Abstract
Objective
Several studies have suggested the efficacy of bupropion and escitalopram on reducing the excessive internet game play. We hypothesized that both bupropion and escitalopram would be effective on reducing the severity of depressive symptoms and internet gaming disorder (IGD) symptoms in patients with both major depressive disorder and IGD. However, the changes in brain connectivity between the default mode network (DMN) and the salience network were different between bupropion and escitalopram due to their different pharmacodynamics.
Methods
This study was designed as a 12-week double blind prospective trial. Thirty patients were recruited for this research (15 bupropion group+15 escitalopram group). To assess the differential functional connectivity (FC) between the hubs of the DMN and the salience network, we selected 12 regions from the automated anatomical labeling in PickAtals software.
Results
After drug treatment, the depressive symptoms and IGD symptoms in both groups were improved. Impulsivity and attentional symptoms in the bupropion group were significantly decreased, compared to the escitalopram group. After treatment, FC within only the DMN in escitalopram decreased while FC between DMN and salience network in bupropion group decreased. Bupropion was associated with significantly decreased FC within the salience network and between the salience network and the DMN, compared to escitalopram.
Conclusion
Bupropion showed greater effects than escitalopram on reducing impulsivity and attentional symptoms. Decreased brain connectivity between the salience network and the DMN appears to be associated with improved excessive IGD symptoms and impulsivity in MDD patients with IGD.
Keywords: Bupropion, Citalopram, Internet, video games, Major depressive disorder, Functional magnetic resonance imaging
INTRODUCTION
Many studies of internet gaming disorder (IGD) have reported correlations between excessive internet game play and depressive symptoms.1–3) In a survey of 1,397 Korean people, problematic game use was associated with nicotine use, depressive disorder, and anxiety disorder.1) Although there has been meaningful debate as to whether IGD is a formal psychiatric disorder with solid diagnostic criteria,4) research has already begun to investigate treatments for the disorder. Several studies have suggested the efficacy of bupropion and escitalopram for reducing excessive internet game play.3,5) Dell’Osso et al.5) reported that escitalopram improved compulsive internet game play in patients with IGD. Han and Renshaw3) reported that bupropion reduced excessive internet game play in patients with both major depressive disorder (MDD) and IGD. Additionally, our previous study found that both bupropion and escitalopram reduced excessive internet game play.6) In a comparison of the efficacy between the two medications, bupropion was more effective than escitalopram in improving attention and impulsivity in patients with IGD.6)
Bupropion, an anti-depressant, is known to inhibit the reuptake of both dopamine and norepinephrine.7) Due to this double action, bupropion was recommended to control withdrawal symptoms in smoking cessation as well as attention and impulsivity in the attention deficit hyper-activity disorder (ADHD) children.8) In our previous study, 6 weeks of bupropion treatment decreased brain activity within the dorsolateral prefrontal cortex in response to game stimulation and reduced the severity of IGD.9) Rzepa et al.10) reported that 7 days of bupropion administration increased brain function connectivity within the central executive network and the default mode network (DMN) in healthy volunteers. Escitalopram, an S-enantiomer of citalopram, is a popular antidepressant for patients with MDD or anxiety disorders.11) Like other selective serotonin reuptake inhibitors (SSRIs), escitalopram inhibits serotonin transporters from reuptaking serotonin, resulting in increased serotonin levels at synapses.12) Wang et al.13) reported that 8 weeks of escitalopram treatment decrease functional connectivity (FC) within the bilateral dorsomedial prefrontal cortex, a part of the DMN, in patients with MDD.
