Abstract
Background
Chronic hepatitis B virus (HBV) infection is the major cause of hepatocellular carcinoma (HCC). HBV X protein (HBx) plays a crucial role in the development of HCC. Moreover, many tripartite motif (TRIM) family proteins exert diverse biological functions in hepatocarcinogenesis. However, as a novel member of this family, the specific effect of TRIM52 is still largely obscure. In the present study, we investigated the expression and function of TRIM52 in HBV-associated HCC.
Material/Methods
Fluorescence quantitative polymerase chain reaction (FQ-PCR) was performed to detect the HBV DNA levels in the peripheral blood of HCC patients. Quantitative real-time PCR (qRT-PCR) and Western blot analysis were performed to detect the expression of TRIM52, HBx, and NF-κB p65. HBx-pcDNA3.1 and TRIM52-shRNA were used to induce HBx ectopic expression and TRIM52 silencing, respectively. Pyrrolidine dithiocarbamate (PDTC) was used to block the activation of NF-κB. Cell proliferation was detected using the Cell Counting Kit-8 (CCK-8) assay.
Results
TRIM52 expression was up-regulated together with HBx in HBV-associated HCC tissues. Ectopic expression of HBx elevated TRIM52 expression in HepG2 cells. TRIM52 silencing repressed the proliferation of HepG2.2.15 cells. Moreover, NF-κB p65 expression was increased in HCC cell lines. Blocking NF-κB activation with PDTC suppressed TRIM52 expression and attenuated the viability of HepG2.2.15 cells.
Conclusions
These findings indicate that TRIM52 can promote cell proliferation and HBx may regulate TRIM52 expression via the NF-κB signaling pathway in HBV-associated HCC.
MeSH Keywords: Cell Proliferation, Hepatitis B Virus, Liver Neoplasms, NF-kappa B, Ubiquitin-Protein Ligases
Background
Liver cancer is one of the most fatal cancers worldwide. According to an estimate of new cases in 2012, it is the fifth most common cancer in men and the ninth in women worldwide [1]. Most primary liver cancers are hepatocellular carcinoma (HCC). Among the many risk factors for HCC, viral infection is the most predominant one. About 73.4% of cases worldwide are attributable to viral infection [2], and chronic hepatitis B virus (HBV) infection is considered as the major one [3]. With the high infection rate of HBV, HCC is much more common in less developed regions, such as Asia and Sub-Saharan Africa [1,4].
HBV is a DNA virus of the Hepadnaviridae family. Its genome encodes 4 overlapping open reading frames (ORFs). HBV X protein (HBx) is a multifunctional protein encoded by the smallest ORF, with a molecular weight of 17 kDa [5]. It has been proved to be oncogenic in transgenic mice [6,7]. Accumulating evidence suggests that HBx can interfere with several cellular processes, such as signal transduction, apoptosis, cell cycle progression, and innate immunity. HBx can activate many transcription factors as a transactivator and interact with AKT, Wnt, STAT, and NF-κB signaling pathways [8,9]. By these pathological functions, HBx exerts its oncogenic effect in HCC.
Tripartite motif (TRIM) family proteins are considered as a kind of E3 ubiquitin ligase, with more than 80 members identified in humans to date [10]. They exert diverse biological functions in human, such as cell proliferation, apoptosis, invasion, innate immunity, and autophagy [11–15]. TRIM52 is a novel TRIM family protein. Because it contains only a unique expanded RING domain and a B-Box2 domain, it is considered as a nonclassical antiviral TRIM protein [16]. A previous study demonstrated the antiviral activity of TRIM52 against Japanese encephalitis virus (JEV) infection by degrading the viral nonstructural protein 2A (NS2A) [17]. Additionally, TRIM52 is a positive regulator of the NF-κB signaling pathway [18]. However, the effect of TRIM52 is still largely unknown.
As both HBx and TRIM52 are involved in the activation of NF-κB, we hypothesize that HBx interacts with TRIM52 and promotes the development of HCC. In the present study, we investigated the expression and function of TRIM52 in HBV-associated HCC.
Material and Methods
Antibodies and chemicals
TRIM52 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HBx antibody was obtained from Abcam (Cambridge, UK). NF-κB p65 and GAPDH antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Pyrrolidine dithiocarbamate (PDTC) was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Clinical samples
All the peripheral blood samples of the HBV-associated HCC patients and the liver tissue samples were obtained from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. This study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from each participant prior to study enrollment.
