Abstract
Objective
Recent studies have reported associations of retinoid-related orphan receptor alpha (RORA) gene single nucleotide polymorphisms (SNPs) with depression and anxiety disorders. Based on these, we attempt to test whether RORA polymorphism is associated with anxiety sensitivity (AS), the intermediate phenotype of depression and anxiety disorders. Considering gene-environment interactions and sex differences in AS, childhood maltreatment (CM) and sex were considered as confounders.
Methods
Two-hundred and five healthy young Korean adults (female: 98, male: 107; age, 23.0±3.2 years) completed genotyping for the RORA SNP rs11071547, as well as measures for AS and CM. Generalized linear models were used to examine the main and interaction effects of RORA genotype, CM, and sex in determining AS.
Results
The main effect of RORA polymorphisms was not found (p=0.760) whereas the main effect of CM and interaction effects among sex, genotype, and maltreatment were significant on AS. In separate analyses by sex, the interaction effect between RORA genotype and maltreatment was significant only in males (p<0.001). In females, the main effects of genotype and CM were significant (both were p<0.001), in which both a history of CM and C genotype tended to be associated with higher AS.
Conclusion
The association between RORA polymorphism and AS might differ by sex. The interaction between RORA polymorphism and CM was significant only in males whereas RORA genotype and CM independently associated with AS in females. Further studies are encouraged to confirm the relationship between RORA polymorphism and AS.
Keywords: Gene-environment interaction, Retinoid acid receptor-related orphan receptor alpha (RORA) gene, Childhood trauma, Sex, Anxiety sensitivity
INTRODUCTION
It has been suggested that retinoid-related orphan receptor alpha (RORA) gene single nucleotide polymorphisms (SNPs) pose a risk for depression1–3) and fear-related internalizing psychopathologies including panic, phobia, and obsessive-compulsive disorders.4) Recent reports have also demonstrated that RORA polymorphisms might be associated with posttraumatic stress disorder after exposure to various types of lifetime trauma5) and a hurricane.6) These results put questions whether RORA polymorphism is associated with an intermediate phenotype of depression and anxiety disorders, as well as how it interacts with traumatic experiences.
Anxiety sensitivity (AS) has been proposed as the potential intermediate phenotype of depression and anxiety disorders.7,8) The individual levels of AS are thought to be influenced by genetic and environmental factors, such as childhood maltreatment (CM).9,10) Regarding genetic factors, the polymorphisms of serotonin transporter gene7,11) and neuropeptide S receptor gene12) have been reported to interact with CM in determining AS.
We aimed to test whether the RORA genotype is associated with AS among healthy young adults. Considering the proposed sex differences in AS13) as well as the gene- environment interaction in determining AS,14,15) CM and sex were regarded as possible confounders.
METHODS
Participants
Healthy young adults were recruited by newspaper advertisement in Seoul, Republic of Korea. Participants were confirmed to be free from lifetime or current diagnosis of any psychiatric disorders and neurologic diseases through an interview with an experienced psychiatrist using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (SCID I and II). Among the 205 unrelated Koreans completed study procedures, 107 (52.2%) were male and a mean age was 23.0 (standard deviation [SD], 3.2; range, 18–31) years. All participants provided written informed consent prior to participation. The study protocol was approved by the Institutional Review Board at Seoul St. Mary’s Hospital, The Catholic University of Korea (KC10TIMI0434).
Measures
Individual AS was measured by Anxiety Sensitivity Index-Revised (ASI-R).16,17) It is rated on a 5-point Likert scale from 0 to 4. Higher total score means higher AS.
The short form of the Childhood Trauma Questionnaire (CTQ) was used to evaluate a history of CM.18,19) The CTQ is a self-report questionnaire consisting of 28 items that measure five categories of CM including emotional and physical neglect as well as emotional, physical, and sexual abuse. Each category consists of five items rated on a 5-point Likert scale. Based on the manual specifying cutoff points for the levels of none, low, moderate, and severe,20) the moderate to severe cutoff scores for each sub-scale were used to determine positive in that category and having one or more positive categories was regarded as the presence of CM.21,22)
Genotyping
The RORA rs11071547 was selected based on the previous study that reported its association with sleep disorder as one of the circadian clock genes.23) The rs11071547 is located in the intron region of RORA gene on chromosome 15. Blood samples (5–10 ml) were collected into an ethylenediaminetetraacetic acid (EDTA) tube, and genomic DNA was isolated using a NucleoSpin® Blood DNA Extraction Kit (Macherey-Nagel, Düren, Germany) according to procedures from the manufacturer’s manual. Genotyping was performed by high-resolution melting curve analysis. Polymerase chain reaction (PCR) was performed in a volume of 20 μl per reaction with a 96-well Bio-Rad CFX96 Real Time PCR system (Bio-Rad, Hercules, CA, USA). Reaction mixtures included 1.5 μl of genomic DNA as a template and each primer for the rs11071547 SNP of RORA at 200 mM (forward 5′-TGC CTA CCG CTT TCC TTT-3′, and reverse 5′-AAA TAA ACT TGG AGT GTT CTG GA-3′), 1×Sso Fast EvaGreen SuperMix (Bio-Rad) and sterile H2O. The amplification protocol started with 98°C/3 minutes followed by 39 cycles of 98°C/10 seconds and 58°C/20 seconds. After an initial step of 95°C/10 seconds and 65°C/10 seconds, melting curves were generated from 65°C to 95°C in increments of 0.3°C/cycle. Melting profiles were analyzed with Bio-Rad Precision Melt software.
