Graphical abstract
Keywords: Benzimidazole, Antibacterial, Activity index, Cycloaddition, Spectroscopy
Abstract
The aim of this present study was to synthesize 2-substituted and 1,2-disubstituted benzimidazole derivatives to investigate their antibacterial diversity for possible future drug design. The structure-based design of precursors 2-(1H-benzimidazol-2-yl)aniline 1, 2-(3,5-dinitro phenyl)-1H-benzimidazole 3 and 2-benzyl-1H-benzimidazole 5 were achieved by the condensation reaction of o-phenylenediamine with anthranilic acid, 3,5-dinitrophenylbenzoic acid, and phenylacetic acid, respectively. The precursors 1, 3 and 5, upon reaction with six different electrophile-releasing agents, furnished the corresponding 2-substituted benzimidazole, 2a-f and 1,2-disubstituted benzimidazole derivatives 4a-f and 6a-f, respectively. The structural identity of the targeted compounds was authenticated by elemental analytical data and spectral information from FT-IR, UV, 1H, and 13C NMR. The outcome of the findings from the in vitro screening unveiled 2-benzyl-1-(phenylsulfonyl)-1H-benzimidazole 6b as the most active derivative with lowest MIC value of 15.63 µg/mL.
Introduction
From time to time, heterocyclic templates have continued to gain respect and much interest among the medicinal chemists, because of their numerous therapeutic applications and effective reported druggability [1]. Benzimidazole is a heterocyclic aromatic organic compound that plays important functions in the development of theory in heterocyclic chemistry and organic synthesis [2]. Benzimidazole is a strongly acidic compound with a pKa of 12.75, while its conjugated acid has a pKa of 5.68, which is less basic than imidazole. Benzimidazole is readily prepared by [4+1]-cycloaddition of o-phenylenediamine with a one carbon donor source in the presence of various heterogeneous catalysts [3], such as H2O2/HCl [4], H2O2/CAN [5], H2O/HCl [6], H2O2/Fe(NO3)3 [7], and H2O2/Bu4NI [8] as efficient oxidative couples. In healthful analysis, the synthesis of novel benzimidazole derivatives remains a focus [9]. Diverse synthetic efforts for accessing benzimidazole derivatives have been documented, however, the commonest technique involves the reaction of o-phenylenediamine with alkanoic acids. From the evaluation of the works of various researchers, benzimidazole derivatives have been reported to possess antimalarial [10], anticancer [11], antimicrobial [12], [13], antioxidant [14], and anticonvulsant [15] activities among others. Some derivatives of benzimidazole are well known in corrosion studies and their corrosion inhibition efficiencies are related to their adsorption properties [16].
The outbreak of new diseases and the increase in population of drug resistant strains of bacteria, such as methicillin-resistant Staphylococcus aureus [17], vancomycin-resistant Enterococci [18], ampicillin-resistant Enterobacter aerogenes [19], gentamicin-resistant Escherichia coli [20], and chloroquine-resistant Plasmodium falciparum [21], have posed great challenges to life and wellbeing of mankind. Based on the existence of antidrug multi-resistant bacteria strains [22], the occurrence of side effects to commercially available drugs [23], adverse drug reaction in elderly patient [24], the emergence of new diseases, and global health threat that have resulted in high mortality rate [25]; it has become highly imperative to consistently and continuously engage in the synthetic preparation of novel heterocyclic templates as highly dynamic biologically active substances for therapeutic uses. Therefore, it is beneficial to design some 2-substituted- and 1,2-disubstituted benzimidazole derivatives by ecofriendly method so as to examine their antimicrobial properties for possible future drug development.
Material and methods
Chemical compounds and reagents were purchased from Sigma-Aldrich Chemicals (St. Louis, Missouri, USA) apart from Tetrahydrofuran (THF), benzenesulfonyl chloride, and anthranilic acid which were supplied by the British Drug Houses (Poole, Dorset, England). All these compounds were then made available by Department of Chemistry, Covenant University for research use. All the chemicals are pure and they were used directly without further purification. The synthesized heterocyclic frameworks were evaluated for their melting point determination using Stuart equipment and the value obtained were recorded directly. Bruker fourier-transform (ft-ir) spectrophotometer was utilized to obtain infrared data. The UV spectra of the solution of the compounds in THF were run in UV Genesys 10 s. The 1H and 13C nuclear magnetic resonance of the heterocycles were NMR Bruker DPX 400 spectrometer at 400 MHz and 100 MHz, respectively in DMSO-d6. The reference utilized was Tetramethylsilane (TMS). The reaction progress as well as the level of purity was routinely checked and monitored with Thin Layer Chromatography (TLC) using CHCl3/CH3OH (9:1, v/v) eluent. After reaction was completed, solvents were evaporated under reduced pressure using IKA® RV 10 Rotary evaporator. In a situation where more than one spots were observed, column chromatography was carried out to get a pure compound.
2-(1H-Benzimizadol-2-yl)aniline as precursor 1
o-Phenylene diamine (15.00 g, 140.00 mmol) was weighed and dissolved in 150 mL of ethanol in a round- bottomed flask. It was stirred for 5 min with the aid of magnetic stirrer after which anthranilic acid (19.20 g, 140.00 mmol) was gradually tipped into the solution followed by the addition of a catalytic amount of NH4Cl (0.75 g, 14.00 mmol). The resulting solution was then heated under reflux at 60–70 °C for 2 h. The TLC was utilized to ascertain the progress of reaction. Upon completion, the resulting solution was allowed to cool down. The flask content was evaporated to dryness and triturated with ice-cold water. The solid mass formed was separated by suction filtration to furnish 2-(1H-benzimizadol-2-yl)aniline 1 (72.68%), mp = 85–87 °C, colour = gray. 1H NMR (400 MHz, DMSO-d6) δH: 4.50 (s-br, 2H, NH2), 6.37–6.39 (dd, J1 = 4.54 Hz, J2 = 8.80 Hz, 1H, Ph-H), 6.49–6.50 (dd, J1 = 4.21 Hz, J2 = 8.87 Hz, 1H, Ph-H), 6.84–6.86 (d, J = 8.80 Hz, 1H, Ph-H), 7.12–7.14 (d, J = 8.00 Hz, 1H, Ph-H), 7.39–7.41 (m, 2H, Ph-H), 7.59–7.63 (d, J = 8.87 Hz, 1H, Ph-H). 13C NMR (100 MHz, DMSO-d6) δC: 109.6, 115.2 (2 × CH), 116.9, 119.4, 123.1 (2 × CH), 125.5, 129.7, 141.9 (2 × C), 145.1, 155.0 ppm. λmax in nm (log εmax): 218 (4.2741), 253 (4.3096), 326 (3.6434). FT-IR ν in cm−1: 3424 (N—H of NH2), 3422 (N—H of NH2), 3405 (N—H), 1620 (C C aromatic). Anal. Calcd for C13H11N3 (209.25): C, 74.62; H, 5.30; N, 20.08%. Found: C, 74.80; H, 5.47; N, 19.96%.
Overall protocol towards accessing 2-substituted benzimidazole 2a-f
Precursor 1 (4.00 g, 19.10 mmol) was dissolved in 20 mL of tetrahydrofuran (THF) in a round-bottomed flask at room temperature. The medium was basified by the addition of Na2CO3 (4.06 g, 38.30 mmol) and cooled to 0–5 °C in ice bath. The corresponding electrophile-releasing substrate a-f (19.10 mmol) was then added and the reacting mixture was maintained on ice bath for additional 15 min after which the medium was warmed up to room temperature and stirred there for 24 h. Monitoring of reaction progress was conducted using TLC and upon reaction completion, the solvent was evaporated at reduced pressure using rotary evaporator. Cold water was added to the resulting mass, filtered by suction, and air-dried to afford crude product which upon column purification afforded 2-substituted benzimidazole derivatives 2a-f.
N-(2-(1H-Benzimidazole-2-yl)phenyl) acetamide 2a
When a = acetyl chloride, yield 57.70%, mp = 253–255 °C, colour = gray. 1H NMR (400 MHz, DMSO-d6) δH: 2.67 (s, 3H, CH3—CO), 6.36–6.39 (dd, J1 = 4.58 Hz, J2 = 8.82 Hz, 1H, Ph—H), 6.51–6.52 (dd, J1 = 4.18 Hz, J2 = 8.89 Hz, 1H, Ph—H), 6.86–6.88 (d, J = 8.82 Hz, 1H, Ph—H), 7.13–7.15 (d, J = 8.00 Hz, 1H, Ph—H), 7.39–7.43 (m, 2H, Ph—H), 7.62–7.65 (d, J = 8.89 Hz, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 26.4 (CH3), 109.6, 115.2 (2 × CH), 116.8, 119.6, 123.6 (2 × CH), 125.6, 129.8, 141.7 (2 × C), 145.3, 156.1, 175.4 (C O) ppm. λmax in nm (log εmax): 218 (4.2988), 248 (4.5563), 323 (3.8261), 464 (2.4771). FT-IR ν in cm−1: 3405 (N—H), 1699 (C O). Anal. Calcd for C15H13N3O (251.11): C, 71.70; H, 5.21; N, 16.72%. Found: C, 71.88; H, 5.09; N, 16.89%.