Cell culture
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Hyclone (Logan, UT, USA). Fetal Bovine Serum (FBS) was obtained from Gibco (Detroit, MI, USA). Penicillin-streptomycin (PS) and 0.25% Trypsin-EDTA were purchased from Solarbio (Beijing, China). Immortalized normal liver cell line LO2 and human HCC cell lines HepG2 and HepG2.2.15 (ATCC, Manassas, VA, USA) were used for the investigation. All the cells were maintained in DMEM containing 10% FBS and 1% PS. A 37°C incubator with 5% carbon dioxide (CO2) was used for cell culture.
Polymerase chain reaction (PCR)
Fluorescence quantitative PCR (FQ-PCR) assay was performed to detect the HBV DNA levels in the peripheral blood of the HCC patients. The serum samples together with HBV positive quality control reference, HBV negative quality control reference, and HBV positive standard samples were treated for FQ-PCR. The results were analyzed using ABI Prism 7300 SDS software (Applied Biosystems, Foster City, CA, USA).
Quantitative real-time PCR (qRT-PCR) assay was performed to detect the mRNA levels of proteins. Total RNA was extracted from the tissue samples and cultured cells by TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA was reverse-transcribed to cDNA using the cDNA Synthesis Kit (Thermo Fisher, St. Louis, MO, USA) according to the manufacturer’s protocol. The qRT-PCR assay was performed on ABI 7300 Real-Time PCR system (Applied Biosystems) using SYBR Green qPCR Master Mixes (Thermo Fisher). The expression of mRNA was normalized to GAPDH and calculated with the 2−ΔΔCt method. The primers sequences were as follows:
Hepatitis B virus (strain ayw) genome (NC_003977.2):
forward: 5′-GACTCTCAGCAATGTCAAC-3′;
reverse: 5′-ACAGCCTCCTAGTACAAAG-3′.
TRIM52 (NM_001346048.1):
forward: 5′-ATGGCTGGTTATGCCACTACT-3′;
reverse: 5′-CTCGTCCTCCTTACTCCACAG-3′.
NF-κB p65 (NM_001145138.1):
forward: 5′-GAATGGCTCGTCTGTAGTG-3′;
reverse: 5′-TGGTATCTGTGCTCCTCTC-3′.
GAPDH (NM_001256799.1):
forward: 5′-CACCCACTCCTCCACCTTTG-3′;
reverse: 5′-CCACCACCCTGTTGCTGTAG-3′.
Cell transfection
The pcDNA3.1(+) was obtained from Addgene (Cambridge, MA, USA). HBx-expressing vector (HBx-pcDNA3.1) was constructed using the primers as follows:
forward: 5′-CCCAAGCTTATGGCTGCTAGGCTGTGCTG-3′;
reverse: 5′-CCGGAATTCGGCAGAGGTGAAAAAGTTG-3′.
HBx-pcDNA3.1 was transiently transfected into HepG2 cells using Lipofectamine 2000 (Invitrogen). The transfection efficiency was confirmed by qRT-PCR and Western blot analysis.
A short hairpin RNA (shRNA) lentiviral vector targeting position 670–692 (GGGCATGTGCTTTAAACAC) of human TRIM52 gene was used to suppress the expression of TRIM52. We designed and synthesized the human TRIM52-shRNA. Then, the annealed TRIM52-shRNA was cloned into linearized pLKO.1-puro (Addgene) and transformed into DH5α cells (TransGen Biotech, Beijing, China). Positive clones were collected for PCR identification. The primer sequences were as follows: forward: 5′-CAAGGTCGGGCAGGAAGAG-3′; reverse: 5′-TAGAAGGCACAGTCGAGG-3′. The endotoxin-free recombinant expression vector was extracted and identified by sequencing. Finally, the recombinant plasmids pLKO.1-shTRIM52, psPAX2, and pMD2G were co-transfected into 293T cells. The supernatant containing viral particles was collected. HepG2.2.15 cells were transfected with recombinant TRIM52-shRNA lentiviral vector or negative control lentiviral vector, respectively. The silencing of TRIM52 was confirmed by qRT-PCR and Western blot analysis.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation of HepG2.2.15 was detected by CCK-8 (Signalway Antibody, College Park, MD, USA) assay. HepG2.2.15 cells were resuspended in DMEM at a concentration of 3×104 cells/ml and seeded in 96-well plates (100 μl/well). Each group had 3 replicate wells. The cells were incubated overnight in a 37°C incubator with 5% CO2. To investigate the effect of TRIM52 on cell proliferation, HepG2.2.15 cells were transfected with negative control lentiviral vector or TRIM52-shRNA lentiviral vector. To investigate the effect of NF-κB inhibitor, HepG2.2.15 cells were treated with different concentrations of PDTC. After transfection or drug treatment for 0, 24, 48, and 72 h, 10 μl of CCK-8 solution was added to each well. Then, the cells were incubated for 1 h. The absorbance at 450 nm was detected by use of a microplate spectrophotometer (Thermo Fisher).
