Abstract
Objectives. To assess the effectiveness of Short Message Service (SMS) reminder messages on antiretroviral and cotrimoxazole prophylaxis adherence among HIV-positive youths as well as the relative effectiveness of SMS with and without a response option.
Methods. Eligible HIV-positive patients aged 15 to 22 years at 2 HIV clinics in Kampala, Uganda, participated in a year-long parallel individual-randomized controlled trial and were assigned in a 1-to-1-to-1 ratio to a weekly SMS message group, weekly SMS message with response option group, or a usual-care control group.
Results. We enrolled 332 participants. Electronically measured mean adherence was 67% in the control group, 64% in the 1-way SMS group (95% confidence interval [CI] = 0.77, 1.14), and 61% in the 2-way SMS group (95% CI = 0.75, 1.12) in an intent-to-treat analysis. Results for secondary outcomes and complete-case analysis were similarly statistically insignificant across groups.
Conclusions. Despite previous evidence that interventions using SMS reminders can promote antiretroviral therapy adherence, this study shows that they are not always effective in achieving behavior change. More research is needed to find out for whom, and under what conditions, they can be beneficial.
Trial registration. ClinicalTrials.gov identifier: NCT00830622.
Ownership of mobile phones has increased dramatically over the past decade and a half, particularly in low-income countries. This growth has spurred the development of mobile health (mHealth) applications including the use of Short Message Service (SMS) text messages as a potentially low-cost and scalable means to reach large fractions of the population in low-resource settings. By 2011, a World Bank report identified more than 500 mHealth projects that have proliferated throughout low-income countries.1
mHealth interventions have been applied to support antiretroviral therapy (ART). Use of ART has been shown to reduce mortality and morbidity as well as prevent transmission of HIV.2–5 However, mean ART dose-taking adherence (percentage of prescribed doses taken) typically ranges from 60% to 80% when measured objectively with the help of electronic monitoring devices, and only 30% to 60% of patients achieve at least 85% adherence.6–8 Incomplete treatment adherence is the major cause of treatment failure, development of drug resistance, HIV disease progression, and death.3–5,9
Two highly cited studies from Kenya showed early promise for the potential of SMS messages to increase rates of adherence to ART among HIV-positive adults.10,11 Lester et al.10 tested a 2-way SMS intervention in which recipients in the intervention group were required to respond to weekly messages inquiring about their well-being within 48 hours, with those indicating not feeling well or not responding at all receiving outreach from clinic staff. They found a 12-percentage-point increase in the likelihood of self-reported adherence greater than 95% among HIV-positive adults in 3 clinics, as well as a 9-percentage-point increase in rates of viral suppression. Pop-Eleches et al.11 reported a 13-percentage-point increase in the likelihood of achieving at least 90% electronically monitored adherence over 48 weeks in the intervention group that received 1-way weekly SMS messages. Both studies were conducted in 2007 and helped spur enthusiasm for mHealth applications in resource-poor settings.12,13
Recent evidence has been more mixed. A randomized controlled trial in Cameroon from 2012 did not show improved adherence outcomes among HIV-positive adults receiving weekly standardized motivational SMS messages.14 Similarly, a large randomized controlled trial conducted across 3 clinics in South India that featured an automated voice reminder accompanied by weekly pictorial SMS messages did not improve outcomes among adult patients beginning first-line ART.15 There remains a need for a robust evidence base regarding the effectiveness of such interventions 7 years after publication of the initial, widely reported studies, including what types of interventions work best among different populations.
Achieving high adherence to ART is of particular importance for adolescents and young adults who face pronounced obstacles to adherence.16,17 Adolescent and young adult populations may be particularly responsive to text messages both because of their heightened struggles with adherence and because they are typically enthusiastic users of modern technologies including mobile phones.18 Yet no previous randomized trials have tested SMS-based interventions targeted at this population.