N-(2-(1H-Benzimidazole-2-yl)phenyl)benzenesulfonamide 2b
When b = benzenesulfonyl chloride, yield 97.21%, mp = N.D. (Oily), colour = black. 1H NMR (400 MHz, DMSO-d6) δH: 6.35–6.38 (m, 3H, Ph—H), 6.46–6.49 (m, 2H, Ph—H), 6.84–6.86 (d, J = 7.16 Hz, 2H, Ph—H), 7.11–7.14 (d, J = 11.96 Hz, 2H, Ph—H), 7.40–7.42 (m, 2H, Ph—H), 7.62–7.65 (d, J = 11.88 Hz, 2H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 109.8, 115.3 (2 × CH), 116.9, 119.5, 123.2 (2 × CH), 125.6, 127.5 (2 × CH), 128.9, 129.8 (2 × CH), 131.9, 154.8, 139.7, 141.6 (2 × C), 145.3 ppm. λmax in nm (log εmax): 221 (4.6609), 251 (4.7332), 317 (4.2878), 428 (3.9294). FT-IR ν in cm−1: 3405 (N—H), 3266 (N—H), 1620 (C C aromatic), 1575 (C N imine), 1376 (SO2), 1185 (SO2). Anal. Calcd for C19H15N3O2S (349.09): C, 65.31; H, 4.33; N, 12.03%. Found: C, 65.20; H, 4.15; N, 11.83%.
N-(2-(1H-Benzimidazole-2-yl)phenyl)-4-methylbenzenesulfonamide 2c
When c = p-toluenesulfonyl chloride, yield 80.03%, mp = N.D. (Oily), colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 2.80 (s, 3H, CH3-Ar), 6.36–6.39 (m, 3H, Ph—H), 6.49–6.51 (m, 2H, Ph—H), 6.84–6.86 (d, J = 7.04 Hz, 1H, Ph—H), 7.11–7.14 (d, J = 11.96 Hz, 2H, Ph—H), 7.40–7.42 (m, 2H, Ph—H), 7.62–7.65 (d, J = 11.88 Hz, 2H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 21.3 (CH3), 109.8, 115.3 (2 × CH), 116.9, 119.5, 123.2 (2 × CH), 125.6, 127.3 (2 × CH), 129.1, 129.8 (2 × CH), 131.9, 139.7, 141.8 (2 × C), 145.3, 155.0 ppm. λmax in nm (log εmax): 212 (4.6343), 233 (5.2124), 311 (4.6750). FT-IR ν in cm−1: 3407 (N—H), 3263 (N—H), 1377 (SO2), 1187 (SO2). Anal. Calcd for C20H17N3O2S (363.43): C, 66.10; H, 4.71; N, 11.56%. Found: C, 65.95; H, 4.63; N, 11.75%.
2-(1H-Benzimidazol-2-yl)-N-(3-chlorobenzyl)aniline 2d
When d = 3-chlorobenzyl chloride, yield 87.90%, mp = N.D. (Oily), colour = black. 1H NMR (400 MHz, DMSO-d6) δH: 3.73 (s, 2H, CH2), 6.31–6.35 (m, 3H, Ph—H), 6.48–6.50 (m, 2H, Ph—H), 6.84–6.87 (d, J = 8.00 Hz, 1H, Ph—H), 7.11–7.14 (d, J = 11.96 Hz, 2H, Ph—H), 7.61–7.63 (d, J = 7.96 Hz, 2H, Ph—H), 7.92–7.94 (d, J = 7.72 Hz, 1H, Ph—H), 8.21 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 48.2 (CH2), 112.3, 114.1, 115.1 (2 × CH), 117.4, 123.2 (2 × CH), 125.0, 125.4, 126.1, 126.8, 129.3, 129.9, 132.2, 134.3, 141.8 (2 × C), 145.4, 154.8 ppm. λmax in nm (log εmax): 224 (5.0026), 251 (4.9633), 302 (4.7279). FT-IR ν in cm−1: 3460, 3354 (N—H), 3107 (C—H aromatic), 2924 (CH aliphatic), 2854 (CH aliphatic), 1624 (C C Aromatic), 1581 (C N). Anal. Calcd for C20H16N3Cl (333.81): C, 71.96; H, 4.84; N, 12.59%. Found: C, 72.14; H, 5.02; N, 12.38%.
5-((2-(1H-Benzimidazole-2-yl)phenyl)amino)pyrimidine-2,4(1H,3H)-dione 2e
When e = 5-bromouracil, yield 83.29%, mp > 300 °C, colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 6.36–6.39 (m, 1H. Ph—H), 6.50–6.52 (m, 1H. Ph—H), 6.83–6.86 (d, J = 11.88 Hz, 1H, Ph—H), 7.11–7.13 (d, J = 8.00 Hz, 2H, Ph—H), 7.39–7.43 (m, 2H, Ph—H), 7.62–7.65 (d, J = 11.88 Hz, 1H, Ph—H), 8.37–8.41 (d, J = 13.92 Hz, 1H, Ph—H), 11.02 (s, IH, NH), 11.56 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δC: 109.2, 110.6, 115.6 (2 × CH), 119.3, 123.1 (2 × CH), 125.3, 125.7, 126.2, 129.4, 141.8 (2 × C), 145.0, 152.5, 169.0 (C O), 169.8 (C O) ppm. λmax in nm (log εmax): 202 (4.5646), 224 (4.9854), 254 (5.1392). FT-IR (νmax in cm−1): 3460, 3354 (N—H), 3107 (C—H aromatic), 2924 (CH aliphatic), 2854 (CH aliphatic), 1624 (C C aromatic), 1581 (C N). Anal. Calcd for C17H13N5O2 (319.32): C, 63.94; H, 4.10; N, 21.93%. Found: C, 63.90; H, 3.99; N, 22.01%.
N-(2-(1H-Benzimidazole-2-yl)phenyl)benzene-1,4-diamine 2f
When f = 4-chloroaniline, yield 80.74%, mp > 300 °C, colour = gray. 1H NMR (400 MHz, DMSO-d6) δH: 5.32 (s, 2H, NH2), 6.33–6.38 (m, 2H, Ph—H), 6.49–6.53 (m, 2H, Ph—H), 6.83–6.86 (d, J = 9.92 Hz, 1H, Ph—H), 7.11–7.14 (d, J = 10.48 Hz, 2H, Ph—H), 7.39–7.41 (d, J = 8.50 Hz, 2H, Ph—H), 7.59–7.62 (d, J = 10.48 Hz, 2H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 109.8, 115.3 (2 × C), 117.2 (2 × CH), 118.1, 119.5, 121.1 (2 × CH), 123.0 (2 × CH), 125.6, 129.8, 132.2, 137.9, 140.9, 141.8 (2 × C), 154.8 ppm. λmax in nm (log εmax): 224 (5.0228), 254 (1.9881), 308 (4.5453). FT-IR (νmax in cm−1): 3436 (N—H of 1° amine), 3406 (N—H of 1° amine), 3330 (N—H of 2° amine), 3060 (C—H aromatic), 1613 (C C aromatic), 1577 (C N). Anal. Calcd for C19H16N4 (300.36): C, 75.98; H, 5.37; N, 18.65%. Found: C, 76.09; H, 5.40; N, 18.45%.