Western blot analysis
Cells or tissues were lysed in RIPA Lysis Buffer (Solarbio). After being centrifuged at 12 000×g for 10 min, the supernatant was collected and the concentration was detected using the BCA Protein Assay Kit (Thermo Fisher). Then, the protein samples were loaded in the 10% polyacrylamide gels and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The target proteins were transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA). Subsequently, the membranes were blocked with 5% nonfat milk or 3% bovine serum albumin (BSA) for 1 h at room temperature. The primary antibodies were diluted to appropriate concentrations with the blocking solution and incubated with the membranes overnight at 4°C. After incubation with the horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime, Nanjing, Jiangsu, China) for 1 h at 37°C, the proteins were visualized and detected using Tanon 5200 chemiluminescent imaging system (Tanon, Shanghai, China).
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. All results were obtained from 3 independent experiments and are presented as mean ± standard deviation (SD). The independent-samples t test (for 2 groups) or one-way ANOVA with Tukey’s multiple comparisons test (for more than 2 groups) were used for statistical evaluations. Two-tailed P<0.05 was considered to be statistically significant.
Results
TRIM52 expression was elevated in HBV-associated HCC tissues
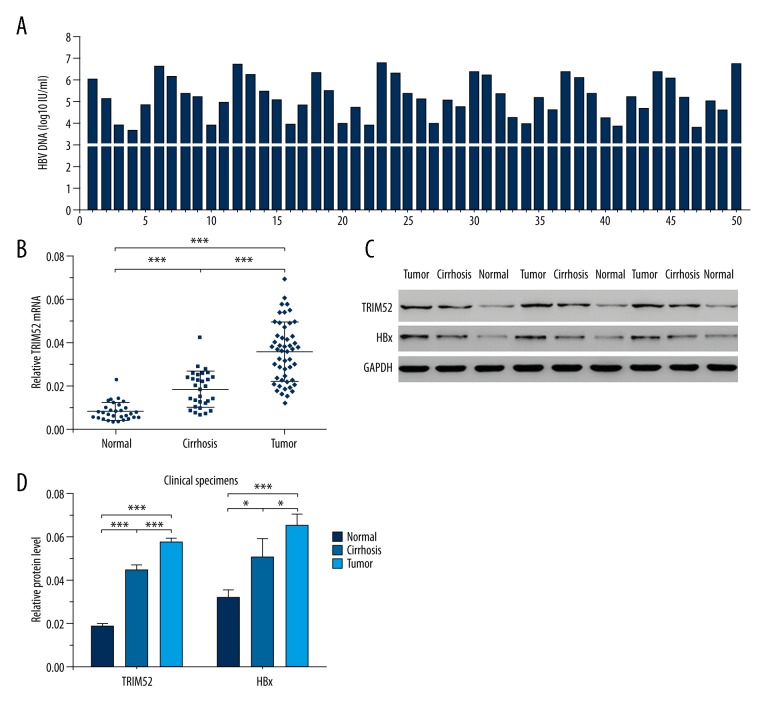
HBV DNA levels in the peripheral blood samples of the HCC patients were detected by FQ-PCR. The results revealed that the serum HBV DNA levels of all the specimens were above 1000 IU/ml (Figure 1A). To investigate the expression of TRIM52 in tumor tissues, HCC tissues, adjacent normal liver tissues and cirrhotic liver tissues were collected for qRT-PCR. As shown in Figure 1B, HCC tissues had the highest mRNA level of TRIM52 and normal liver tissues had the lowest mRNA level. Subsequently, we explored the expression of TRIM52 in tissue samples by Western blot analysis, and the results were consistent with qRT-PCR (Figure 1C, 1D). Moreover, the change trend of HBx expression was similar with TRIM52 (Figure 1C, 1D). In general, TRIM52 expression was up-regulated in HBV-associated HCC tissues.
Figure 1.
