al: A multicenter randomized phase III trial of neoadjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. J Clin Oncol 24, 2016 (suppl; abstr 4000) [Google Scholar]
- 19. http://clinicaltrials.gov/show/NCT01924819 ClinicalTrials.gov: Trial of Preoperative Therapy for Gastric and Esophagogastric Junction Adenocarcinoma (TOPGEAR).
- 20. https://clinicaltrials.gov/ct2/show/NCT01761461 ClinicalTrials.gov: Phase III Randomized Trial of Adjuvant Chemotherapy With S-1 vs S-1/Oxaliplatin ± Radiotherapy for Completely Resected Gastric Adenocarcinoma: The ARTIST II Trial (ARTIST-II).
- 21. https://clinicaltrials.gov/ct2/show/NCT02661971 ClinicalTrials.gov: FLOT vs. FLOT/Ramucirumab for Perioperative Therapy of Gastric or GEJ Cancer (RAMSES) (RAMSES/FLOT7).
- 22.Cancer Genome Atlas Research Network : Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202-209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]