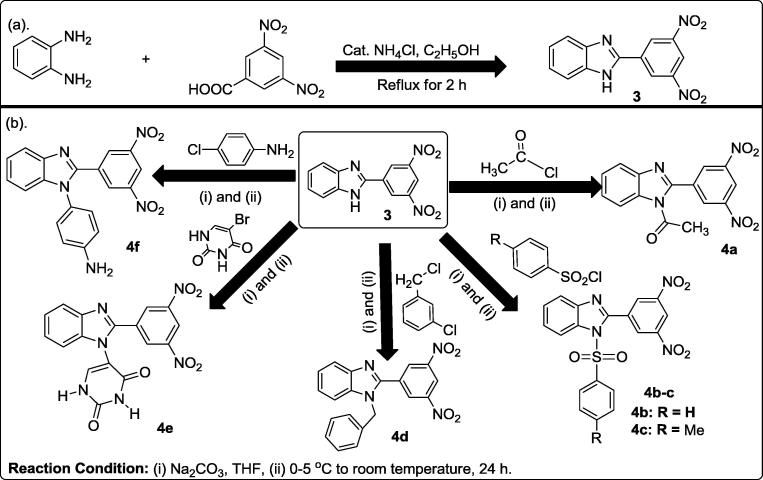
2-(3,5-Dinitrophenyl)-1H-benzimidazole as precursor 3
Procedure for the synthesis of precursor 1 was repeated for the reaction of o-phenylenediamine with 3,5-dinitrophenylbenzoic acid to afford precursor 3 (90.07%), mp = 177–179 °C, colour = yellow. 1H NMR (400 MHz, DMSO-d6) δH: 7.40–7.42 (d, J = 8.00 Hz, 1H, Ph—H), 7.44–7.46 (d, J = 8.00 Hz, 1H, Ph—H), 7.72–7.74 (t, J = 7.58 Hz, 1H, Ph—H), 7.94–7.96 (m, 1H, Ph—H), 8.64 (s, 2H, Ph—H), 8.85 (s, 1H, Ar—H). 13C NMR (100 MHz, DMSO-d6) δC: 115.2 (2 × CH), 125.8, 128.7 (2 × CH), 129.4 (2 × CH), 134.8, 144.2 (2 × C), 150.7 (2 × C), 156.1 ppm. λmax in nm (log εmax): 218 (4.6522), 248 (4.6365). FT-IR (νmax in cm−1): 3349 (N—H), 3172 (C—H aromatic), 3101 (C—H aromatic), 1606 (C C), 1572 (C N), 1543 (NO2 asym.), 1344 (NO2 sym.). Anal. Calcd for C13H8N4O4 (284.22): C, 55.13; H, 2.49; N, 19.78%. Found: C, 54.98; H, 2.54; N, 19.92%.
Overall protocol towards accessing 1,2-disubstituted-1H-benzimidazole 4a-f
Similar procedure for 2a-f was repeated herein using 2-(3,5-dinitrophenyl)-1H-benzimidazole 3 as the precursor which reacted with substrates a-f to afford 1-substituted-2-(3,5-dinitrophenyl)-1H-benzimidazoles 4a-f.
1-(2-(3,5-Dinitrophenyl)-1H-benzimidazole-1-yl)ethanone 4a
Yield 72.58%, mp = 250–252 °C, colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 2.59 (s, 3H, CH3—CO), 7.40–7.42 (d, J = 8.00 Hz, 1H, Ph—H), 7.44–7.46 (d, J = 8.00 Hz, 1H, Ph—H), 7.72–7.74 (t, J = 7.56 Hz, 1H, Ph—H), 7.94–7.96 (m, 1H, Ph—H), 8.64 (s, 2H, Ph—H), 8.85 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 28.9 (CH3), 116.6 (2 × CH), 126.1, 128.9 (2 × CH), 129.7 (2 × CH), 135.0, 144.3 (2 × C), 150.5 (2 × C), 156.3, 174.1 (C O) ppm. λmax in nm (log εmax): 218 (4.3729), 248 (4.3050). FT-IR (νmax in cm−1): 3106 (C—H aromatic), 2854 (C—H aliphatic), 1699 (C O amide), 1622 (C C), 1580 (C N), 1541 (NO2asym.), 1346 (NO2 sym.). Anal. Calcd for C15H10N4O5 (326.26): C, 55.22; H, 3.09; N, 17.17%. Found: C, 55.13; H, 2.89; N, 17.08%.
2-(3,5-Dinitrophenyl)-1-(phenylsulfonyl)-1H-benzimidazole 4b
Yield 62.77%, mp > 300 °C, colour = gray. 1H NMR (400 MHz, DMSO-d6) δH: 7.15–7.19 (m, 5H, Ph—H), 7.40–7.46 (m, 2H, Ph—H), 7.72–7.74 (t, J = 7.60 Hz, 1H, Ph—H), 7.94–7.96 (m, 1H, Ph—H), 8.64 (s, 2H, Ph—H), 8.85 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 116.6 (2 × CH), 119.6 (2 × CH), 126.1, 128.9 (2 × CH), 129.7 (2 × CH), 132.5, 135.0 (2 × C), 137.5 (2 × CH), 144.3 (2 × C), 150.5, 152.0, 156.3 ppm. λmax in nm (log εmax): 215 (3.9395), 251 (4.1492). FT-IR (νmax in cm−1): 3050 (C—H aromatic), 2855 (C—H aliphatic), 1620 (C C), 1580 (C N), 1541 (NO2asym.), 1376 (SO2), 1346 (NO2 sym.), 1185 (SO2 2nd band). Anal. Calcd for C19H12N4O6S (434.39): C, 53.77; H, 2.85; N, 13.20%. Found: C, 53.95; H, 3.03; N, 13.31%.
2-(3,5-Dinitrophenyl)-1-tosyl-H-benzimidazole 4c
Yield 98.77%, mp = 112 °C, colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 2.60 (s, 3H, CH3—Ar), 6.90–6.92 (d, J = 8.00 Hz, 2H, Ph—H), 7.15–7.17 (d, J = 8.00 Hz, 2H, Ph—H), 7.40–7.44 (m, 2H, Ph—H), 7.73–7.75 (t, J = 7.54 Hz, 1H, Ph—H), 7.93–7.95 (m, 1H, Ph—H), 8.60 (s, 2H, Ph—H), 8.84 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 21.7 (CH3), 116.6 (2 × CH), 119.6 (2 × CH), 124.8, 156.3, 152.1, 150.5, 144.3 (2 × C), 137.5 (2 × CH), 135.0, 132.5, 129.5 (2 × CH), 126.0, 128.9 (2 × CH) ppm. λmax in nm (log εmax): 242 (4.6138). FT-IR (νmax in cm−1): 3105 (C—H aromatic), 2852 (C—H aliphatic), 1620 (C C), 1575 (C N), 1540 (NO2 asym.), 1377 (SO2), 1345 (NO2 sym.). Anal. Calcd for C20H14N4O6S (438.41): C, 54.79; H, 3.22; N, 12.78%. Found: C, 54.62; H, 3.16; N, 12.94%.
1-(3-Chlorobenzyl)-2-(3,5-dinitrophenyl-1H-benzimidazole 4d
Yield 71.16%, mp > 300 °C, colour = black. 1H NMR (400 MHz, DMSO-d6) δH: 3.72 (s, 2H, CH2—Ar), 7.11–7.14 (m, 1H, Ph—H), 7.25–7.27 (d, J = 7.56 Hz, 2H, Ph—H), 7.40–7.46 (m, 2H, Ph—H), 7.72–7.74 (t, J = 7.60 Hz, 1H, Ph—H), 7.94–7.96 (d, J = 7.22 Hz, 1H, Ph—H), 8.21 (s, 1H, Ph—H), 8.64 (s, 2H, Ph—H), 8.85 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 48.0 (CH2), 115.2, 116.2 (2 × CH), 119.2, 125.1, 126.1, 128.9 (2 × CH), 129.7 (2 × CH), 130.0, 135.0, 139.1, 144.0 (2 × C), 150.4 (2 × C), 156.1 ppm. λmax in nm (log εmax): 212 (3.7076), 248 (4.1986), 467 (2.6020), 470 (2.6020). FT-IR (νmax in cm−1): 2924, 2854 (CH aliphatic), 1612 (C C Aromatic), 1584 (C N imine), 1501 (NO2 asym.). Anal. Calcd for C20H13N4O4Cl (408.79): C, 58.76; H, 3.21; N, 13.71%. Found: C, 58.88; H, 3.32; N, 13.89%.
5-(2-(3,5-Dinitrophenyl)-1H-benzimidazol-1-yl)pyrimidine-2,4(1H,3H)-dione 4e
Yield 61.04%, mp > 300 °C, colour = gray. 1H NMR (400 MHz, DMSO-d6) δH: 7.40–7.42 (t, J = 7.24 Hz, 1H, Ph—H), 7.44–7.46 (m, 1H, Ph—H), 7.72–7.74 (d, J = 8.00 Hz, 2H, Ph—H), 7.94–7.96 (s, 1H, Ph—H), 8.64 (s, 2H, Ph—H), 8.85 (s, 1H, Ph—H), 11.05 (s, IH, NH), 11.55 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δC: 116.6 (2 × CH), 126.1, 128.9 (2 × CH), 129.7 (2 × CH), 135.0, 138.7, 140.1, 144.3 (2 × C), 150.5 (2 × C), 156.3, 169.0 (C O), 169.7 (C O) ppm. λmax in nm (log εmax): 220 (4.0234), 242 (4.8730), 311 (4.0790). FT-IR (νmax in cm−1): 3362 (N—H), 3217 (N—H), 3050 (C—H aromatic), 1685 (C O amide), 1612 (C C aromatic), 1575 (C N), 1536 (NO2 asym), 1344 (NO2 sym.). Anal. Calcd for C17H10N6O6 (394.30): C, 51.78; H, 2.56; N, 21.31%. Found: C, 51.97; H, 2.69; N, 21.51%.