TRIM52 expression was elevated in HBV-associated HCC tissues. (A) HBV DNA levels in the serum specimens of the HCC patients (n=50). (B) TRIM52 mRNA levels were detected by qRT-PCR in HCC tissues (n=50), adjacent normal liver tissues (n=30) and cirrhotic liver tissues (n=30). (C) The expression of TRIM52 and HBx was detected by Western blot analysis in HCC tissues, adjacent normal liver tissues, and cirrhotic liver tissues, respectively. GAPDH was used as the loading control. (D) Statistical analysis of the relative protein levels of TRIM52 and HBx. Data are presented as mean ±SD. * P<0.05, *** P<0.001.
TRIM52 expression was modulated by HBx
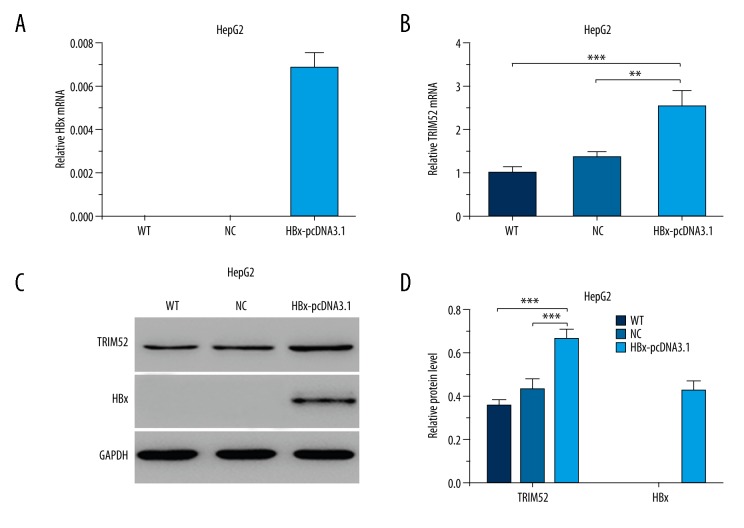
Due to the high expression of TRIM52 and HBx in HCC tumor tissues, we speculated that HBx interacted with TRIM52 either directly or indirectly. Thus, we further investigated the regulatory effect of HBx on TRIM52 expression in HCC cell lines. The ectopic HBx-expressing cellular model was established by transfecting HBx-expressing vectors (HBx-pcDNA3.1) into HepG2 cells. qRT-PCR and Western blot analysis revealed the steady expression of HBx in HepG2 cells transfected with HBx-pcDNA3.1 (Figure 2A, 2C, 2D). Furthermore, we detected the mRNA and protein levels of TRIM52 in ectopic HBx-expressing cells. As shown in Figure 2B–2D, TRIM52 expression was significantly increased in HBx-pcDNA3.1-transfected HepG2 cells. The results indicate that HBx stimulates the expression of TRIM52.
Figure 2.
TRIM52 expression was modulated by HBx. HBx-expressing vectors (HBx-pcDNA3.1) were transfected into HepG2 cells. The expression of HBx and TRIM52 in HBx-pcDNA3.1-transfected HepG2 cells was detected by qRT-PCR and Western blot analysis. (A) The relative HBx mRNA level. (B) The relative TRIM52 mRNA levels. (C) The representative Western blot results of TRIM52 and HBx expression. GAPDH was used as the loading control. (D) Statistical analysis of the relative protein levels of TRIM52 and HBx. WT – untransfected HepG2 cells; NC – negative control plasmid-transfected HepG2 cells; HBx-pcDNA3.1 – HBx-pcDNA3.1-transfected HepG2 cells. Data are presented as mean ±SD. ** P<0.01, *** P<0.001.