We conducted a year-long randomized controlled trial in Uganda that sent SMS messages to HIV-positive adolescents and young adults aged 15 to 22 years and followed the timing, frequency, and overall design of the 2 successful earlier Kenya studies. A key novelty is that our study directly tested 1-way against 2-way SMS messages to determine their relative effectiveness. We hypothesized that 1-way SMS could remind and motivate participants to take their medicines, and that the additional response option offered by 2-way messages would be perceived as offering additional functionality (allowing one to report one’s health status) and potentially increase perceived social support as a 2016 study in Uganda found that such SMS messages increased patient perception of being “cared for” by the health care system.19 A review by Wald et al. of SMS messages found that 2-way messages were more effective in improving adherence; the studies reviewed had diverse implementation approaches including both “pure” SMS-based messages as tested in our study as well as more resource-intensive ones that use participant response as an entry point for phone or personal counseling.20 In this study, we investigated specifically whether 2-way messages with limited other communication are more effective for improving ART adherence when directly compared with 1-way messages.
METHODS
This individually randomized, parallel, multisite controlled trial was conducted at 2 HIV care facilities in Kampala, Uganda: the Infectious Diseases Institute and Mildmay Uganda. Both clinics are nonprofit organizations that serve the general population in and around Kampala. Antiretroviral therapy and other services are provided free of charge. Patients from both clinics on ART or cotrimoxazole prophylaxis against common opportunistic infections were eligible for study participation if they were aged 15 to 22 years, able to access a mobile phone on a daily basis, and were familiar with SMS text messaging. Individuals who did not own mobile phones were eligible if they had shared access for at least 5 days per week. Mobile phones or phone airtime were not provided. Exclusion criteria included attending boarding school or expecting to attend one in the next 2 years, because mobile phone use is commonly prohibited in these institutions. Recruitment was done on a rolling basis beginning in April 2014. Patients aged 18 years or older provided written informed consent at enrollment in either English or Luganda, the most commonly spoken languages. Patients younger than 18 years provided verbal assent and their parents or caretakers provided written consent. All participants were given a copy of the consent form in their language of choice.
Randomization and Masking
The principal investigator (S. L.) conducted the randomization. Participants were randomly assigned by simple randomization in a 1-to-1-to-1 ratio to control group (care as usual) or 1 of 2 SMS intervention groups21: 1-way SMS (message only) or 2-way SMS (message plus response option) via a random number generator based on lists of eligible clients in the electronic records. Study participants and the study coordinators could not be masked to intervention allocation because participants had to be informed of their study group assignment, and on the basis of that, either received text messages or did not. However, data analyses performed by investigators were masked to study group assignment.
Procedures
During recruitment, all participants were provided a pill bottle with an electronic medication event monitoring system (MEMS) cap. The MEMS data provide a more objective measure of adherence than does self-report, which has been shown to overestimate adherence.22,23 Phone numbers were updated and MEMS cap information electronically downloaded during routine clinic visits, which occur once every 1 to 3 months depending on the patient. During these visits, study coordinators did not provide feedback to participants regarding their MEMS measured adherence to minimize potential confounding of intervention effects with “MEMS-use” effects. If MEMS caps were reported lost or broken, replacement caps were provided.
The SMS procedures were explained to patients in the intervention arms at recruitment. Before the study, focus group discussions investigated feasibility and acceptability of weekly SMS messages, and generated input for the appropriate wording of messages.24 The date and time of sending SMS were also determined during these focus groups. Every Sunday at 9 am, the program manager dispatched the text messages to patients in both intervention groups. For the 1-way group, the message was “We hope you are feeling well today.” For the 2-way group, the message was “We hope you are feeling well today. Reply 1 if well, 2 if unwell.” Responding by pressing a single button (“1” or “2”), rather than asking participants to text back a word (“well,” “unwell”) was implemented to reduce the (time) burden on the participant, and to avoid errors related to typos.