4-(2-(3,5-Dinitrophenyl)-1H-benzimidazole-1-yl)aniline 4f
Yield 66.85%, mp > 300 °C, colour = gray. 1H NMR (400 MHz, DMSO-d6) δH: 4.50 (s, 2H, NH2), 6.90–6.92 (d, J = 8.00 Hz, 2H, Ph—H), 7.15–7.17 (d, J = 8.00 Hz, 2H, Ph—H), 7.40–7.44 (m, 2H, Ph—H), 7.73–7.76 (t, J = 7.84 Hz, 1H, Ph—H), 7.93–7.96 (m, 1H, Ph—H), 8.69 (s, 2H, Ph—H), 8.90 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 115.2, 116.6 (2 × CH), 118.3 (2 × CH), 125.3, 126.1, 128.9 (2 × CH), 129.7 (2 × CH), 135.0, 144.1 (2 × C), 147.0, 147.8, 150.5 (2 × C), 155.4 ppm. λmax in nm (log εmax): 235 (5.4130), 302 (4.8040). FT-IR (νmax in cm−1): 3472 (N—H), 3363 (N—H), 3214, 1620 (C C aromatic), 1536 (NO2 asym), 1321, 1377 (NO2sym). Anal. Calcd for C19H13N5O4 (375.34): C, 60.80; H, 3.49; N, 18.66%. Found: C, 61.00; H, 3.68; N, 18.84%.
2-Benzyl-1H-benzimidazole as precursor 5
Procedure for the synthesis of precursor 1 was repeated for the reaction of o-phenylenediamine with phenyl acetic acid to afford precursor 5 (79.12%), mp = 108–110 °C, colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 3.56 (s, 2H, CH2—Ar), 6.35–6.38 (dd, J1 = 3.48 Hz, J2 = 9.12 Hz, 1H, Ph—H), 6.48–6.50 (dd, J1 = 3.48 Hz, J2 = 8.00 Hz, 1H, Ph—H), 7.12 (s, 5H, Ph—H), 7.24–7.26 (d, J = 9.12 Hz, 1H, Ph—H), 7.29–7.31 (d, J = 8.00 Hz, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 34.9 (CH2), 115.2 (2 × CH), 123.4 (2 × CH), 125.8, 128.7 (2 × CH), 129.4 (2 × CH), 136.9, 138.8 (2 × C), 142.7 ppm. λmax in nm (log εmax): 257 (5.4181), 275 (5.4133), 314 (4.4842). FT-IR (νmax in cm−1): 3415 (N—H), 2924 (C—H aliphatic), 2854 (C—H aliphatic), 1638 (C C), 1587 (C N imine). Anal. Calcd for C14H12N2 (208.26): C, 80.74; H, 5.81; N, 13.45%. Found: C, 80.80; H, 6.01; N, 13.44%.
Overall protocol towards accessing 1,2-disubstituted-1H-benzimidazole 6a-f
Similar procedure for the synthesis of 2a-f was repeated herein using 2-benzyl-1H-benzimidazole 5 as the precursor which reacted with substrates a-f to afford 1-substituted-2-benzyl-1H-benzimidazoles 6a-f.
1-(2-Benzyl-1H-benzimidazole-1-yl)ethanone 6a
Yield 90.11%, mp = N.D. (Oily), colour = black. 1H NMR (400 MHz, DMSO-d6) δH: 2.67 (s, 3H, CH3CO), 3.56 (s, 2H, CH2—Ar), 6.36–6.39 (dd, J1 = 3.48 Hz, J2 = 9.12Hz, 1H, Ph—H), 6.48–6.50 (dd, J1 = 3.48 Hz, J2 = 8.00Hz, 1H, Ph—H), 7.09 (s, 5H, Ph—H), 7.24–7.26 (d, J = 9.12 Hz, 1H, Ph—H), 7.29–7.31 (d, J = 8.00 Hz, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 28.9 (CH3), 34.9 (CH2), 115.2 (2 × CH), 123.5 (2 × CH), 125.9, 128.7 (2 × CH), 129.5 (2 × CH), 136.9, 138.9 (2 × C), 142.8 ppm. λmax in nm (log εmax): 227 (2.2300), 315 (2.5057). FT-IR (νmax in cm−1): 2922 (C—H aliphatic), 2855 (C—H aliphatic), 1685 (C O), 1620 (C C), 1575 (C N imine). Anal. Calcd for C16H14N2O (250.30): C, 76.78; H, 5.64; N, 11.19%. Found: C, 76.94; H, 5.59; N, 11.00%.
2-Benzyl-1-(phenylsulfonyl)-1H-benzimidazole 6b
Yield 87.48%, mp = N.D. (Oily), colour = black. 1H NMR (400 MHz, DMSO-d6) δH: 3.56 (s, 2H, CH2—Ar), 6.35–6.38 (dd, J1 = 3.60 Hz, J2 = 9.26 Hz, 1H, Ph—H), 6.48–6.50 (dd, J1 = 3.60 Hz, J2 = 8.00 Hz, 1H, Ar—H), 6.91–6.95 (m, 3H, Ph—H), 7.12 (s, 5H, Ph—H), 7.13–7.17 (m, 3H, Ph—H), 7.22–7.24 (d, J = 9.26 Hz, 1H, Ph—H), 7.30–7.32 (d, J = 8.00 Hz, 1H, Ph—H), 7.44–7.46 (d, J = 8.66 Hz, 2H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 34.7 (CH2), 115.2 (2 × CH), 123.4 (2 × CH), 125.8, 128.1 (2 × CH), 128.7 (2 × CH), 129.0 (2 × CH), 129.9 (2 × CH), 133.9, 136.8, 137.9, 138.7 (2 × C), 142.9 ppm. λmax in nm (log εmax): 215 (4.4669), 254 (4.4540). FT-IR (νmax in cm−1): 2922, 2850 (CH aliphatic), 1620 (C C aromatic), 1575 (C N imine), 1375 (SO2), 1185 (SO2 2nd band). Anal. Calcd for C20H16N2O2S (348.42): C, 68.94; H, 4.63; N, 8.04%. Found: C, 69.00; H, 4.82; N, 7.89%.
2-Benzyl-1-tosyl-1H-benzimidazole 6c
Yield 93.25%, mp = N.D. (Oily), colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 2.76 (s, 3H, CH3—Ar), 3.56 (s, 2H, CH2—Ar), 6.35–6.38 (dd, J1 = 3.60 Hz, J2 = 9.26 Hz, 1H, Ph—H), 6.48–6.49 (dd, J1 = 3.60 Hz, J2 = 8.00 Hz, 1H, Ph—H), 6.92–6.94 (d, J = 8.76 Hz, 2H, Ph—H), 7.12 (s, 5H, Ph—H), 7.22–7.24 (d, J = 9.26 Hz, 1H, Ph—H), 7.30–7.32 (d, J = 8.00 Hz, 1H, Ph—H), 7.44–7.46 (d, J = 8.76 Hz, 2H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 21.5(CH3), 34.9 (CH2), 115.2 (2 × CH), 123.4 (2 × CH), 125.8, 128.1, (2 × CH), 128.7 (2 × CH), 129.5 (2 × CH), 130.1 (2 × CH), 134.7, 136.7, 138.9 (2 × C), 139.6, 142.6 ppm. λmax in nm (log εmax): 233 (5.3679), 299 (4.7896). FT-IR (νmax in cm−1): 2924, 2854 (CH aliphatic), 1648 (C C aromatic), 1562 (C N imine), 1376 (SO2), 1185 (SO2 2nd band). Anal. Calcd for C21H18N2O2S (362.44): C, 69.59; H, 5.01; N, 7.73%. Found: C, 69.51; H, 4.88; N, 7.82%.