Statistics
The Hardy-Weinberg equilibrium for genotype distribution was tested by the χ2 test. Between-group comparisons were performed with independent t-test or one-way analysis of variance and χ2 test for continuous and categorical variables, respectively. The relationship between continuous variables was examined by Pearson’s correlation analysis. Generalized linear models were used to explore the main effects of sex, RORA genotype, and CM with all possible two- and three-way interaction effects on AS. Data were analyzed with SAS software ver. 9.0 (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-tailed, and α was set at 0.05.
RESULTS
Distributions of the alleles and genotypes of RORA rs11071547 were in Hardy-Weinberg equilibrium (χ2=0.480, p=0.488). Among 205 participants, the allelic frequencies of C and T were 36.1% (n=148) and 63.9% (n=262), respectively. These frequencies are similar to reported distributions among general populations of China and Japan in the HapMap database (http://www.hapmap.org). The genotype frequencies of C/C, C/T, and T/T were 14.1% (n=29), 43.9% (n=90), and 42.0% (n=86), respectively. We reclassified the three genotypes into two groups, C (C/C and C/T, n=119) and T/T (n=86).
Mean scores of AS was 20.8 (SD=14.8). One or more types of CM were reported among 87 (42.4%) participants, in which 32, 30, 11, 54, and 24 individuals reported a history of emotional neglect, physical neglect, emotional abuse, physical abuse, and sexual abuse, respectively. Participants with CM showed significantly higher AS than those without (p=0.015). The frequencies of RORA genotype and sex as well as the mean ages did not differ significantly by a history of CM (p=0.668, 0.114, 0.791, respectively). Age showed significant negative association with AS (r=−0.178, p=0.010).
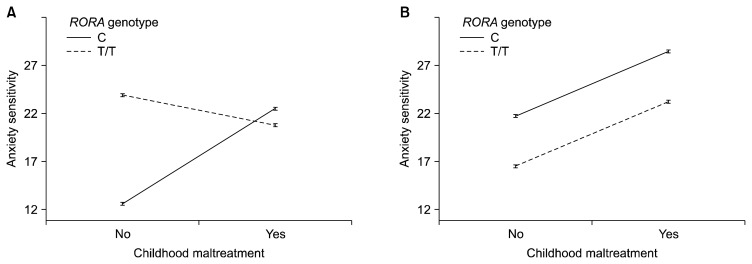
In the generalized linear model controlling for age (Table 1), the main effect of RORA genotype was not significant whereas that of CM was significant in predicting AS. There were significant three-way interaction among sex, genotype, and CM. In separate models by sex (Fig. 1), the interaction effect between genotype and CM was significant only in males (χ2=64.70, p<0.001). The T/T genotype was associated with the higher AS than C genotype among males without CM (p=0.016), whereas there was no difference in AS by genotype among males with CM (p=0.565). In females, the main effects of genotype and CM were significant (χ2=19.73, p<0.001 and χ2=27.25, p< 0.001, respectively), in which the history of CM and C genotype were associated with higher AS.
Table 1.
Results of generalized linear model examining the effect of sex, childhood maltreatment, and RORA genotype on anxiety sensitivity (WALD type 3 statistic)
| Source | df | χ2 test | p value |
|---|---|---|---|
| Age | 1 | 83.02 | <0.001 |
| Sex | 1 | 13.36 | <0.001 |
| Genotype (RORA polymorphism) | 1 | 0.09 | 0.760 |
| Maltreatment | 1 | 58.13 | <0.001 |
| Sex×genotype | 1 | 59.46 | <0.001 |
| Sex×maltreatment | 1 | 2.61 | 0.106 |
| Genotype×maltreatment | 1 | 20.49 | <0.001 |
| Sex×genotype×maltreatment | 1 | 34.43 | <0.001 |
Omnibus test (p<0.001).
df, degree of freedom.
Fig. 1.
Interaction effects between RORA polymorphism and childhood maltreatment on anxiety sensitivity by sex. The interaction effect was significant in male subects (A), whereas main effects of RORA genotype and childhood maltreatment were significant in female subjects (B).