Those in the 2-way group who did not respond within 48 hours received a follow-up message asking “How are you? We have not heard back from you. Reply 1 if well, 2 if unwell.” No additional messages or follow-up calls were provided after this to the nonresponders, in contrast to Lester et al., which used the response feature as an entry point for further communication.10 Our definition of 2-way messaging is narrower and reflects the resource scarcity at the implementing clinics. Those who responded that they were unwell received a call from a study coordinator within 24 hours. During these conversations, study coordinators were instructed to find out why participants responded feeling unwell but not to provide lengthy counsel. Instead, study coordinators were advised to refer patients with serious cases such as severe depression or anxiety or illness to regular clinic counseling to maintain the focus of integrating SMS into care as usual; this occurred in less than 2% of the sample.
Study coordinators conducted 3 rounds of surveys (at baseline, month 6, and month 12) to collect socioeconomic and demographic information. Those unable to be traced within 6 months of the last clinic visit (as the time between scheduled clinic visits varies across patients) were defined as lost to follow-up.
Outcomes
We calculated MEMS adherence over the 48-week period since enrollment as the number of recorded bottle openings divided by the number of prescribed bottle openings. We set the maximum number of daily openings to 1 for patients on once-daily regimens, and equal to 2 for patients on twice-daily regimens to prevent inflating MEMS adherence by extra openings. For those who changed regimens over the course of the study, study coordinators placed the MEMS cap on the new pill bottle after the regimen change. We used pharmacy patient records and patient self-report at each clinic visit to create adherence measures that account for these regimen changes. Our primary outcome was mean adherence measured by MEMS caps over 48 weeks, with secondary outcomes of adherence of at least 90%, as well as self-reported adherence (percentage of doses taken as prescribed in the last month collected in the surveys), and a binary indicator of whether MEMS data indicated a treatment interruption of 48 or more hours during each period of analysis.
Statistical Analysis
We calculated that a sample size of 110 in each intervention arm would be required to detect a 7-percentage-point difference in mean adherence between the 2 intervention groups and the control group, with 80% power and a .05 significance. We also powered the study to detect an 8-percentage-point difference in mean adherence between the 2 intervention groups.
The analysis of primary outcomes was by intention to treat. We did not adjust means by using covariates in the primary analysis, as is conventional and recommended.25 Following Lester et al.10 and Pop-Eleches et al.,11 we classified all individuals lost to follow-up as nonadherent for the period since their last recorded MEMS opening. Our secondary analysis was per-protocol (complete-case) analysis of outcomes, in which we included only participants who had complete MEMS outcome data. We compared differences in outcomes between each of the intervention groups and control group by t test (for continuous outcomes) and χ2 test of proportion (for binary outcomes) for the full 48-week follow-up period. We also report overall differences across the 3 groups by using an F test. As a robustness check, we tested differences in outcomes for smaller time intervals (12-week, monthly, and weekly), as well as for treatment heterogeneity on subgroup variables, such as clinic, gender, age, housing quality, literacy, and ownership of a mobile phone. We also tested whether loss to follow-up varied by intervention group with χ2 test. We performed all statistical analyses with Stata version 13.0 (StataCorp LP, College Station, TX).