2-Benzyl-1-(3-chlorobenzyl)-1H-benzimidazole 6d
Yield 91.83%, mp = N.D. (Oily), colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 3.42 (s, 2H, CH2—Ar), 3.58 (s, 2H, CH2—Ar), 6.35–6.38 (dd, J1 = 3.48 Hz, J2 = 9.18 Hz, 1H, Ph—H), 6.48–6.50 (dd, Jm1 = 3.48 Hz, J2 = 8.00 Hz, 1H, Ph—H), 7.10 (s, 5H, Ph—H), 7.17–7.19 (dd, J1 = 7.20 Hz, J2 = 7.82 Hz, 1H, Ph—H), 7.23–7.25 (d, J = 9.18 Hz, 1H, Ph—H), 7.31–7.33 (d, J = 8.00 Hz, 1H, Ph—H), 7.37–7.38 (d, J = 7.82 Hz, 1H, Ph—H), 7.51–7.52 (d, J = 7.20 Hz, 1H, Ph—H), 8.21 (s, 1H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 33.8 (CH2), 48.0 (CH2), 115.2 (2 × CH), 123.4 (2 × CH), 125.1 125.6, 125.8, 128.7 (2 × CH), 128.9, 129.5 (2 × CH), 130.2, 134.4, 136.7, 137.8, 138.9 (2 × C), 142.5 ppm. λmax in nm (log εmax): 233 (5.3877), 251 (5.0266). FT-IR (νmax in cm−1): 2984 (C—H aliphatic), 1646 (C C). 1565 (C N), 652 (C—Cl). Anal. Calcd for C20H17ClN2 (332.83): C, 75.78; H, 5.15; N, 8.42%. Found: C, 75.70; H, 4.01; N, 8.25%.
5-(2-Benzyl-1H-benzimidazole-1-yl)pyrimidine-2,4(1H,3H)-dione 6e
Yield 80.58%, mp = N.D. (Oily), colour = black. 1H NMR (400 MHz, DMSO-d6) δH: 3.56 (s, 2H, CH2—Ar), 6.34–6.37 (dd, J1 = 3.44 Hz, J2 = 9.18 Hz, 1H, Ph—H), 6.48–6.50 (dd, J1 = 3.44 Hz, J2 = 8.00 Hz, 1H, Ph—H), 7.11 (s, 5H, Ph—H), 7.23–7.25 (d, J = 9.18 Hz, 1H, Ph—H), 7.30–7.32 (d, J = 8.00 Hz, 1H, Ph—H), 7.95–7.97 (s, 1H, Ph—H), 11.05 (s, IH, NH), 11.55 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δC: 34.8 (CH2), 115.2 (2 × CH), 119.9, 123.3 (2 × CH), 125.7, 128.7 (2 × CH), 129.4 (2 × CH), 136.7, 138.5 (2 × C), 142.9, 150.0, 169.2 (C O), 169.7 (C O) ppm. λmax in nm (log εmax): 218 (4.6532), 248 (4.5775), 293 (4.1399), 437 (3.4914). FT-IR (νmax in cm−1): 3360 (N—H), 3217 (N—H), 3050 (C—H aromatic), 1685 (C O amide), 1615 (C C aromatic), 1575 (C N). Anal. Calcd for C18H14N4O2 (318.33): C, 67.91; H, 4.43; N, 17.60%. Found: C, 68.10; H, 4.57; N, 17.77%.
4-(2-Benzyl-1H-benzimidazole-1-yl) aniline 6f
Yield 86.24%, mp = N.D. (Oily), colour = brown. 1H NMR (400 MHz, DMSO-d6) δH: 3.56 (s, 2H, CH2—Ar), 4.50 (s-br, 2H, NH2), 6.35–6.38 (dd, J1 = 3.46 Hz, J2 = 9.12 Hz, 1H, Ph—H), 6.48–6.50 (dd, J1 = 3.46 Hz, J2 = 8.00 Hz, 1H, Ph—H), 7.11 (s, 5H, Ph—H), 7.16–7.18 (d, J = 8.20 Hz, 2H, Ph—H), 7.24–7.26 (d, J = 9.12 Hz, 1H, Ph—H), 7.29–7.31 (d, J = 8.00 Hz, 1H, Ph—H), 7.44–7.46 (d, J = 8.20 Hz, 2H, Ph—H). 13C NMR (100 MHz, DMSO-d6) δC: 34.9 (CH2), 115.2 (2 × CH), 118.7 (2 × CH), 123.4 (2 × CH), 125.8, 126.2 (2 × CH), 128.7 (2 × CH), 129.4 (2 × CH), 136.9, 138.8 (2 × C), 142.7, 146.8, 148.7 ppm. λmax in nm (log εmax): 230 (5.2753), 251 (5.1322), 299 (4.8401). FT-IR (νmax in cm−1): 3404 (N—H interfered by OH), 2985 (C—H aliphatic), 1610 (C C aromatic), 1572 (C N). Anal. Calcd for C20H17N3 (299.37): C, 80.24; H, 5.72; N, 14.04%. Found: C, 80.21; H, 5.90; N, 13.85%.
Antibacterial sensitivity testing of compounds 1–6f
All the synthesized compounds (1–6f) and gentamicin were screened for antibacterial activity on four bacterial strains using agar well diffusion method [26]. The detailed was as attached in Supplementary Materials.
Minimum inhibitory/bactericidal concentration (MIC and MBC) testing
The minimum inhibitory concentration (MIC) test on the chosen organisms was carried out via serial dilution technique [27] and the concentration range was from 500.00 to 15.63 µg/mL, while the minimum bactericidal concentration (MBC) was determined by a standard method [26]. The detailed description of the procedures for the determination of MIC and MBC were as presented in the supplementary material.
Results and discussion
Based on the enthusiastic outcome of an extensive review on functionalized benzimidazole [3] and in the continuation of the research effort in the area of benzo-fused imidazole moieties [6], [28], the synthesis of functionalized 2-substituted and 1,2-disubstituted benzimidazole derivatives was herein reported to evaluate their antibacterial activities. Although, various derivatives of benzimidazole moieties have been synthesized and reported in literature; high temperature for the reflux process and the uses harsh reaction condition in the presence of strong and concentrated acids such as HCl [4], [6] have been involved. On the contrary, the synthesis of 2-(1H-benzimizadol-2-yl)aniline precursor 1, was achieved herein by the use of eco-friendly reaction of o-phenylenediamine with anthranilic acid using a catalytic amount of NH4Cl in the presence of ethyl alcohol as a solvent at refluxing temperature of 60–70 °C (Scheme 1a). The synthetic modification of NH2 functional side chain of the reactive intermediate 1 was conducted by reacting it with six different electrophile-releasing substrates to furnish 2a-f. Prior to this, the reaction optimization study was carried out using two main parameters. First, solvent dependent condition was investigated using the synthesis of N-(2-(1H-benzimidazole-2-yl)phenyl)acetamide 2a via the reaction of 1 with acetyl chloride in either tetrahydrofuran (THF), ethanol or acetonitrile at room temperature for 24 h; this gave yield of 87.7%, 30%, and 25% respectively. In addition, the thermodynamic dependent kinetic of the synthesis of 2a was evaluated using the comparative study of the synthesis in THF at room temperature, 60 °C, and 120 °C. It was unveiled that room temperature gave the highest yield (87.71%) followed by 60 °C (38.24%), while there was no isolated product at 120 °C. Thus, the same reaction condition was adopted for reaction of 1 with the remaining five electrophile-releasing substrates b-f to produce diverse functionalized 2-substitutedbenzimidazole derivatives 2b-f (Scheme 1b). According to another route [29], the o-phenylenediamine reacted with 3,5-dinitrobenzoic acid to achieve 3 Scheme 2a, which was subsequently treated with the earlier reported six electrophile-releasing substrates to furnish 1,2-disubstituted benzimidazole derivatives 4a-f (Scheme 2b) in varying yields. Finally, the last precursor 5 in this present work was prepared by the condensation of o-phenylenediamine with phenyl acetic acid (Scheme 3a). Precursor 5 was eventually reacted with the six electrophile-releasing substrates to access the 1,2-disubstituted benzimidazole derivatives 6a-f (Scheme 3b).
Scheme 1.
(a) Synthesis of the precursor 1 (b) Synthesis of 2-substitutedbenzimidazoles 2a-f.
Scheme 2.
(a) Synthesis of the precursor 3 (b) Synthesis of 1,2-disubstitutedbenzimidazoles 4a-f.
Scheme 3.
(a) Synthesis of the precursor 5 (b) Synthesis of 1,2-disubstitutedbenzimidazoles 6a-f.