TRIM52 and NF-κB p65 were up-regulated in HepG2.2.15 cells
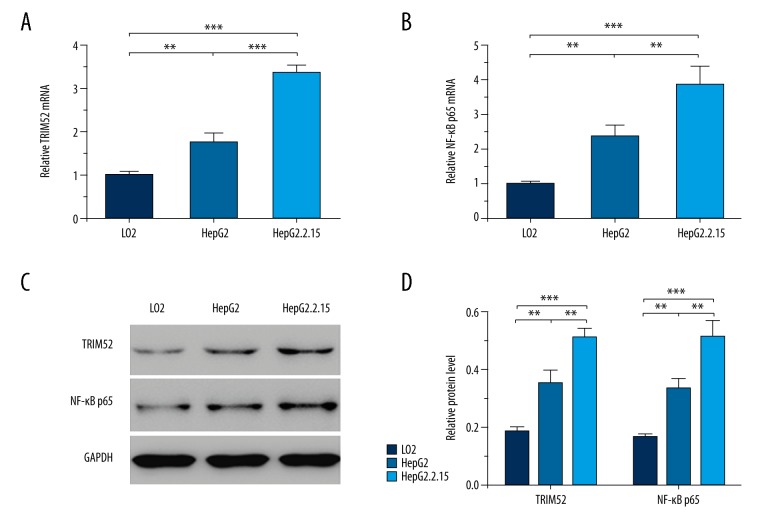
HepG2.2.15 is a stable HBx-expressing cell line. The expression of TRIM52 and NF-κB p65 was detected by qRT-PCR and Western blot analysis in LO2, HepG2, and HepG2.2.15 cells. Both the mRNA and protein levels of TRIM52 were up-regulated in HepG2 and HepG2.2.15 cells (Figure 3A, 3C, 3D). Moreover, the elevation was more obvious in HepG2.2.15 cells, corresponding to the above results. All of these results suggest that HBx elevates the expression of TRIM52. Similarly, NF-κB p65 expression was also significantly increased in HepG2 and HepG2.2.15 cells. The latter revealed a higher expression of NF-κB p65 (Figure 3B–3D). Our findings indicate that HBx stimulates NF-κB p65 expression.
Figure 3.
TRIM52 and NF-κB p65 were up-regulated in HepG2.2.15 cells. TRIM52 and NF-κB p65 expression was detected by qRT-PCR and Western blot analysis in LO2, HepG2, and HepG2.2.15 cells. (A) The relative TRIM52 mRNA levels. (B) The relative NF-κB p65 mRNA levels. (C) The representative Western blot results of TRIM52 and NF-κB p65 expression. GAPDH was used as the loading control. (D) Statistical analysis of the relative protein levels of TRIM52 and NF-κB p65. Data are presented as mean ±SD. ** P<0.01, *** P<0.001.
TRIM52 silencing inhibited HepG2.2.15 cell proliferation
To further identify the specific function of TRIM52 in HCC cell lines, the lentiviral vector for TRIM52 shRNA was constructed. HepG2.2.15 cells were transfected with TRIM52 shRNA for the subsequent study. TRIM52 expression was detected by qRT-PCR and Western blot analysis. As shown in Figure 4A–4C, TRIM52 expression was specifically inhibited by the shRNA against TRIM52 compared with the control groups, indicating TRIM52 silencing in HepG2.2.15 cells. Furthermore, we examined the effect of TRIM52 on cell proliferation by performing CCK-8 assay. After transfection with TRIM52 shRNA for 24, 48, and 72 h, the viability of HepG2.2.15 cells was significantly attenuated. Moreover, the anti-proliferation effect was time-dependent (Figure 4D). In general, TRIM52 acted as an oncoprotein in HBV-associated HCC cells, and TRIM52 silencing repressed the proliferation of HCC cells.
Figure 4.
TRIM52 silencing inhibited HepG2.2.15 cell proliferation. HepG2.2.15 cells were transfected with TRIM52-shRNA through lentiviral vectors. TRIM52 expression was detected by qRT-PCR and Western blot analysis. (A) The relative TRIM52 mRNA levels. (B) The representative Western blot results of TRIM52 expression. GAPDH was used as the loading control. (C) Statistical analysis of the relative protein levels of TRIM52. (D) CCK-8 assay of HepG2.2.15 cells after transfection with TRIM52-shRNA for 24, 48, and 72 h. WT – untransfected HepG2.2.15 cells; NC – negative control lentiviral vector-transfected HepG2.2.15 cells; TRIM52-shRNA – TRIM52 shRNA-transfected HepG2.2.15 cells. Data are presented as mean ± SD. ** P<0.01, *** P<0.001.