DISCUSSION
This preliminary study did not found the association between RORA rs11071547 genotype and AS. However, we demonstrated the sex-specific interactions between RORA genotype and CM in determining AS. The RORA genotype interacted with CM in males, whereas each of RORA genotype and CM were significant predictor of AS in females. These results suggest the possible role of sex in interaction between RORA polymorphism and CM to determine AS.
RORA protein plays a role in maintaining circadian rhythm.24) Clinical studies have shown that disruptions in circadian rhythms may contribute to anxiety-prone traits and/or anxiety disorders.25) Among circadian-clock genes, the SNPs of ARNTL2, DRD2, BCL2, and PAWR have been reported to be associated with AS.26,27) In addition, RORA protein is widely expressed in brain regions including the frontal cortex,28,29) and participates in protecting neurons and glial cells from oxidative stress-induced apoptosis.30) Oxidative stress is one of the potential mechanisms implicated in pathophysiology of depression and anxiety disorders. In animal studies, high levels of oxidative stress induced anxiogenic behaviors31) and vulnerability to depression.32) These roles of RORA protein might support the biological plausibility of the association between RORA polymorphism and AS.
Results of the present study emphasize the importance of the sex in gene-environment interaction in determining AS. As in previous studies,9,10) CM was a significant environmental factor influencing AS. However, we found that CM interacts with RORA genotype in males but not in females. Among females, the main effects of genotype and maltreatment were significant. These results suggested that the level AS might be influenced differently to CM according to RORA genotype in males whereas it might be expected by the genotype and the experience of CM in females. These results are partly in line with that of a twin study, in which AS is heritable only in women and better explained by environmental factors in men.33) It has also been reported that heritability and the impact of genetic factors might be greater among females than those among males in the studies of anxiety disorder and neuroticism.14,15) Regarding the behavioral impact of circadian genes, a the CLOCK gene mutation has been reported to induce more pronounced behavioral changes of increased exploratory behaviors in female relative to male mice.34) Based on the results of previous and present studies, we could hypothesize that the impact of related genes including circadian genes might be differently influenced by environmental factor on AS by sex. Further studies on the effect of sex hormones and sexual difference in epigenetic processes on brain and behaviors35) would be required to attest our hypothesis.
There are several limitations. Sample size is small and the study design is cross-sectional. A retrospective assessment of CM may be biased by individual levels of emotionality36) though recall bias has been reported to account for less than 1% of variance in non-clinical young adults.37) In addition, the clinical significance of RORA rs11071547 is yet to be determined and we did not analyze other RORA SNPs, which have been reported the associations with depression and anxiety disorders.4–6) Recently suggested critical issues of G×E approach need to be duly considered.38)
In summary, we did not found the association between RORA rs11071547 and AS among healthy young adults. However, we provide the evidence of the sex-specific interaction between RORA polymorphism and CM in determining AS.
Acknowledgments
This work was supported by a grant from the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI) (grant number: HM15C1054) and the Korea Science and Engineering Foundation (KOSEF), funded by the Korean government (NRF-2015R1A2A2A01003564).
REFERENCES
- 1.Lavebratt C, Sjöholm LK, Partonen T, Schalling M, Forsell Y. PER2 variantion is associated with depression vulnerability. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:570–581. doi: 10.1002/ajmg.b.31021. [DOI] [PubMed] [Google Scholar]
- 2.Terracciano A, Tanaka T, Sutin AR, Sanna S, Deiana B, Lai S, et al. Genome-wide association scan of trait depression. Biol Psychiatry. 2010;68:811–817. doi: 10.1016/j.biopsych.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maglione JE, Nievergelt CM, Parimi N, Evans DS, Ancoli-Israel S, Stone KL, et al. Associations of PER3 and RORA circadian gene polymorphisms and depressive symptoms in older adults. Am J Geriatr Psychiatry. 2015;23:1075–1087. doi: 10.1016/j.jagp.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MW, Wolf EJ, Logue MW, Baldwin CT. The retinoid- related orphan receptor alpha (RORA) gene and fear- related psychopathology. J Affect Disord. 2013;151:702–708. doi: 10.1016/j.jad.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2013;18:937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amstadter AB, Sumner JA, Acierno R, Ruggiero KJ, Koenen KC, Kilpatrick DG, et al. Support for association of RORA variant and post traumatic stress symptoms in a population-based study of hurricane exposed adults. Mol Psychiatry. 2013;18:1148–1149. doi: 10.1038/mp.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33:312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: prospective prediction of panic attacks and Axis I pathology. J Psychiatr Res. 2006;40:691–699. doi: 10.1016/j.jpsychires.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Stein MB, Jang KL, Livesley WJ. Heritability of anxiety sensitivity: a twin study. Am J Psychiatry. 1999;156:246–251. doi: 10.1176/ajp.156.2.246. [DOI] [PubMed] [Google Scholar]