Physicochemical parameter data were presented in experimental section alongside the elemental analysis data. The result of elemental analysis for the % calculated agreed with % found for C, H N of all the synthesized compounds with high state of accuracy (the difference was not more that ±0.20 in all cases). Furthermore, the spectroscopic characterization of the targeted templates was carried out using IR and UV, 1H, and 13C NMR analysis. The 1H NMR spectra of the compounds were run in DMSO-d6 at 400 MHz with chemical shift values recorded in ppm. The aryl-linked CH3 of 2c, 4c, and 6c resonated upfield at δ 2.60–2.80 ppm as 3H singlets and acetyl-linked CH3 of 2a, 4a, and 6a were seen upfield as 3H singlets at δ 2.60–2.67 ppm. The aryl-linked CH2 of 2d, 4d, and 5 as well as 6a-f were noticed as singlets at δ 3.42–3.73 ppm. Signals of all aryl H appeared downfield of TMS around δ 6.31–8.90 ppm, NH2 of amine in 1, 2f, 4f, and 6f were broad singlets at δ 4.50–5.32 ppm. The most downfield signals were that of N—H of amide found as singlets in compounds 2e, 4e, and 6e at δ 11.02–11.56 ppm. The 13C NMR spectra were run in DMSO-d6 at 100 MHz with chemical shift values recorded in ppm. On the overall, the 13C NMR spectra of the structure-based benzimidazole derivatives varied from 21.3 ppm for CH3 of compound 2c to 175.4 ppm for C O of 2a. Specifically, the aryl-linked CH3 of 2c, 4c, and 6c appeared at δ 21.7–21.3 ppm, whereas the CH2 signals of 2d, 4d, and 6d resonated at δ 48.0–48.2 ppm. The formation of acetamide in 2a, 4a, and 6a was validated by presence of CH3 intense singlet signal at δ 26.4–28.9 ppm, which was absent in the precursors 1, 3 and 5, respectively from which they were derived. The UV transition was run in tetrahydrofuran (THF) for the precursors 1, 2, and 3 and their final compounds 2a-f, 4a-f, and 6a-f. The lowest wavelengths observed at 202–224 nm, were due to electronic excitation of π→π∗ peculiar to C C, which depicted the presence of benzene ring in those structures. Bathochromic shifts observed herein led to the presence of other peaks at higher wavelengths (233 nm to 470 nm). Some of these shifts were because of π→n transition, which was attributable to the presence of auxochromic C N group; which belong to K bands [28], [30]. The FT-IR data of the benzimidazole derivatives 4a-f revealed the stretching frequencies of C—H aromatic, C C and C N at 3106–3050, 1622–1600, and 1580–1575 cm−1, respectively [28]. Additional bands were noticed in 2a, 4a, and 6a at 1699–1685 cm−1, which represents the C O of amide. The two bands at 1377–1375 and 1187–1185 cm−1, which were domiciled in sulfonamide 2b-c, 4b-c, and 6b-c, were peculiarly assigned to SO2 functionality. The bands at 1543–1540 cm−1 depicted the presence of NO2 (asym.) in compounds 3 and 4a-f. Therefore, the extrapolated spectroscopic information of targeted benzimidazole motifs was in concordance with the proposed structures.
Antibacterial activity
The general sensitivity testing was evaluated using the in vitro screening of the synthesized compounds against four bacterial isolates (Staphylococcus aureus, Bacillus licheniformis, Proteus vulgaris, and Pseudomonas aeruginosa). Gentamicin was used as the positive control in this study. The justification of gentamicin as a clinical standard was due to the mode of action, which involved irreversible binding at ribosomal level, thereby signaling to obstruct and interrupt protein synthesis [31]. Agar diffusion method was used for the sensitivity testing and the diameters of zones of inhibition (Z. O. I) were documented in millimeter (Table 1). Although, large zones of inhibition were noticed for most of the targeted benzimidazole derivatives final products against the screened organisms, resistance was observed in few cases such as 6c against S. aureus; 4d-f, 6a against Bacillus licheniformis; 2a, 2c, 4a, 4d, and 6f against Proteus vulgaris; 2e, 4d-f, and 6a against Pseudomonas aeruginosa, and 6f developed resistant against the effect of gentamicin. Overall, the largest zone of inhibition (40.00 ± 0.10 mm) was recorded for 1 against S. aureus, while the lowest zone of inhibition (15.00 ± 0.08 mm) was recorded for 3 against S. aureus. In comparison with gentamicin, all synthesized compounds, except 3, had better activity with higher zones of inhibition against growth potential of S. aureus. This means that the array of compounds synthesized herein might be a possible replacement for gentamicin on infectious disease caused by the S. aureus or enhance the potency of gentamicin where resistance issues occur. Compared to gentamicin, all compounds except 3 and 6c showed larger zones of inhibition against growth of S. aureus (i.e. >16 mm); and except 4d-f and 6c against B. licheniformis (i.e. >16 mm), while 3 and 4c exhibited approximately the same Z.O.I. as gentamicin against S. aureus (15 mm) and B. licheniformis (16 mm), respectively. Interestingly, more than 75% of the targeted benzimidazole derivatives were active on P. vulgaris with large zones of inhibition, whereas this organism was resistant to gentamicin (Table 1). Similarly, more than 75% of the targeted benzimidazole derivatives (Z.O.I = 25.00 ± 0.08 to 38.00 ± 0.12 mm) were more active than gentamicin (20.00 ± 0.08 mm) upon P. aeruginosa. In the present study, the choice of S. aureus and E. coli was due to the broad array of pathogenic infections and precarious health issue that are associated with these bacterial strains [32] and wide reported occurrence of resistant strain of S. aureus [1]. S. aureus has been reported to have strong relationship with death rate increase in human population, prolong admission of patients in hospitals, and the infections caused by this bacterial strain are expensive to treat [33]. Due to heat stable toxin production, S. aureus is enlisted as one of the highly invasive organisms referred to as pyogenic cocci involved in numerous adverse infectious conditions in humans [28], [32], [34]. In view of the reported predicament aforementioned and large zones of inhibition experienced via action of the benzimidazole framework herein on S. aureus, motivation was enhanced to investigate the activity index (A.I.) of the synthesized compounds against this organism (Fig. 1). It is quite impressive to note that all the synthesized benzimidazole motifs herein, showed better activity indices than gentamicin against S. aureus except 3 and 6c. The compound 3 competed favourably with gentamicin (A.I. ≈ 1.00) while 6c developed resistance; hence could not have activity index.
Table 1.
Antibacterial sensitivity testing with zones of inhibition in millimetre.
| Compound No↓ | S. aureus | B. licheniformis | P. vulgaris | P. aeruginosa |
|---|---|---|---|---|
| 1 | 40.00 ± 0.10 | 26.00 ± 0.08 | 35.00 ± 0.09 | 38.00 ± 0.12 |
| 2a | 27.00 ± 0.09 | 26.00 ± 0.08 | R | 28.00 ± 0.08 |
| 2b | 30.00 ± 0.09 | 20.00 ± 0.08 | 25.00 ± 0.09 | 30.00 ± 0.08 |
| 2c | 35.00 ± 0.09 | 25.00 ± 0.08 | R | 28.00 ± 0.08 |
| 2d | 30.00 ± 0.10 | 28.00 ± 0.09 | 30.00 ± 0.09 | 30.00 ± 0.09 |
| 2e | 32.00 ± 0.12 | 26.00 ± 0.08 | 35.00 ± 0.08 | R |
| 2f | 30.00 ± 0.11 | 30.00 ± 0.12 | 28.00 ± 0.08 | 30.00 ± 0.08 |
| 3 | 15.00 ± 0.08 | 20.00 ± 0.08 | 30.00 ± 0.12 | 28.00 ± 0.09 |
| 4a | 20.00 ± 0.10 | 24.00 ± 0.09 | R | 25.00 ± 0.08 |
| 4b | 30.00 ± 0.10 | 18.00 ± 0.08 | 20.00 ± 0.08 | 25.00 ± 0.08 |
| 4c | 25.00 ± 0.10 | 16.00 ± 0.08 | 20.00 ± 0.08 | 25.00 ± 0.08 |
| 4d | 30.00 ± 0.09 | R | R | R |
| 4e | 30.00 ± 0.10 | R | 20.00 ± 0.08 | R |
| 4f | 30.00 ± 0.12 | R | 23.00 ± 0.08 | R |
| 5 | 30.00 ± 0.09 | 28.00 ± 0.09 | 32.00 ± 0.12 | 38.00 ± 0.08 |
| 6a | 19.00 ± 0.08 | R | 18.00 ± 0.08 | R |
| 6b | 35.00 ± 0.09 | 22.00 ± 0.08 | 30.00 ± 0.10 | 28.00 ± 0.08 |
| 6c | R | 18.00 ± 0.08 | 32.00 ± 0.08 | 26.00 ± 0.08 |
| 6d | 32.00 ± 0.10 | 26.00 ± 0.08 | 35.00 ± 0.12 | 30.00 ± 0.08 |
| 6e | 25.00 ± 0.08 | 24.00 ± 0.08 | 25.00 ± 0.08 | 30.00 ± 0.09 |
| 6f | 20.00 ± 0.08 | 26.00 ± 0.08 | R | 28.00 ± 0.08 |
| Gentamicin | 16.00 ± 0.09 | 16.00 ± 0.08 | R | 20.00 ± 0.08 |
R = Resistance. Mean ± SD of triplicate determination.