In a review of resting state FC in patients with MDD, Mulders et al.14) reported four consistent findings: (1) increased brain FC within the anterior DMN, (2) increased FC between the salience network and the anterior DMN, (3) changed FC between the anterior DMN and the posterior DMN, and (4) decreased FC between the posterior DMN and the salience network. The DMN was defined as areas that are synchronically deactivated during task performance and prominently activated during rest.15) The DMN includes the posterior cingulate cortex (PCC), precuneus, medial frontal cortex, ventral anterior cingulate cortex, and lateral and inferior parietal cortices.15) A failure of deactivation of DMN was thought to be associated with impulsivity, risky decision, and attention deficit.2,14,16) In patients with substance dependence, the brain connectivity within the DMN was positively connected with impulsivity.16) In response to the Wisconsin Card Sorting Test, depressed adolescents with compulsive IGD showed a failure to suppress DMN activity.2) Wang et al.17) reported that IGD subjects showed higher FC within the DMN in response to risky decision stimulation. The salience network is a collection of brain regions in response to stimuli which are deserving of our attention.18) The salience network is thought to reflect paralimbic emotional processing and emotional control.18) In addition, the salience network is implicated in switching between the DMN and the central executive network.19) The salience network consists of the fronto-insular cortex, dorsal anterior cingulate cortex (dACC), amygdala, and temporal pole.20)
We hypothesized that both bupropion and escitalopram would be effective for reducing the severity of depressive and IGD symptoms. However, the changes in brain connectivity between the DMN and the salience network were different between bupropion and escitalopram due to their different pharmacodynamics.
METHODS
Participants
Thirty-four patients with both problematic internet game play and MDD agreed to participate in the current study. All patients were screened with the structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) and diagnosed by a psychiatrist. Problematic internet game play was defined as excessive internet game play of more than 4 hours per day or 30 hours per week,21) Young Internet Addiction Scale (YIAS) scores of more than 50,22) and maladaptive and disruptive behavior in general life due to excessive internet game play. All patients were randomly assigned to bupropion+education regarding internet game play or escitalopram+education regarding internet game play in a 1:1 ratio. Of the 34 initial participants, one patient in the bupropion group and one patient in the escitalopram group discontinued medication due to nausea and diarrhea. One patient in the bupropion group stopped medication due to palpitation. One patient in the escitalopram group was excluded due to mood change from depression to mania. Finally, 30 patients participated in this research (15 in the bupropion group, 15 in the escitalopram group). The Chung-Ang University Hospital Institutional Review Board approved the study protocol (C2011131) for this research and written informed consent was provided by all patients.
Study Procedure
This study was designed as a 12-week double blind prospective trial. At baseline and 12-week follow up, all patients were asked to complete questionnaires comprising demographic data questions, the YIAS, the Beck Depressive Inventory (BDI), the Korea Attention Deficit Hyperactivity Disorder Scale (K-ARS), and the Behavioral Inhibition and Activation Scales (BIS-BAS). In addition, all patients were scanned to assess their brain FC using resting state functional magnetic resonance imaging at baseline and 12-week follow up. The patients in the bupropion group were started on bupropion sustained-release 150 mg/day and increased to 300 mg/day during the first week of treatment. The patients in the escitalopram group were started on escitalopram 10 mg/day and increased to 20 mg/day during the first week of treatment. During weeks 2 to 12, the patients in both groups were asked to maintain a consistent medication dose. The YIAS is a self-report scale for the severity of internet use, and the internal consistency of the Korea YIAS has ranged from 0.90 to 0.91.23) The BDI, which has an internal consistency ranging from 0.75 to 0.85, was used to assess depressive symptoms.24) The K-ARS, with an internal consistency ranging from 0.77 to 0.89, was applied to assess inattention and hyperactivity.25) The BIS-BAS, with a consistency ranging from 0.78 to 0.79, is a self-report scale used to estimate impulsiveness.26)