- 10.Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 11.Klauke B, Deckert J, Reif A, Pauli P, Zwanzger P, Baumann C, et al. Serotonin transporter gene and childhood trauma--a G × E effect on anxiety sensitivity. Depress Anxiety. 2011;28:1048–1057. doi: 10.1002/da.20840. [DOI] [PubMed] [Google Scholar]
- 12.Klauke B, Deckert J, Zwanzger P, Baumann C, Arolt V, Pauli P, et al. Neuropeptide S receptor gene (NPSR) and life events: G × E effects on anxiety sensitivity and its subdimensions. World J Biol Psychiatry. 2014;15:17–25. doi: 10.3109/15622975.2011.646302. [DOI] [PubMed] [Google Scholar]
- 13.Stewart SH, Taylor S, Baker JM. Gender differences in dimensions of anxiety sensitivity. J Anxiety Disord. 1997;11:179–200. doi: 10.1016/S0887-6185(97)00005-4. [DOI] [PubMed] [Google Scholar]
- 14.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 15.Lake RI, Eaves LJ, Maes HH, Heath AC, Martin NG. Further evidence against the environmental transmission of individual differences in neuroticism from a collaborative study of 45,850 twins and relatives on two continents. Behav Genet. 2000;30:223–233. doi: 10.1023/A:1001918408984. [DOI] [PubMed] [Google Scholar]
- 16.Taylor S, Cox BJ. An expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sample. J Anxiety Disord. 1998;12:463–483. doi: 10.1016/S0887-6185(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 17.Lim YJ, Yu BH, Kim JH. Korean Anxiety Sensitivity Index-Revised: its factor structure, reliability, and validity in clinical and nonclinical samples. Depress Anxiety. 2007;24:331–341. doi: 10.1002/da.20210. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Park SC, Yang H, Oh DH. Reliability and validity of the korean version of the childhood trauma questionnaire-short form for psychiatric outpatients. Psychiatry Investig. 2011;8:305–311. doi: 10.4306/pi.2011.8.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein DP, Fink L. Childhood trauma questionnaire: a restrospective self-report: manual. Orlando: Psychological Corporation; 1998. [Google Scholar]
- 21.Grabe HJ, Schwahn C, Mahler J, Appel K, Schulz A, Spitzer C, et al. Genetic epistasis between the brain-derived neuro-trophic factor Val66Met polymorphism and the 5-HTT promoter polymorphism moderates the susceptibility to depressive disorders after childhood abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:264–270. doi: 10.1016/j.pnpbp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry. 2009;66:72–80. doi: 10.1001/archgenpsychiatry.2008.508. [DOI] [PubMed] [Google Scholar]
- 23.Kripke DF, Kline LE, Nievergelt CM, Murray SS, Shadan FF, Dawson A, et al. Genetic variants associated with sleep disorders. Sleep Med. 2015;16:217–224. doi: 10.1016/j.sleep.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Coles ME, Schubert JR, Nota JA. Sleep, circadian rhythms, and anxious traits. Curr Psychiatry Rep. 2015;17:73. doi: 10.1007/s11920-015-0613-x. [DOI] [PubMed] [Google Scholar]
- 26.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm (Vienna) 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 27.Sipilä T, Kananen L, Greco D, Donner J, Silander K, Terwilliger JD, et al. An association analysis of circadian genes in anxiety disorders. Biol Psychiatry. 2010;67:1163– 1170. doi: 10.1016/j.biopsych.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Ino H. Immunohistochemical characterization of the orphan nuclear receptor ROR alpha in the mouse nervous system. J Histochem Cytochem. 2004;52:311–323. doi: 10.1177/002215540405200302. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010;24:3036–3051. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boukhtouche F, Vodjdani G, Jarvis CI, Bakouche J, Staels B, Mallet J, et al. Human retinoic acid receptor-related orphan receptor alpha1 overexpression protects neurones against oxidative stress-induced apoptosis. J Neurochem. 2006;96:1778–1789. doi: 10.1111/j.1471-4159.2006.03708.x. [DOI] [PubMed] [Google Scholar]
- 31.Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.144. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang KL, Stein MB, Taylor S, Livesley WJ. Gender differences in the etiology of anxiety sensitivity: a twin study. J Gend Specif Med. 1999;2:39–44. [PubMed] [Google Scholar]
- 34.Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183X.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 35.Ratnu VS, Emami MR, Bredy TW. Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. J Neurosci Res. 2017;95:301–310. doi: 10.1002/jnr.23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fergusson DM, Horwood LJ, Boden JM. Structural equation modeling of repeated retrospective reports of childhood maltreatment. Int J Methods Psychiatr Res. 2011;20:93–104. doi: 10.1002/mpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]