RESULTS
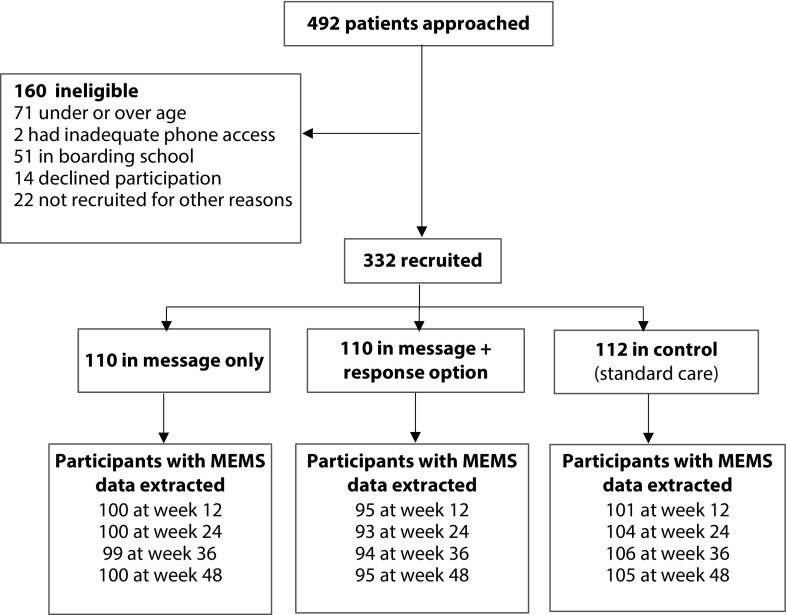
Between April and November 2014, 492 HIV clients eligible on the basis of criteria observed in the clinic electronic records data were approached and 332 were enrolled (64.2%; Figure 1). One-hundred-sixty were approached but not enrolled because they were in boarding school (n = 51), over- or underage (n = 71), declined (n = 14), or had some other reason for not participating (n = 22), such as being on another research study at the clinic, transferring clinics in the foreseeable future, or having difficulty understanding the consenting or study procedures. Only 2 participants out of 160 (1.3%) were turned away because of inadequate phone access. In the 48 weeks following enrollment, 17 people left the study (loss to follow-up, transferred to a different clinic, or died) and 28 did not have MEMS outcomes collected throughout because of broken or malfunctioning MEMS, losing their MEMS caps, or repeatedly not bringing it to clinic visits. These constitute 13.6% of participants who were enrolled, which is slightly lower than loss-to-follow-up rates in similar programs.11,26,27 Seventy-one participants got a replacement MEMS cap during study because of malfunctioning or lost caps. There were no statistically significant differences between groups in terms of loss to follow-up or incomplete MEMS data. No adverse event directly attributable to the intervention was reported during the study period.
FIGURE 1—
Flowchart of Randomized Controlled Trial of Text Messages for Improving Antiretroviral Therapy Adherence: Kampala, Uganda, 2014
Note. MEMS = medication event monitoring system.
The mean age of recruited participants was 18 years; 61% were female; and 74% were at Mildmay with the remainder at Infectious Diseases Institute. At baseline, 60% of participants owned a phone and 96% had completed primary education. Participants’ demographic and socioeconomic characteristics such as age, gender, marital status, education, housing quality, and self-reported adherence were distributed similarly across both intervention groups and the control group (Table 1).
TABLE 1—
Demographics and Baseline Characteristics of Study Participants in Randomized Controlled Trial of Text Messages for Improving Antiretroviral Therapy Adherence: Kampala, Uganda, 2014
| Characteristic | Message Only (n = 110) | Message + Response Option (n = 110) | Control (n = 112) | P |
| Female, % | 63 | 61 | 57 | .65 |
| Age, mean, y | 18.5 | 18.3 | 18.2 | .58 |
| Married, % | 35 | 30 | 28 | .54 |
| Clinic, % | ||||
| Mildmay | 70 | 76 | 75 | .50 |
| Infectious Diseases Institute | 30 | 24 | 25 | .50 |
| Years since starting clinic, mean | 6.8 | 6.9 | 6.3 | .55 |
| Literacy, % | ||||
| Can read | 88 | 92 | 91 | .67 |
| Can write | 91 | 93 | 94 | .77 |
| Reads easily | 65 | 72 | 61 | .23 |
| Writes easily | 64 | 71 | 61 | .26 |
| Education, % | ||||
| Completed primary | 96 | 98 | 97 | .51 |
| Completed secondary | 61 | 61 | 53 | .37 |
| Housing, % | ||||
| Self-rated house as “poor” | 13 | 9 | 6 | .29 |
| Has electricity | 78 | 80 | 79 | .91 |
| Has piped water | 66 | 75 | 73 | .28 |
| Weekly income in Ugandan shillings (USH)a | ||||
| Mean | 21 790 | 21 005 | 16 927 | .31 |
| Income > 50 000 USH, % | 46 | 44 | 39 | .61 |
| Income > 75 000 USH, % | 28 | 26 | 19 | .37 |
| Mobile phone access, % | ||||
| Owns phone | 63 | 61 | 52 | .22 |
| Shares phone | 37 | 39 | 48 | .22 |
| Self-reported adherence,b % | 81 | 81 | 81 | .96 |
Note. The last column shows the P value from the F statistic of the mean comparison between groups.