Fig. 1.
Comparative study of the activity index of synthesized benzimidazoles and gentamicin.
Furthermore, based on high susceptibility of the organisms to the synthesized benzimidazole templates 1-6f and broad spectrum of activity observed herein, the MIC testing was carried out to determine the lowest concentration of the compound solution that conveniently inhibited the bacterial growth (Table 2). It was carried out using serial dilution method (500, 250, 125, 62.50, 31.25, and 15.63 µg/mL) via an earlier reported method [27]. The MIC of benzimidazole derivatives upon S. aureus varied from 15.63 ± 1.63 to 250 ± 2.66 µg/mL; against B. licheniformis varied from 62.50 ± 2.04 to 250 ± 2.65 µg/mL; against P. vulgaris varied from 31.25 ± 1.94 to 250 ± 2.65 µg/mL; and against P. aeruginosa ranged from 15.63 ± 1.63 to 125 ± 2.45 µg/mL. The best activity against S. aureus was observed in the precursor 1 among the series of the 2-substituted benzimidazoles 1-2f. The presence of amino functionality in 1 and the ready availability of its lone pair of electron for coordination led to improved activity. The activity decreases after the NH2 had underwent substitution as contained in 2a-f, except for 2c and 2e, which competed favorably with 1. This means that incorporation of p-toluenesulfonamido (in 2c) and pyrimidinedione (in 2e) played significant role as essential pharmacophores in increased activity observed in 2c and 2e against S. aureus. On the contrary, the series of 1,2-disubstituted benzimidazoles 4a-f were more active than the precursor 3 from which they were derived. This showed that additional substitution on position 1 of compound 3 to afford 1,2-disubstitution in 4a-f, was a worthwhile adventure in increasing the bioactivity of precursor 3, since the series of 1,2-disubstituted benzimidazole products 4a-f resulted in drastic improvement of growth inhibition in S. aureus. In addition to MIC testing, the minimum bactericidal concentration (MBC) testing was determined to authenticate the lowest concentration of the benzimidazole solution that causes death of the bacterium targeted per time and the results are shown in Fig. 2. Apart from where the MBC was not determined (N.D.) due to occurrence of resistance, all other cases showed that the MBC values were double the MIC in each consideration, except in 6a alone where MBC (500 µg/mL) was 4 times that of MIC (125 µg/mL) against P. vulgaris. From all indications, the lowest MBC trend was observed for the bio-assay screening of benzimidazole derivatives upon S. aureus, whereas the highest MBC was reported against P. aeruginosa as shown in Fig. 2.
Table 2.
Minimum inhibitory concentration (MIC) in µg/mL of targeted benzimidazoles, 1-6f.
| Compound No↓ | S. aureus | B. licheniformis | P. vulgaris | P. aeruginosa |
|---|---|---|---|---|
| 1 | 15.63 ± 1.64 | 125.00 ± 2.44 | 62.50 ± 2.04 | 31.25 ± 1.98 |
| 2a | 31.25 ± 1.98 | 125.00 ± 2.43 | N.D. | 62.50 ± 2.01 |
| 2b | 31.25 ± 1.97 | 250.00 ± 2.67 | 125.00 ± 2.44 | 31.25 ± 1.94 |
| 2c | 15.63 ± 1.67 | 125.00 ± 2.44 | N.D. | 62.50 ± 2.03 |
| 2d | 31.25 ± 1.96 | 125.00 ± 2.44 | 62.50 ± 2.01 | 62.50 ± 2.03 |
| 2e | 15.63 ± 1.63 | 250.00 ± 2.67 | 62.50 ± 2.01 | N.D. |
| 2f | 31.25 ± 1.94 | 62.50 ± 2.04 | 125.00 ± 2.44 | 31.25 ± 1.94 |
| 3 | 250.00 ± 2.66 | 250.00 ± 2.67 | 125.00 ± 2.44 | 62.50 ± 2.01 |
| 4a | 125.00 ± 2.45 | 125.00 ± 2.44 | N.D. | 62.50 ± 2.02 |
| 4b | 31.25 ± 1.95 | 250.00 ± 2.66 | 250.00 ± 2.67 | 62.50 ± 2.01 |
| 4c | 62.50 ± 2.01 | 250.00 ± 2.66 | 250.00 ± 2.66 | 31.25 ± 1.97 |
| 4d | 31.25 ± 1.98 | N.D. | N.D. | N.D. |
| 4e | 15.63 ± 1.65 | N.D. | 250.00 ± 2.65 | N.D. |
| 4f | 31.25 ± 1.94 | N.D. | 250.00 ± 2.67 | N.D. |
| 5 | 15.63 ± 1.67 | 62.50 ± 2.04 | 62.50 ± 2.03 | 15.63 ± 1.63 |
| 6a | 250.00 ± 2.66 | N.D. | 125.00 ± 2.44 | N.D. |
| 6b | 15.63 ± 1.63 | 250.00 ± 2.65 | 62.50 ± 2.04 | 62.50 ± 2.02 |
| 6c | N.D. | 250.00 ± 2.67 | 31.25 ± 1.94 | 125.00 ± 2.45 |
| 6d | 31.25 ± 1.94 | 125.00 ± 2.44 | 31.25 ± 1.95 | 31.25 ± 1.96 |
| 6e | 125.00 ± 2.45 | 125.00 ± 2.45 | 125.00 ± 2.44 | 62.50 ± 2.02 |
| 6f | 125.00 ± 2.43 | 125.00 ± 2.45 | N.D. | 62.50 ± 2.01 |
| Gentamicin | 5.00 ± 1.23 | 2.5 ± 1.09 | N.D. | 7.5 ± 1.45 |
N.D. = Not Determined. Mean ± SD of triplicate determination.
Fig. 2.
Graphical representation of minimum bactericidal concentration (MBC).
Structure activity relationship (SAR) study
From the overview of the structure activity relationship (SAR) study, it was found that the nature of substituent on 1-position and 2-positions of the benzimidazole nucleus had significant effect on the antibacterial activity of the entire structures. The compounds series 2a-f were structurally related in the core pharmacophoric 2-phenylbenzimidazole; the SAR study showed their activity against S. aureus to be in the order 2c ≈ 2e > 2a ≈ 2b ≈ 2d ≈ 2f. This means that the presence of electron donating CH3 on p-toluenesulfonamide moieties in the 2-position of anilino side chain played a significant role in the improvement of activity, since its counterpart 2b without CH3 was far less active and stayed in the categories of 2a, 2d, and 2f in its activity upon S. aureus growth inhibition. Considering 4a-f on S. aureus, the activity varied in the order of: 4e > 4b ≈ 4d ≈ 4f > 4c > 4a. Thus, electron withdrawing ability and π-π stacking character in pyrimidine-dione at 1-position of 2-(3,5-dinitrophenyl)benzimidazole core worked synergistically with the electron withdrawing NO2 on benzene at 2-position to increase the activity of 4e, thereby causing it to exhibit outstanding activity against S. aureus among the 4a-f series. Based on the in vitro screening of 6a-f against S. aureus, the order of activity was 6b > 6d > 6e ≈ 6f > 6a > 6c. Hence, presence of benzenesulfonamido group on 1-position of 2-benzylbenzimidazole in series 6a-f played a crucial role in activity boosting, making 6b to be the most active among the group and more active as compared to its isomorphic template 6c where no activity was noticed. It was interesting to note that p-methyl group in 6c which was the only group absent in 6b provided the framework 6a-f with antagonistic effect thereby causing total activity loss in 6c as compared to 6b against S. aureus.
Conclusions
Benzimidazole is an essential heterocyclic framework in agrochemicals, pharmaceuticals, and medicinal chemistry research. NH4Cl catalyzed strategy was found to be efficient approach for accessing the reported benzimidazole precursor in good yield. Thus, mono- and disubstituted benzimidazole derivatives with improved medicinal potential were successfully synthesized via an elegant pathway. The findings of the in vitro screening unveiled the broad spectrum of activity of the synthesized benzimidazole templates in the present study. Among the series, the highest potency was exerted and experienced in 2-(1H-benzimizadol-2-yl)aniline, 1 and 2-benzyl-1-(phenylsulfonyl)-1H-benzimidazole, 6b. It will be a worthwhile adventure to advance the work further for more guidance on the pharmacokinetic and pharmacodynamic study to ascertain probable candidature of the templates for future drug design.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
OOA is thankful to The World Academy of Sciences for the sponsorship of this project under the TWAS Research Grants Programme in Basic Sciences for Individual Scientists (Grant No. 14-069 RG/CHE/AF/AC_1). Covenant University is also gratefully acknowledged for support for this present work.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jare.2017.09.003.