Brain Imaging Data Analysis
We used a Achieva 3.0 Tesla TX magnetic resonance imaging (MRI) scanner (Philips, Eindhoven, the Nether-lands) to obtain resting state brain activity. Acquisition was performed during a resting-state scan, yielding a total of 240 volumes. Sagittal three-dimensional magnetization prepared rapid acquisition gradient echo (MPRAGE) images were acquired with an isotropic in-plane resolution of 1×1×1 (TR=3 seconds, 12-minute scan, 128×128 matrix, 40 slices at 4.0 mm slice thickness). All imaging data were preprocessed with the following processing: despiking, motion correction, co-registration to the MPRAGE image, normalization to Montreal Neurological Institute (MNI) space, temporal detrending, bandpass filtering, and voxel-wise regression of an identically bandpass filtered time series of 6 head motion parameters, degraded cere-brospinal fluid, degraded white matter, and facial soft tissues, as previous studies have described.27,28) To correct for head movements, censoring of time points with head motion >0.2 mm was used.29,30)
No regression of the global signal was performed.31,32) To assess the differential FC between the hubs of the DMN and the salience network, we extracted 12 regions from the automated anatomical labeling in the PickAtals software (ANSIR Laboratory, Wake Forest University School of Medicine)33,34). The DMN includes the left/right middle prefrontal cortex (mPFC), left/right PCC, left/right parietal cortex, and left/right precuneus. The salience network includes the left/right dACC and left/right insular. Correlation coefficients were measured for each pair of regions of interest in each participant.35–37)
Statistical Analysis
We analyzed the demographic and clinical characteristics using chi-square tests and Mann-Whitney U tests with significance set at p<0.05. We used paired t-tests to evaluate the changes in FC for patients in both the bupropion and the escitalopram groups. Additionally, we used repeated measures ANOVA to compare changes in FC between the two groups. To correct for multiple comparisons over the 78 pairs in 12 regions, we used an acceptable false discovery rate of q<0.05. We calculated the correlation between the changes in BDI scores and the changes in functional correlation with a threshold of p<0.05, and used commercially available software (IBM SPSS Statistics, version 20; IBM Co., Armonk, NY, USA).
RESULTS
Demographic Characteristics
There were no group differences in age (z=−1.43, p=0.15), school year (z=0.29, p=0.77), genre of game (χ2=0.72, p=0.87), YIAS score (z=−0.62, p=0.53), BDI score (z=1.92, p=0.06), K-ARS score (z=−1.43, p=0.15), or BIS-BAS score (z=1.20, p=0.23) at baseline (Table 1).
Table 1.
The comparison of demographic characteristics and psychological condition between bupropion group an escitalopram group
| Characteristic | Bupropion (n=15) | Escitalopram (n=15) | Statistics |
|---|---|---|---|
| Dose (mg/day) | 175 | 13.8 | - |
| Age (yr) | 22.9±1.9 | 23.9±1.6 | z=−1.43, p=0.15 |
| School (yr) | 12.5±1.6 | 12.4±1.6 | z=0.29, p=0.77 |
| Genre of game | |||
| RPG | 6 (40.0) | 7 (46.7) | χ2=0.72, p=0.87 |
| FPS | 2 (13.3) | 1 (6.7) | |
| RTS | 5 (33.3) | 4 (26.7) | |
| Others* | 2 (13.3) | 3 (20.0) | |
| YIAS | |||
| Baseline | 63.6±13.9 | 67.5±11.4 | z=−0.62, p=0.53 |
| Follow up | 41.2±18.0 | 51.0±16.0 | z=−1.51, p=0.13 |
| BDI | |||
| Baseline | 18.6±2.8 | 16.0±4.0 | z=1.92, p=0.06 |
| Follow up | 10.2±4.8 | 8.3±3.2 | z=1.16, p=0.24 |
| K-ARS | |||
| Baseline | 10.1±2.5 | 10.4±3.2 | z=−1.43, p=0.15 |
| Follow up | 3.6±3.1 | 8.5±3.4 | z=−3.19, p<0.01 |
| BIS-BAS | |||
| Baseline | 56.0±8.8 | 52.5±7.4 | z=1.20, p=0.23 |
| Follow up | 43.6±12.0 | 46.7±8.1 | z=−0.60, p=0.55 |
Values are presented as mean ± standard deviation or number (%).