At an exchange rate of 1 USD to 2519 USH around the time of recruitment April 2014, the mean weekly income reported is about 8 USD.
Self-reported adherence is the share of doses taken as prescribed in the past month, as reported by the participant during the baseline survey.
Weekly messages were sent with an average sending success rate of 86.4%, meaning that the messages were marked as “sent” or “delivered” to the recipient’s phone in Telerivet, the online software used to send messages. Undelivered messages were typically attributable to the recipient’s phone being switched off at the time of sending and the mobile network deleting the message after a period of time if the phone is not switched back on. Among those in the 2-way group who were required to respond, response rates averaged 28.4% (calculated as the number of response messages divided by the number of expected response messages since time of recruitment; Figure A, available as a supplement to the online version of this article at http://www.ajph.org).
We found no statistically significant difference in outcomes between the intervention groups compared with the control group over the 48-week period by intention-to-treat or by complete case analysis (Table 2). In the intent-to-treat analysis, mean adherence was 64% for the 1-way group (P value of comparison with control = .27) and 61% for the 2-way group (P value of comparison with control = .15), compared with 67% in the control group.
TABLE 2—
Antiretroviral Therapy Adherence Outcomes Among Study Participants Over 48 Weeks: Kampala, Uganda, 2014
| Mean Adherencea |
Proportion Adhering at Least 90% |
Proportion With 48 Hours Treatment Interruption |
||||
| Intervention Group | ITT, Mean (95% CI) or No. | Complete Case, Mean (95% CI) or No. | ITT, Proportion (95% CI) or No. | Complete Case, Proportion (95% CI) or No. | ITT, Proportion (95% CI) or No. | Complete Case, Proportion (95% CI) or No. |
| Control | ||||||
| Mean or proportion | 0.67 (0.62, 0.72) | 0.75 (0.71, 0.80) | 0.24 (0.16, 0.32) | 0.29 (0.20, 0.39) | 0.92 (0.87, 0.97) | 0.92 (0.86, 0.97) |
| Sample size | 112 | 95 | 112 | 95 | 112 | 107 |
| Message only | ||||||
| Mean or proportion | 0.64 (0.58, 0.70) | 0.72 (0.69, 0.78) | 0.24 (0.16, 0.32) | 0.28 (0.19, 0.37) | 0.90 (0.84, 0.96) | 0.90 (0.84, 0.96) |
| Sample size | 110 | 92 | 110 | 92 | 110 | 106 |
| Message + response option | ||||||
| Mean or proportion | 0.61 (0.56, 0.67) | 0.74 (0.69, 0.78) | 0.20 (0.12, 0.28) | 0.26 (0.16, 0.35) | 0.95 (0.90, 0.99) | 0.94 (0.89, 0.99) |
| Sample size | 110 | 86 | 110 | 86 | 110 | 100 |
| Pooled intervention | ||||||
| Mean or proportion | 0.63 (0.59, 0.67) | 0.73 (0.69, 0.76) | 0.22 (0.16, 0.27) | 0.27 (0.20, 0.34) | 0.92 (0.89, 0.96) | 0.92 (0.88, 0.96) |
| Sample size | 220 | 178 | 220 | 178 | 220 | 206 |
| Overall Pb | .35 | .57 | .73 | .84 | .46 | .53 |
Note. CI = confidence interval; ITT = intent-to-treat. The overall F test tests equality of means across the 3 groups and the P value is presented.