NF-κB inhibitor suppressed TRIM52 expression and then reduced HepG2.2.15 cell proliferation
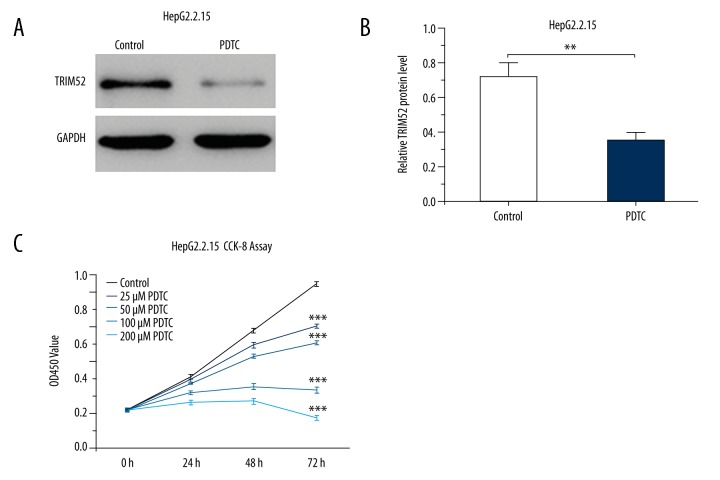
As a specific NF-κB inhibitor, PDTC can inhibit the activation of NF-κB. Interestingly, in the presence of PDTC, TRIM52 expression was significantly reduced in HepG2.2.15 cells (Figure 5A, 5B). In addition, CCK-8 assay demonstrated that cell growth was significantly inhibited after pretreatment with varying concentrations of PDTC for 24, 48, and 72 h. Similarly, the effect of PDTC was dose-dependent (Figure 5C). These results suggest that blocking the activation of NF-κB signaling suppresses TRIM52 expression and then inhibits HCC cell growth.
Figure 5.
NF-κB inhibitor suppressed TRIM52 expression and then reduced HepG2.2.15 cell proliferation. HepG2.2.15 cells were treated with PDTC. TRIM52 expression was detected by Western blot analysis. (A) The representative Western blot results of TRIM52 expression. GAPDH was used as the loading control. (B) Statistical analysis of the relative protein levels of TRIM52. (C) CCK-8 assay of HepG2.2.15 cells after treatment with different concentrations of PDTC for 24, 48, and 72 h. Data are presented as mean ±SD. ** P<0.01, *** P<0.001 compared with the control group.
Discussion
Many TRIM proteins are involved in tumorigenesis. A number of recent studies have focussed on the function of TRIM proteins in HCC. The complexes of TRIM24, TRIM28, and TRIM33 can suppress HCC formation in mice [19]. Down-regulation of TRIM3 is associated with the poor prognosis in HCC patients [20]. In addition, TRIM26 and TRIM16 are significantly down-regulated in HCC tissues and are correlated with poor clinical outcomes. Both of them inhibit the formation and progression of HCC as tumor suppressors [21,22]. On the contrary, TRIM11 expression is elevated in HCC tissues and cell lines, which contributes to HCC cell proliferation, invasion, epithelial-mesenchymal transition (EMT), and poor prognosis of HCC patients [13,23]. TRIM52 is a novel TRIM family member. Except for the antiviral activity against JEV infection and regulatory function in NF-κB activation [17,18], no other effect has been reported, including in tumorigenesis. In this study, we investigated the specific function of TRIM52 in HBV-associated HCC.
After infection with HBV, HBx can be detected in liver tissues, especially in cirrhosis and HCC caused by chronic HBV infection [24–26]. However, Wang et al. demonstrated that the HBx-positive rate decreased in HBV-associated HCC tissues compared with the corresponding normal liver tissues as indicated by immunohistochemistry [27]. In another study, the transcriptional level of HBx detected by qRT-PCR was lower in HBV-associated HCC tissues than that in adjacent normal liver tissues [28]. However, Western blot analysis showed that HBx was up-regulated in tumor tissues of HBV-associated HCC patients [29]. These data are contradictory. In our study, the protein level of HBx was increased in HBV-associated HCC tissues compared with cirrhotic liver tissues and adjacent normal liver tissues. Further detection should be performed to confirm the expression level of HBx in clinical samples.
In the present study, a significant elevation of TRIM52 expression was observed in HBV-associated HCC tissues, which prompted us to investigate the interrelation between HBx and TRIM52. We constructed an HBx-expressing cellular model by transfecting HBx-pcDNA3.1 into HepG2 cells. TRIM52 expression was increased in the presence of HBx. Furthermore, the same result was obtained in stable HBx-expressing HepG2.2.15 cells. Functionally, RNA interference targeting TRIM52 mRNA repressed the proliferation of HepG2.2.15 cells. Next, we studied the mechanism by which HBx regulates TRIM52 expression in HCC. Previous studies have indicated that HBx induces activation of NF-κB in HCC tissues and cell lines, contributing to hepatocarcinogenesis [30–32]. In agreement with this, we found that NF-κB p65 expression was up-regulated in HCC cell lines, especially in HepG2.2.15 cells. Furthermore, restricting NF-κB activation with PDTC suppressed TRIM52 expression and cell proliferation in HepG2.2.15 cells, indicating that HBx regulates TRIM52 expression via the NF-κB signaling pathway. Further investigations are needed to fully elucidate the molecular mechanisms involved.