RPG, role playing game; FPS, first person shooting game; RTS, real time strategy; YIAS, Young Internet Addiction Scale Score; BDI, Beck Depressive Inventory; K-ARS, Korean Attention Deficit Hyperactivity Disorder Scale; BIS-BAS, Behavioral Inhibition and Activation Scales.
Sports, casual, etc.
Changes in Clinical Symptoms
After the 12-week drug treatments, the BDI scores (bupropion: z=3.1, p<0.01; escitalopram: z=3.6, p< 0.01) and YIAS scores (bupropion: z=3.1, p<0.01; escitalopram: z=3.1, p<0.01) in both groups decreased; however, there was no significant difference between the changes in BDI (F=0.25, p=0.62) and YIAS scores (F=0.91, p=0.35) between the two groups. The BIS-BAS scores (bupropion: z=3.6, p<0.01; escitalopram: z=3.6, p< 0.01) were also decreased in both groups. However, the BIS-BAS scores were significantly decreased in the bupropion group compared to the escitalopram group (F=6.0, p=0.02). The K-ARS scores in the bupropion group decreased (z=2.6, p<0.01), whereas the K-ARS scores in the escitalopram group did not change (z=1.3, p=0.18) (Table 1).
Changes in Functional Correlations
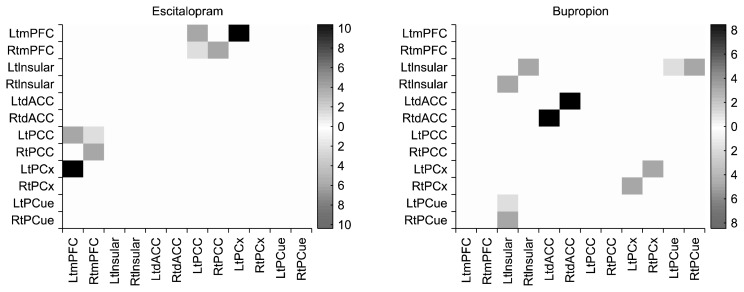
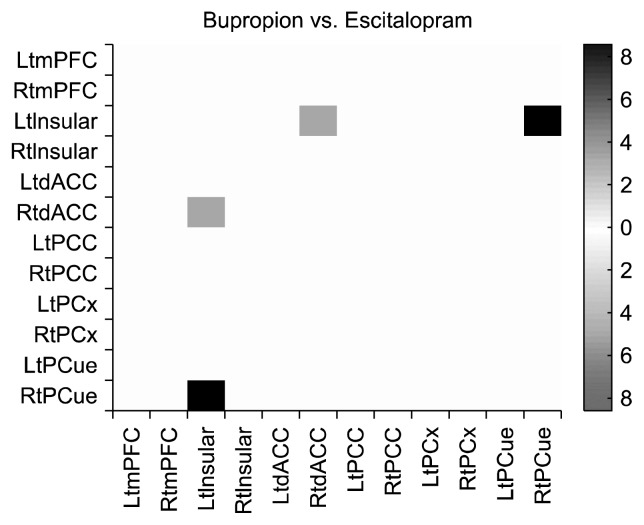
At baseline, there were no significant differences in functional correlations between the bupropion and escitalopram groups. After the 12-week drug therapy, the escitalopram group showed decreased functional correlations between four pairs of regions, satisfying uncorrected p< 0.05: left mPFC to left PCC (F=4.52, p=0.04), left mPFC to left parietal cortex (F=8.22, p<0.01), right mPFC to left PCC (F=4.14, p=0.04), and right mPFC to right PCC (F=4.60, p=0.03) (Fig. 1). After the 12-week drug therapy, the bupropion group showed decreased functional correlations between five pairs of regions, satisfying uncorrected p<0.05: left precuneus to left insular (F=4.12, p=0.04), right precuneus to left insular (F=4.67, p=0.03), right parietal cortex to left parietal cortex (F=4.64, p= 0.03), left insular to right insular (F=4.79, p=0.03), and left dACC to right dACC (F=7.16, p=0.01) (Fig. 1). In a comparison of bupropion and escitalopram, bupropion was associated with significantly decreased functional correlations between two pairs of regions: right dACC to left insular (F=6.12, p<0.01) and right precuneus to left insular (F=7.24, p<0.01), which satisfied the q<0.05 false discovery rate (Fig. 2).