Appendix A. Supplementary material
References
- 1.Ajani O.O., Familoni O.B., Aderohunmu D.V., Ogunniran K.O., Adekoya J.A., Olanrewaju I.O. Comparative study of the antibacterial activity of N,N-diethylamido substituted p-toluenesulfonamides to their α-toluenesulfonamide counterparts. Pak J Biol Sci. 2015;18:166–172. doi: 10.3923/pjbs.2015.166.172. [DOI] [PubMed] [Google Scholar]
- 2.Martins P., Jesus J., Santos S., Raposo L.R., Roma-Rodrigues C., Baptista P.V., et al. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules. 2015;20:16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajani O.O., Aderohunmu D.V., Ikpo C.O., Adedapo E.A., Olanrewaju I.O. Functionalized benzimidazole scaffolds: privileged heterocycle for drug design in therapeutic medicine. Arch Pharm. 2016;349:1–32. doi: 10.1002/ardp.201500464. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami K., Khodaei M.M., Kavianinia I. A simple and efficient one-pot synthesis of 2-substituted benzimidazoles. Synthesis. 2007;4:547–550. [Google Scholar]
- 5.Bahrami K., Khodaei M.M., Naali F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J Org Chem. 2008;73:6835–6837. doi: 10.1021/jo8010232. [DOI] [PubMed] [Google Scholar]
- 6.Ajani O.O., Ezeoke E.K., Edobor-Osoh A., Ajani O.A. Facile synthesis and characterization of new 2,3-disubstituted benzimidazole derivatives. Int Res J Pure Appl Chem. 2013;3:10–21. [Google Scholar]
- 7.Bahrami K., Khodaei M.M., Naali F. H2O2/Fe(NO3)3-promoted synthesis of 2-aryl benzimidazoles and 2-arylbenzothiazoles. Synlett. 2009;4:569–572. [Google Scholar]
- 8.Zhu C.J., Wei Y.Y. An inorganic iodine-catalyzed oxidative system for the synthesis of benzimidazoles using hydrogen peroxide under ambient conditions. Chemsuschem. 2011;4:1082–1086. doi: 10.1002/cssc.201100228. [DOI] [PubMed] [Google Scholar]
- 9.Goswami B., Singh A.K. Pharmacological activities of benzimidazole derivatives – Overview. Int J Sci Inno Discov. 2012;2(1):121–136. [Google Scholar]
- 10.Kondaparla S., Agarwal P., Srivastava K., Puri S.K., Katti S.B. Design, synthesis and in vitro antiplasmodial activity of some bisquinolines against chloroquine-resistant strain. Chem Biol Drug Des. 2017;89(6):901–906. doi: 10.1111/cbdd.12914. [DOI] [PubMed] [Google Scholar]
- 11.Patil S.A., Patil S.A., Patil R. Medicinal application of (benz)imidazole- and indole-based macrocycles. Chem Biol Drug Des. 2017;89:639–649. doi: 10.1111/cbdd.12802. [DOI] [PubMed] [Google Scholar]
- 12.El-Gohary N.S., Shaaban M.I. Synthesis and biological evaluation of a new series of benzimidazole derivatives as antimicrobial, antiquorum-sensing and antitumor agents. Eur J Med Chem. 2017;131:255–262. doi: 10.1016/j.ejmech.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Alasmary F.A.S., Snelling A.M., Zain M.E., Alafeefy A.M., Awaad A.S., Karodia N. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules. 2015;20:15206–15223. doi: 10.3390/molecules200815206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavrova A.T., Yancheva D., Anastassova N., Anichina K., Zvezdanovic J., Djordjevic A., et al. Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazole. Bioorg Med Chem. 2015;23(19):6317–6326. doi: 10.1016/j.bmc.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Shaharyar M., Mazumder A., Salahuddin, Garg R., Pandey R.D. Synthesis, characterization and pharmacological screening of novel benzimidazole derivatives. Arab J Chem. 2016;9 S342–S47. [Google Scholar]
- 16.Tang Y., Zhang F., Hu S., Cao Z., Wu Z., Jing W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: gravimetric, electrochemical, SEM and XPS studies. Corros Sci. 2013;74:271–282. [Google Scholar]
- 17.Abdel-Maksoud M., El-Shokry M., Ismail G., Hafez S., El-Kholy A., Attia E., et al. Methicillin -resistant Staphylococcus aureus recovered from healthcare- and community-associated infections in Egypt. Int J Bacteriol. 2016;2016:5. doi: 10.1155/2016/5751785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alotaibi F.E., Bukhari E.E. Emergence of Vancomycin-resistant Enterococci at a teaching hospital, Saudi Arabia. Chin Med J (Engl) 2017;130(3):340–346. doi: 10.4103/0366-6999.198923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davin-Regli A., Pagès J.M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392. doi: 10.3389/fmicb.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mwanyika G.O., Subbiah M., Buza J., Rugumisa B.T., Call D.R. A systematic review of antibiotic-resistant Escherichia coli and Salmonella data obtained from Tanzanian healthcare settings (2004–2014) Afr J Microbiol Res. 2017;11(2):45–54. [Google Scholar]
- 21.Mwanza S., Joshi S., Nambozi M., Chileshe J., Malunga P., Kabuya J.B.B., et al. The return of chloroquine-susceptible Plasmodium falciparum malaria in Zambia. Malar J. 2016;15:584. doi: 10.1186/s12936-016-1637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayandiran T.A., Dahunsi S.O. Microbial evaluation and occurrence of antidrug multi-resistant organisms among the indigenous Clarias species in River Oluwa, Nigeria. J King Saud Univ Sci. 2017;29:96–105. [Google Scholar]
- 23.More D. Sulpha medication allergies in: sulpha drug allergy. Available online at: <http://allergies.about.com/od/medicationallergies/a/sulfa.htm> [accessed on 1st of June, 2016].
- 24.Routledge P.A., O'Mahony M.S., Woodhouse K.W. Adverse drug reactions in elderly patients. Br J Clin Pharmacol. 2004;57(2):121–126. doi: 10.1046/j.1365-2125.2003.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslan M.M., Westerhaus M., Herce M., Nakashima K., Farmer P.E. Poverty, global health and infectious disease: lessons from Haiti and Rwanda. Infect Dis Clin North Am. 2011;25(3):611–622. doi: 10.1016/j.idc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajani O.O., Nwinyi O.C. Microwave assisted synthesis and evaluation of antimicrobial activity of 3-{3-(s-aryl and s-heteroaromatic) acryloyl}-2H-chromen-2-one derivatives. J Heterocycl Chem. 2010;47:179–187. [Google Scholar]
- 27.Ajani O.O., Obafemi C.A., Nwinyi O.C., Akinpelu D.A. Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioorg Med Chem. 2010;18(1):214–221. doi: 10.1016/j.bmc.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 28.Ajani O.O., Aderohunmu D.V., Olorunshola S.J., Ikpo C.O., Olanrewaju I.O. Facile synthesis, characterization and antimicrobial activity of 2-alkanamino benzimidazole derivatives. Orient J Chem. 2016;32(1):109–120. [Google Scholar]
- 29.Rithe S.R., Jagtap R.S., Ubarhande S.S. One pot synthesis of substituted benzimidazole derivatives and their characterization. Rasayan J Chem. 2015;8:213–217. [Google Scholar]
- 30.Komurcu S.G., Rollas S., Uglen M., Gorrod J.W. Evaluation of some aryl hydrazones of p-aminobenzoic acid hydrazide as antimicrobial agents and their in-vitro hepatic microsomal metabolism”. Boll Chim Farmac. 1995;134:375–379. [PubMed] [Google Scholar]
- 31.Prescott L.M., Harley J.P., Donald K.A. 6th ed. McGraw Hill; 2005. In microbiology. [Google Scholar]
- 32.Nwinyi O.C., Chinedu S.N., Ajani O.O., Ikpo C.O., Ogunniran K.O. Antibacterial effects of extracts of Ocimum gratissimum and Piper guineense on Escherichia coli and Staphylococcus aureus. Afr J Food Sci. 2009;3(3):77–81. [Google Scholar]
- 33.Nwinyi O.C., Chinedu N.S., Ajani O.O. Evaluation of antibacterial activity of Pisidium guajava and Gongronema latifolium. J Med Plant Res. 2008;2(8):189–192. [Google Scholar]
- 34.Ajani O.O., Familoni O.B., Wu F., Echeme J.O., Sujiang Z. Room temperature synthesis and antibacterial activity of new sulfonamides containing N,N-diethyl-substituted amido moieties. Int J Med Chem. 2012;2012:13. doi: 10.1155/2012/36781. Article ID 367815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.