For patients with HCC, chemotherapy, radiotherapy, and surgery are still the most common therapies. Recent studies have shown that anti-HBV therapy is effective for HBV-associated HCC patients [33–35]. In addition, sorafenib can suppress HBx expression and overcome drug resistance induced by HBx [36]. The currently available anti-HBV drugs exert their anticancer effects in HBV-associated HCC through down-regulation of HBx and the related transcription factors, such as NF-κB and AP-1 [37]. The present study demonstrated that HBx expression was increased in HBV-associated HCC tissues. HBx regulated TRIM52 expression via the NF-κB signaling pathway and TRIM52 silencing repressed cell proliferation. Therefore, TRIM52 decrease mediated by HBx down-regulation may be a potential action mechanism for HBx-targeting therapy.
Although we demonstrated some important discoveries in the present study, there are also limitations. Functionally, we only examined the pro-proliferation effect of TRIM52. Mechanistically, the underlying molecular mechanisms have not been comprehensively elucidated. Further investigations should be performed on this topic in diverse human tumors.
Conclusions
In summary, our study provides insights into the specific function of TRIM52 in HCC. TRIM52 functions as an oncoprotein in HBV-associated HCC cells. It can promote cell proliferation and may be a potentially therapeutic target for HCC. HBx may regulate TRIM52 expression via the NF-κB signaling pathway.
Footnotes
Conflict of interest
None.
Source of support: This study was partly supported by the Scientific Research Fund of Shanghai Municipal Health and Family Planning Commission for Traditional Chinese Medicine (No. 2014JQ019A) and the Medical Education Research Project of Shanghai Jiao Tong University School of Medicine (No. YB150712)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob Health. 2016;4:e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 3.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang A, Hallouch O, Chernyak V, et al. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2017 doi: 10.1007/s00261-017-1209-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Lamontagne RJ, Bagga S, Bouchard MJ. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163–86. doi: 10.20517/2394-5079.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim CM, Koike K, Saito I, et al. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–20. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 7.Koike K, Moriya K, Iino S, et al. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology. 1994;19:810–19. [PubMed] [Google Scholar]
- 8.Geng M, Xin X, Bi LQ, et al. Molecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesis. World J Gastroenterol. 2015;21:10732–38. doi: 10.3748/wjg.v21.i38.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang XD, Wang Y, Ye LH. Hepatitis B virus X protein accelerates the development of hepatoma. Cancer Biol Med. 2014;11:182–90. doi: 10.7497/j.issn.2095-3941.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatakeyama S. TRIM family proteins: Roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42:297–311. doi: 10.1016/j.tibs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Ren H, Xu Y, Wang Q, et al. E3 ubiquitin ligase tripartite motif-containing 71 promotes the proliferation of non-small cell lung cancer through the inhibitor of kappaB-α/nuclear factor kappaB pathway. Oncotarget. 2017 doi: 10.18632/oncotarget.19075. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Takayama KI, Fujimura T, et al. A novel prognostic factor TRIM44 promotes cell proliferation and migration, and inhibits apoptosis in testicular germ cell tumor. Cancer Sci. 2017;108:32–41. doi: 10.1111/cas.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Xu C, Zhang X, et al. TRIM11 upregulation contributes to proliferation, invasion, and EMT of hepatocellular carcinoma cells. Oncol Res. 2017;25:691–99. doi: 10.3727/096504016X14774897404770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MM, Shu HB. Multifaceted roles of TRIM38 in innate immune and inflammatory responses. Cell Mol Immunol. 2017;14:331–38. doi: 10.1038/cmi.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandell MA, Jain A, Arko-Mensah J, et al. TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev Cell. 2014;30:394–409. doi: 10.1016/j.devcel.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malfavon-Borja R, Sawyer SL, Wu LI, et al. An evolutionary screen highlights canonical and noncanonical candidate antiviral genes within the primate TRIM gene family. Genome Biol Evol. 2013;5:2141–54. doi: 10.1093/gbe/evt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan W, Wu M, Qian S, et al. TRIM52 inhibits Japanese encephalitis virus replication by degrading the viral NS2A. Sci Rep. 2016;6:33698. doi: 10.1038/srep33698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan W, Liu T, Li X, et al. TRIM52: A nuclear TRIM protein that positively regulates the nuclear factor-kappa B signaling pathway. Mol Immunol. 