Fig. 1.
Changes in functional correlations after 12 week treatment.
Lt, left; Rt, right; mPFC, middle prefrontal cortex; dACC, dorsal anterior cingulate cortex; PCC, posterior cingulate cortex; PCx, parietal cortex; PCue, precuneus.
Fig. 2.
Comparison of functional correlations between bupropion and escitalopram.
Lt, left; Rt, right; mPFC, middle prefrontal cortex; dACC, dorsal anterior cingulate cortex; PCC, posterior cingulate cortex; PCx, parietal cortex; PCue, precuneus.
Correlations between the Changes in Clinical Scales Scores and Functional Correlations
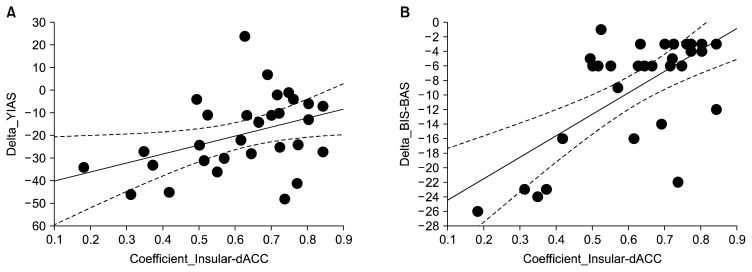
Improvement in YIAS scores was positively correlated with decreased functional correlation between the right dACC and the left insular in all MDD patients with problematic internet game play (r=0.40, p=0.03). Improvement in BIS-BAS scores was positively correlated with decreased functional correlation within the right dACC and the left insular in all MDD patients with problematic internet game play (r=0.66, p<0.01) (Fig. 3). Changes in YIAS scores were positively correlated with changes in BIS-BAS scores (r=0.50, p<0.01) (Fig. 3).
Fig. 3.
Correlations between the changes in clinical symptoms and functional correlations. (A) The correlation between the changes in Young Internet Addiction Scale (YIAS) scores and functional correlations from right dorsal anterior cingulate (dACC) and the left insular, r=0.40, p=0.03. (B) The correlation between the changes in Behavioral Inhibition and Activation Scales (BIS-BAS) scores and functional correlations from right dACC and the left insular, r=0.66, p<0.01.
DISCUSSION
The results in this study showed that 12-week bupropion or escitalopram treatment improved depressive symptoms and reduced the severity of IGD in patients with MDD and IGD. Compared to escitalopram, bupropion was associated with significantly greater decreased BIS-BAS and K-ARS scores. Escitalopram decreased FC within the DMN while bupropion decreased FC within the DMN and the salience networks. In addition, bupropion greatly decreased functional correlations within the salience network as well as between the salience and the DMNs.