Proportion of pills taken over total prescribed.
P values determined by F test for equality of means across the 3 groups.
The proportion of participants achieving adherence of at least 90% over the 48-week period of analysis was 28% (1-way group) and 26% (2-way group), and this was not significantly different from the 29% in the control group (P = .85 and .69, respectively). Complete-case results showed marginally higher overall adherence, but group differences were again nonsignificant.
Mean self-reported adherence, defined as the share of doses taken as prescribed over the past month, was higher than MEMS-measured adherence—averaging 82% at the month-12 survey. Again, there was no statistically significant difference across arms in any survey round (Table A, available as a supplement to the online version of this article at http://www.ajph.org). Showing at least 1 treatment interruption of 48 hours or more also did not differ across groups (Table 2).
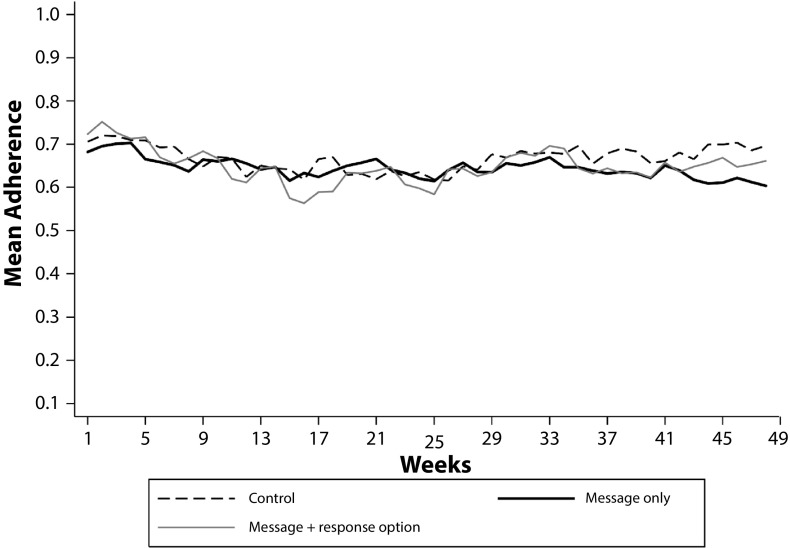
When we investigated potential temporal patterns for more granular time periods, we again did not find any results different from zero. Figure 2 plots weekly mean adherence across the 3 groups over the intervention period and shows no apparent temporal patterns, which was confirmed when we ran our regression analysis for 12-week, monthly, and weekly intervals. An analysis of treatment heterogeneity revealed that none of the subgroup variables had an effect when interacted with the intervention variable at conventional levels of significance (Table B, available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 2—
Weekly Mean Adherence to Antiretroviral Therapy Over 48 Weeks Among Study Participants: Kampala, Uganda, 2014
DISCUSSION
In this study, we found that SMS reminder messages targeted at HIV-positive adolescent and young adult patients at 2 clinics in Uganda did not have an effect on multiple measures of their adherence to ART or prophylaxis medications. Furthermore, there was no differential effect on adherence between 1-way and 2-way messages. These findings are robust to both intent-to-treat and complete-case analyses, and were further confirmed by self-reported adherence. Limitations of this study include the absence of viral load as a biological endpoint, but given that we did not find results for any of our adherence measures, there is little reason to expect a treatment impact on biological outcomes. Although the study was conducted at 2 urban clinics in Uganda, we believe that the clinic set-up is typical of many African settings and should therefore be generalizable to other (urban) settings with similar characteristics.