2017;82:114–22. doi: 10.1016/j.molimm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Herquel B, Ouararhni K, Khetchoumian K, et al. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci USA. 2011;108:8212–17. doi: 10.1073/pnas.1101544108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao J, Zhang XF, Pan QZ, et al. Decreased expression of TRIM3 is associated with poor prognosis in patients with primary hepatocellular carcinoma. Med Oncol. 2014;31:102. doi: 10.1007/s12032-014-0102-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, He D, Yang L, et al. TRIM26 functions as a novel tumor suppressor of hepatocellular carcinoma and its downregulation contributes to worse prognosis. Biochem Biophys Res Commun. 2015;463:458–65. doi: 10.1016/j.bbrc.2015.05.117. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Dong L, Qu X, et al. Tripartite motif 16 inhibits hepatocellular carcinoma cell migration and invasion. Int J Oncol. 2016;48:1639–49. doi: 10.3892/ijo.2016.3398. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Li L, Qian X, et al. High expression of TRIM11 correlates with poor prognosis in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017;41:190–96. doi: 10.1016/j.clinre.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Diamantis ID, McGandy CE, Chen TJ, et al. Hepatitis B X-gene expression in hepatocellular carcinoma. J Hepatol. 1992;15:400–3. doi: 10.1016/0168-8278(92)90077-3. [DOI] [PubMed] [Google Scholar]
- 25.Su Q, Schröder CH, Hofmann WJ, et al. Expression of hepatitis B virus X protein in HBV-infected human livers and hepatocellular carcinomas. Hepatology. 1998;27:1109–20. doi: 10.1002/hep.510270428. [DOI] [PubMed] [Google Scholar]
- 26.Wei Y, Liu D, Zhou F, et al. Identification of beta-1,4-galactosyltransferase I as a target gene of HBx-induced cell cycle progression of hepatoma cell. J Hepatol. 2008;49:1029–37. doi: 10.1016/j.jhep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Wang Y, Wu MC, et al. Activating mechanism of transcriptor NF-kappaB regulated by hepatitis B virus X protein in hepatocellular carcinoma. World J Gastroenterol. 2004;10:356–60. doi: 10.3748/wjg.v10.i3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, He Z, Cao Y, et al. TAGLN2, a novel regulator involved in hepatitis B virus transcription and replication. Biochem Biophys Res Commun. 2016;477:1051–58. doi: 10.1016/j.bbrc.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Lu Y, Toh ST, et al. Lethal-7 is down-regulated by the hepatitis B virus X protein and targets signal transducer and activator of transcription 3. J Hepatol. 2010;53:57–66. doi: 10.1016/j.jhep.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 30.Chan CF, Yau TO, Jin DY, et al. Evaluation of nuclear factor-kappaB, urokinase-type plasminogen activator, and HBx and their clinicopathological significance in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4140–49. doi: 10.1158/1078-0432.CCR-03-0574. [DOI] [PubMed] [Google Scholar]
- 31.Ye L, Dong N, Wang Q, et al. Progressive changes in hepatoma cells stably transfected with hepatitis B virus X gene. Intervirology. 2008;51:50–58. doi: 10.1159/000120289. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WY, Cai N, Ye LH, Zhang XD. Transformation of human liver L-O2 cells mediated by stable HBx transfection. Acta Pharmacol Sin. 2009;30:1153–61. doi: 10.1038/aps.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Gao JY, Wang J, Cheng J. The impact of anti-HBV treatment on the occurrence and recurrence of hepatocellular carcinoma: Focus on Asian studies. Discov Med. 2015;19:89–99. [PubMed] [Google Scholar]
- 34.Chan AC, Chok KS, Yuen WK, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–81. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 35.Yu LH, Li N, Shi J, et al. Does anti-HBV therapy benefit the prognosis of HBV-related hepatocellular carcinoma following hepatectomy? Ann Surg Oncol. 2014;21:1010–15. doi: 10.1245/s10434-013-3320-z. [DOI] [PubMed] [Google Scholar]
- 36.Kim HY, Jung HU, Yoo SH, et al. Sorafenib overcomes the chemoresistance in HBx-expressing hepatocellular carcinoma cells through down-regulation of HBx protein stability and suppresses HBV gene expression. Cancer Lett. 2014;355:61–69. doi: 10.1016/j.canlet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Gao S, Zhao M, et al. Anti-HBV drugs suppress the growth of HBV-related hepatoma cells via down-regulation of hepatitis B virus X protein. Cancer Lett. 2017;392:94–104. doi: 10.1016/j.canlet.2017.02.003. [DOI] [PubMed] [Google Scholar]