The results in this study showed that 12-week administration of bupropion or escitalopram improved depressive symptoms and reduced IGD severity. Compared to escitalopram, bupropion was associated with significantly improved BIS-BAS and K-ARS scores. These results are consistent with those of our previous study6) of 119 adolescent and young adults with IGD, in which both bupropion and escitalopram were effective in treating IGD. Moreover, bupropion was more effective in improving attention and impulsivity in IGD, compared to escitalopram.6) The effectiveness of bupropion on IGD symptoms in 50 patients with MDD was also shown in our previous study,3) in which 8-week administration of bupropion decreased BDI and YIAS scores in MDD patients with IGD.3) Two open-label studies reported that escitalopram reduced the severity of IGD and impulsivity.5,38) Due to the dual action of bupropion (norepinephrine and dopamine reuptake inhibition), bupropion is more effective for improving impulsivity and attention than escitalopram.39) Increased noradrenergic activity induced by bupropion is known to be associated with a reduction in impulsivity in patients with MDD.40) Release of dopamine by bupropion has been used to treat children with ADHD.8)
In the current results, bupropion decreased FC within the DMN and the salience network as well as between the DMN and the salience network, whereas escitalopram decreased FC only within the DMN. There has been controversy regarding the effects of bupropion on altering brain activity within the DMN.10,41) Costello et al.41) reported that bupropion reduced brain activity within the DMN in response to goal-oriented tasks. In contrast, Rzepa et al.10) reported that 7-day bupropion administration increased the brain function connectivity from the dorsal medial prefrontal cortex to the PCC and the precuneus cortex in healthy volunteers. Decreased FC between the DMN and the salience network after bupropion treatment may be related to the results seen in atomoxetine and modafinil studies. Atomoxetine, a first-line treatment medication for attention deficit hyperactivity disorder, is thought to have the dual action of increasing both nor-epinephrine and dopamine.42) An 8-week administration of atomoxetine decreased FC between the task-positive network and the DMN.43) Modfinil also increased norepinephrine and dopamine in synapses by inhibiting nor-epinephrine transporters and dopamine transporters.44) Minzenberg et al.45) reported that modafinil administration led to task-induced deactivation of the DMN in healthy adults. Repeated social stress is thought to alter norepinephrine release in the locus ceruleus, which is associated with reward salience in addictive behaviors.46) Dopamine also has a crucial role in incentive-sensitization in behavioral addiction.47) Like atomoxetine and modafinil, increased norepinephrine and dopamine by bupropion administration may decrease the activity of the salience network. Increased FC between the DMN and the salience network was found in IGD patients with childhood ADHD history.48) In our results, decreased FC between the DMN and the salience network after bupropion treatment, which was greater than the changes after escitalopram administration, was associated with decreased YIAS scores and impulsivity scores.
van de Ven et al.49) reported that escitalopram decreased FC within the DMN, including the anterior and PCC, hippocampal complex, and lateral parietal regions. These results are consistent with those of previous studies of regional changes in brain activity after serotonin challenge.50–52) In task-related functional MRI studies, decreased brain activity was found after SSRI administration.50,51) In a positron emission tomography study, administration of the SSRI, fenfluramine, decreased activity within the PCC.52) In the current study, we found no correlation between clinical scale scores and FC within the DMN in the escitalopram group.
In summary, increased serotonin in the brain decreased brain connectivity only within the DMN, whereas increased norepinephrine and dopamine in the brain decreased brain connectivity between the salience network and the DMN. This decreased brain connectivity was associated with improvement in excessive internet game playing and impulsivity in MDD patients with IGD.
There were several limitations to the current study. First, the small number of subjects is insufficient to support generalizing the results to other brain networks. Second, the short duration of the study did not allow full documentation of the effects of the drug treatments. Longer periods of medication treatment and follow-up are needed to better elucidate responses and potential relapse in MDD patients with IGD.
Both bupropion and escitalopram decreased depressive symptoms and IGD symptoms in MDD patients with IGD. Moreover, bupropion showed greater effects on reducing impulsivity and attentional symptoms. Decreased brain connectivity between the salience network and the DMN were associated with improvement in excessive internet game playing and impulsivity in MDD patients with IGD.
Acknowledgments
This work was supported by a Korea Creative Content Agency grant (R2014040055).