Our trial is unique in that it is the first study to report the effect of an SMS reminder intervention on ART adherence specifically among adolescent and young adult populations. Our result stands in contrast to those in 2 published trials in Kenya that targeted adults. In addition to the difference in our target population, there are other noteworthy differences between the 2 early successful published trials and ours not finding an effect of SMS on ART adherence. The Pop-Eleches et al. trial tested 1-way messaging only and provided mobile phones to all study participants,11 which we did not. They did this at a time (2007) when less than a third of the population in Kenya owned a mobile phone,28 and at baseline, fewer than half their sample owned such a device. By contrast, in the study by Mbuagbaw et al., 95% of participants approached owned a phone, and in our study, just 2 of 492 (less than 1%) patients approached were ruled ineligible because of inadequate phone access.14 In the earlier days of mobile technology in Africa, the novelty of receiving messages on a mobile phone may have been higher and text recipients may have been more likely to pay attention to and fully digest the contents of a message. In the 12 months of our study from 2014 to 2015, texting activity increased from just 27% of participants reporting sending a text within the past 24 hours at baseline to 41% at the 12-month survey. Because novelty likely correlates with user engagement (for example, our study found a substantially lower response rate of 28% compared with the 60% to 70% found in the Lester et al. study10), this could be one factor differentiating our study from past ones, and contributing to lower effectiveness in more recent SMS studies.
Another difference is that, in the Lester et al. trial, study coordinators followed up with everyone who did not respond to weekly messages and used the 2-way messaging as an entry point for more involved communication and care with the participant.10 By contrast, we only followed up with those who responded “unwell” among our 2-way intervention group. We did this both to prevent confounding the effect of SMS with personal communication over the phone and to preserve the low-cost, scalable properties of the SMS reminder intervention being investigated.
A third difference is that our intervention targeted clients who on average had been at the clinic for 6 years, in contrast to SMS reminder studies targeting treatment initiators as in Lester et al.10 and Pop-Eleches et al.11 The SMS messages may play a more important role for those initiating treatment at a time when they are forming habits. In this regard, our target population is more similar to that in the study by Mbuagbaw et al., who recruited participants at various points since initiating ART and found no effect of weekly SMS messages on (self-reported) adherence over a 6-month period.14
The failure of SMS messages to improve adherence in our study suggests that it may be time to reconsider the potential such messages hold in the long term, and under what circumstances they are effective. Nearly 10 years have passed since the first, oft-cited studies were conducted that led to much enthusiasm for using mHealth for improving health in low-resource settings. Since then, phone ownership and access have become nearly universal, and text-message volumes continue to grow by leaps and bounds in both developed and developing countries.29 In this changing technological landscape, it is possible that we must update our expectations about what simple text messages can achieve, and how they must be designed to ensure continued effectiveness over the long term. Simple reminder messages may not be enough to capture the attention of recipients, and thinking carefully about what other functions SMS messages can take on (such as using them to communicate adherence feedback, or send small incentives conditional on high observed adherence), or how they can be integrated into a more involved mHealth approach may lead to more fruitful future interventions.
ACKNOWLEDGMENTS
This research was funded by National Institutes of Health grant R01 HD074925.
We are grateful to the study coordinating team, Peter Wabukala, Gloria Abura, and Halima Nakawooza, for their excellent work. Our thanks to the study participants who so generously gave of their time and insights.
Note. The funder of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all study data and final responsibility for the decision to submit for publication.
HUMAN PARTICIPANT PROTECTION
The study protocol was approved by the ethics review boards at Mildmay Uganda and Infectious Diseases Institute (who referred to the institutional review board of the Joint Clinical Research Centre), the Uganda National Council for Science and Technology, and the Human Subjects Protection Committee of the RAND Corporation.