REFERENCES
- 1.Park S, Jeon HJ, Son JW, Kim H, Hong JP. Correlates, comorbidities, and suicidal tendencies of problematic game use in a national wide sample of Korean adults. Int J Ment Health Syst. 2017;11:35. doi: 10.1186/s13033-017-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Han DH, Kim SM, Bae S, Renshaw PF, Anderson JS. A failure of suppression within the default mode network in depressed adolescents with compulsive internet game play. J Affect Disord. 2016;194:57–64. doi: 10.1016/j.jad.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Han DH, Renshaw PF. Bupropion in the treatment of problematic online game play in patients with major depressive disorder. J Psychopharmacol. 2012;26:689–696. doi: 10.1177/0269881111400647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aboujaoude E, Koran LM, Gamel N, Large MD, Serpe RT. Potential markers for problematic internet use: a telephone survey of 2,513 adults. CNS Spectr. 2006;11:750–755. doi: 10.1017/S1092852900014875. [DOI] [PubMed] [Google Scholar]
- 5.Dell’Osso B, Hadley S, Allen A, Baker B, Chaplin WF, Hollander E. Escitalopram in the treatment of impulsive- compulsive internet usage disorder: an open-label trial followed by a double-blind discontinuation phase. J Clin Psychiatry. 2008;69:452–456. doi: 10.4088/JCP.v69n0316. [DOI] [PubMed] [Google Scholar]
- 6.Song J, Park JH, Han DH, Roh S, Son JH, Choi TY, et al. Comparative study of the effects of bupropion and escitalopram on Internet gaming disorder. Psychiatry Clin Neurosci. 2016;70:527–535. doi: 10.1111/pcn.12429. [DOI] [PubMed] [Google Scholar]
- 7.Hou YC, Lai CH. The accompanying changes in brain structure of a remitted depression patient with the bupropion treatment. Clin Psychopharmacol Neurosci. 2015;13:319–320. doi: 10.9758/cpn.2015.13.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conners CK, Casat CD, Gualtieri CT, Weller E, Reader M, Reiss A, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35:1314–1321. doi: 10.1097/00004583-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Han DH, Hwang JW, Renshaw PF. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. Exp Clin Psychopharmacol. 2010;18:297–304. doi: 10.1037/a0020023. [DOI] [PubMed] [Google Scholar]
- 10.Rzepa E, Dean Z, McCabe C. Bupropion administration increases resting-state functional connectivity in dorsomedial prefrontal cortex. Int J Neuropsychopharmacol. 2017;20:455–462. doi: 10.1093/ijnp/pyx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh KS, Shin E, Ha J, Shin D, Shin Y, Lim SW. Early improvement in one week predicts the treatment response to escitalopram in patients with social anxiety disorder: A preliminary study. Clin Psychopharmacol Neurosci. 2016;14:161–167. doi: 10.9758/cpn.2016.14.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong H, Haddjeri N, Sánchez C. Escitalopram, an anti-depressant with an allosteric effect at the serotonin transporter--a review of current understanding of its mechanism of action. Psychopharmacology (Berl) 2012;219:1–13. doi: 10.1007/s00213-011-2463-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W, et al. The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum Brain Mapp. 2015;36:768–778. doi: 10.1002/hbm.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regner MF, Saenz N, Maharajh K, Yamamoto DJ, Mohl B, Wylie K, et al. Top-down network effective connectivity in abstinent substance dependent individuals. PLoS One. 2016;11:e0164818. doi: 10.1371/journal.pone.0164818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Wu L, Lin X, Zhang Y, Zhou H, Du X, et al. Dysfunctional default mode network and executive control network in people with Internet gaming disorder: Independent component analysis under a probability discounting task. Eur Psychiatry. 2016;34:36–42. doi: 10.1016/j.eurpsy.2016.01.2424. [DOI] [PubMed] [Google Scholar]
- 18.Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, et al. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Ha JH, Yoo HJ, Cho IH, Chin B, Shin D, Kim JH. Psychiatric comorbidity assessed in Korean children and adolescents who screen positive for Internet addiction. J Clin Psychiatry. 2006;67:821–826. doi: 10.4088/JCP.v67n0517. [DOI] [PubMed] [Google Scholar]
- 23.Kim JW, Kim SY, Choi JW, Kim KM, Nam SH, Min KJ, et al. Differences in resting-state quantitative electroencephalography patterns in attention deficit/hyperactivity disorder with or without comorbid symptoms. Clin Psychopharmacol Neurosci. 2017;15:138–145. doi: 10.9758/cpn.2017.15.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uh D, Jeong HG, Choi KY, Oh SY, Lee S, Kim SH, et al. Dehydroepiandrosterone sulfate level varies nonlinearly with symptom severity in major depressive disorder. Clin Psychopharmacol Neurosci. 2017;15:163–169. doi: 10.9758/cpn.2017.15.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean parent and teacher ADHD rating scale. J Korean Neuropsychiatr Assoc. 2002;41:283–289. [Google Scholar]
- 26.Kim K, Kim W. [Korean-BAS/BIS scale]. Korean J Health Psychol. 2001;6:19–37. Korean. [Google Scholar]
- 27.Anderson JS, Nielsen JA, Ferguson MA, Burback MC, Cox ET, Dai L, et al. Abnormal brain synchrony in Down Syndrome. Neuroimage Clin. 2013;2:703–715. doi: 10.1016/j.nicl.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134:3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 34.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Park SY, Kim YI, Son YD, Chung US, Min KJ, et al. Affective network and default mode network in depressive adolescents with disruptive behaviors. Neuropsychiatr Dis Treat. 2015;12:49–56. doi: 10.2147/NDT.S95541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- 37.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sattar P, Ramaswamy S. Internet gaming addiction. Can J Psychiatry. 2004;49:869–870. doi: 10.1177/070674370404901225. [DOI] [PubMed] [Google Scholar]
- 39.Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11:133–141. doi: 10.1038/npp.1994.43. [DOI] [PubMed] [Google Scholar]
- 40.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 41.Costello MR, Mandelkern MA, Shoptaw S, Shulenberger S, Baker SK, Abrams AL, et al. Effects of treatment for tobacco dependence on resting cerebral glucose metabolism. Neuropsychopharmacology. 2010;35:605–612. doi: 10.1038/npp.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 43.Lin HY, Gau SS. Atomoxetine treatment strengthens an anti-correlated relationship between functional brain networks in medication-naïve adults with attention-deficit hyperactivity disorder: A randomized double-blind placebo-controlled clinical trial. Int J Neuropsychopharmacol. 2015;19:pyv094. doi: 10.1093/ijnp/pyv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 45.Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215:23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaijale NN, Snyder K, Arner J, Curtis AL, Valentino RJ. Repeated social stress increases reward salience and impairs encoding of prediction by rat locus coeruleus neurons. Neuropsychopharmacology. 2015;40:513–523. doi: 10.1038/npp.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71:670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee D, Lee J, Lee JE, Jung YC. Altered functional connectivity in default mode network in Internet gaming disorder: Influence of childhood ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:135–141. doi: 10.1016/j.pnpbp.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 49.van de Ven V, Wingen M, Kuypers KP, Ramaekers JG, Formisano E. Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS One. 2013;8:e68355. doi: 10.1371/journal.pone.0068355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Psychopharmacology (Berl) 2006;185:339–347. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- 51.Wingen M, Kuypers KP, van de Ven V, Formisano E, Ramaekers JG. Sustained attention and serotonin: a pharmaco-fMRI study. Hum Psychopharmacol. 2008;23:221–230. doi: 10.1002/hup.923. [DOI] [PubMed] [Google Scholar]
- 52.Mann JJ, Malone KM, Diehl DJ, Perel J, Nichols TE, Mintun MA. Positron emission tomographic imaging of serotonin activation effects on prefrontal cortex in healthy volunteers. J Cereb Blood Flow Metab. 1996;16:418–426. doi: 10.1097/00004647-199605000-00008. [DOI] [PubMed] [Google Scholar]