REFERENCES
- 1.Qiang CZ, Yamamichi M, Hausman V, Altman D. Mobile Applications for the Health Sector. Washington, DC: World Bank; 2011. [Google Scholar]
- 2.Li JZ, Paredes R, Ribaudo HJ et al. Relationship between minority nonnucleoside reverse transcriptase inhibitor resistance mutations, adherence, and the risk of virologic failure. AIDS. 2012;26(2):185–192. doi: 10.1097/QAD.0b013e32834e9d7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy JV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35(3):261–268. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hogg RS, Heath K, Bangsberg D et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16(7):1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR, Perry S, Charlebois ED et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Shuter J, Sarlo JA, Stubbs RO, Rode RA, Zingman BS. Sequential antiretroviral adherence measurement using electronic bottle cap monitors in a cohort of HIV-infected adults. J Int Assoc Physicians AIDS Care (Chic) 2012;11(2):94–97. doi: 10.1177/1545109711420498. [DOI] [PubMed] [Google Scholar]
- 7.Ortego C, Huedo-Medina TB, Llorca J et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–1396. doi: 10.1007/s10461-011-9942-x. [DOI] [PubMed] [Google Scholar]
- 8.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palella FJ, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 10.Lester RT, Ritvo P, Mills EJ et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomized trial. Lancet. 2010;376(9755):1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 11.Pop-Eleches C, Thirumurthy H, Habyarimana JP et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25(6):825–834. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;(3):CD009756. doi: 10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeKoekkoek T, Given B, Given CW, Ridenour K, Schueller M, Spoelstra SL. mHealth SMS text messaging interventions and to promote medication adherence: an integrative review. J Clin Nurs. 2015;24(19-20):2722–2735. doi: 10.1111/jocn.12918. [DOI] [PubMed] [Google Scholar]
- 14.Mbuagbaw L, Thabane L, Ongolo-Zogo P et al. The Cameroon Mobile Phone SMS (CAMPS) trial: a randomized trial of text messaging versus usual care for adherence to antiretroviral therapy. PLoS One. 2012;7(12):e46909. doi: 10.1371/journal.pone.0046909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shet A, De Costa A, Kumarasamy N et al. Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomized controlled trial in India. BMJ. 2014;349:g5978. doi: 10.1136/bmj.g5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer J, Vachon L, Desforges C, Sussman NM. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical-trial. Epilepsia. 1995;36(11):1111–1117. doi: 10.1111/j.1528-1157.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting [erratum in Child Dev. 2010;81(3):1024] Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell KJ, Bull S, Kiwanuka J, Ybarra ML. Cell phone usage among adolescents in Uganda: acceptability for relaying health information. Health Educ Res. 2011;26(5):770–781. doi: 10.1093/her/cyr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware NC, Pisarski EE, Tam M et al. The meanings in the messages: how SMS reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. AIDS. 2016;30(8):1287–1294. doi: 10.1097/QAD.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald DS, Butt S, Bestwick JP. One-way versus two-way text messaging on improving medication adherence: meta-analysis of randomized trials. Am J Med. 2015;128(10):1139.e1–5. doi: 10.1016/j.amjmed.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Schulz KF, Grimes DA. Unequal group sizes in randomized trials: guarding against guessing. Lancet. 2002;359(9310):966–970. doi: 10.1016/S0140-6736(02)08029-7. [DOI] [PubMed] [Google Scholar]
- 22.Thirumurthy H, Siripong N, Vreeman RC et al. Differences between self-reported and electronically monitored adherence among patients receiving antiretroviral therapy in a resource-limited setting. AIDS. 2012;26(18):2399–2403. doi: 10.1097/QAD.0b013e328359aa68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rana Y, Haberer J, Huang HJ et al. Short Message Service (SMS)-based intervention to improve treatment adherence among HIV-positive youth in Uganda: focus group findings. PLoS One. 2015;10(4):e0125187. doi: 10.1371/journal.pone.0125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perneger TV. Adjusting for multiple testing in studies is less important than other concerns. BMJ. 1999;318(7193):1288. doi: 10.1136/bmj.318.7193.1288a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wools-Kaloustian K, Kimaiyo S, Diero L et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20(1):41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 28. Cell phones in Africa: communication lifeline. Pew Research Center. April 2015.
- 29.Aker JC, Mbiti IM. Mobile phones and economic development in Africa. J Econ Perspect. 2010;24(3):207–232. [Google Scholar]