Abstract
The discovery of endocannabinoid’s role within the central nervous system and its potential therapeutic benefits have brought forth rising interest in the use of cannabis for medical purposes. The present review aimed to synthesize and evaluate the available evidences on the efficacy of cannabis and its derivatives for psychiatric, neurodegenerative and movement disorders. A systematic search of randomized controlled trials of cannabis and its derivatives were conducted via databases (PubMed, Embase and the Cochrane Central Register of Controlled Trials). A total of 24 reports that evaluated the use of medical cannabis for Alzheimer’s disease, anorexia nervosa, anxiety, dementia, dystonia, Huntington’s disease, Parkinson’s disease, post-traumatic stress disorder (PTSD), psychosis and Tourette syndrome were included in this review. Trial quality was assessed with the Cochrane risk of bias tool. There is a lack of evidence on the therapeutic effects of cannabinoids for amyotrophic lateral sclerosis and dystonia. Although trials with positive findings were identified for anorexia nervosa, anxiety, PTSD, psychotic symptoms, agitation in Alzheimer’s disease and dementia, Huntington’s disease, and Tourette syndrome, and dyskinesia in Parkinson’s disease, definitive conclusion on its efficacy could not be drawn. Evaluation of these low-quality trials, as rated on the Cochrane risk of bias tools, was challenged by methodological issues such as inadequate description of allocation concealment, blinding and underpowered sample size. More adequately powered controlled trials that examine the long and short term efficacy, safety and tolerability of cannabis for medical use, and the mechanisms underpinning the therapeutic potential are warranted.
Keywords: Cannabis, Cannabinoids, Randomized controlled trial, Mental disorders, Movement disorders, Neurodegenerative diseases
INTRODUCTION
Cannabis (marijuana) has long been used for medical and recreational purposes. The Cannabis sativa and Cannabis indica are two common species used for consumption. Between the two species, C. sativa has comparatively higher delta-9-tetrahydrocannabinol (THC) concentration while C. indica has comparatively higher cannabidiol concentration. Cannabinoids can be classified into three subtypes, endocannabinoids (naturally present in human body), phytocannabinoids (present in cannabis plant) and synthetic cannabinoids (produced chemically). Presently, over 60 different types of pharmacologically active cannabinoids have been identified and isolated from the cannabis plant.1) These include the exogenous cannabinoids such as the psychoactive THC and non-psychoactive cannabidiol, as well as the endogenous cannabinoids such as anandamide, which affects most systems in the human body, especially the central nervous system. The cannabinoid binds to two types of G protein-coupled receptors: CB1, which are most abundant in the brain, and CB2, which are expressed on cells in the immune system where inflammation is modulated.1) Hence, cannabinoids are involved in psychomotor coordination, memory, mood, and pain.2) Given the expression of these receptors in the human body, and the interactions between cannabinoids with neurotransmitters and neuromodulators, such as dopamine, glutamate, serotonin, gamma-amino-butyric acid (GABA), it has been thought that cannabis may potentially confer some degree of medical benefit.
Medical cannabis refers to the use of cannabis and its derivatives to treat disease and relieve symptoms.3) Common commercially available cannabinoids for medical use are presented in Table 1.4–6) Testing of other synthetic cannabinoid compounds such as Epidiolex (GW Pharmaceuticals, Cambridge, UK), Namisol (Echo Pharmaceuticals, Weesp, the Netherlands) and Cannador (Society for Clinical Research, Berlin, Germany) are currently underway. These cannabinoid formulations of varying THC or cannabidiol concentration and/or ratio have been widely studied for a variety of illnesses, most notably somatic conditions like pain and spasticity.3) More recently, there has been a growing interest in the neuroprotective potential of cannabinoids for neurological conditions, and the antipsychotic properties of cannabidiol. Preclinical evidences suggest that cannabinoids may attenuate neurodegeneration by reducing excitotoxicity and oxidative damage via CB1 and CB2 receptors and receptor-independent mechanisms.7,8) In the case of cannabidiol, there are indications that cannabidiol modulates the endocannabinoids system by enhancing anandamine levels, thereby reducing psychotic symptoms.9) Although reviews of preclinical and clinical studies have been conducted on movement disorders8) and psychosis,10,11) the aim of the present review is to provide a more in-depth evaluation of the efficacy of medical cannabinoids by appraising the quality of evidences from clinical studies across a broader range of neurodegenerative disorders and psychiatric conditions.
Table 1.
Summary of cannabinoids
| Generic name | Trade name | Administration method | Formulation | Dosage | Pharmacokinetics |
|---|---|---|---|---|---|
| Dronabinol | Marinol | Oral capsule | Synthetic THC | 2.5 mg, 5 mg, 10 mg | tmax=2–4 hr. Completely absorbed (90–95%) after a single dose Alpha (plasma) half-life: 4 hr Beta (tissue) half-life: 25–36 hr* |
| Nabilone | Cesamet | Oral capsule | Synthetic structural analogue of THC Methylgroup at C9 and pentyl side chain in THC substituted with a ketone group and a dimethyl heptyl side chain respectively. |
1 mg | tmax=2 hr Alpha (plasma) half-life: 2 hr Beta (tissue) half-life: 35 hr* |
| Nabiximols | Sativex | Oromucosal spray | Whole plant cannabis extract | 2.7 mg THC and 2.5 mg CBD, per spray (100 μl) | tmax=98–253 min Variable plasma half-life of 85–130 min Clearance within 12–24 hr after dose* |
| CBD | None | Oral capsule | Cannabis plant extract | Variable | No available information in humans4) |
| THC | Namisol | Oral capsule | Cannabis plant extract | Variable | tmax=1–2 hr Half-life: 72–80 min5,6) |
THC, tetrahydrocannabinol; CBD, cannabidiol.
Marinol (Abbott Products, 2010), Cesamet (Valeant Canada, 2009), and Sativex (GW Pharmaceutical, 2010).
METHODS
Types of Studies
Randomized controlled trials that compared and examined the pharmacological intervention of cannabis (in any preparation form, and any route of administration) with placebo or other active treatments were included. Other quantitative study designs such as cohort studies, retrospective chart review studies, and case studies were excluded. Opinion and discussion papers were also excluded. This review only considered studies on human participants that were published in English-language.
Types of Participants
People of any age and sex with any of the following conditions, and/or clinically diagnosed with movement disorders (e.g., dystonia, Huntington’s disease, Parkinson’s disease, Tourette syndrome), neurological conditions (e.g., Alzheimer’s disease, dementia, amyotrophic lateral sclerosis [ALS]) and psychiatric condition (e.g., psychosis, schizophrenia, anxiety).
Types of Interventions
Any form of cannabis for medical use irrespective of the route of administration, duration of intervention or dosage: Smoked cannabis, natural or synthetic cannabinoid including, THC, cannabinol (CBN), cannabidiol, or combinations of abovementioned agents. The comparators included placebo, usual care, other types of active treatments, or derivatives of cannabis.
Search Strategy
An electronic search of human studies published in English-language was conducted in PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from its inception to present (April 2017), using the following keywords: “randomized controlled trial (RCT), cannabinoids, cannabis, tetrahydrocannabinol, THC, cannabidiol, movement disorder, neurodegenerative, psychiatric, dystonia, Huntington’s disease, Parkinson’s disease, Tourette syndrome, Alzheimer’s disease, dementia, ALS, psychosis, schizophrenia, anxiety”. The reference lists of retrieved papers were also reviewed for additional papers. The full texts retrieved were assessed for relevance based on the objectives and inclusion criteria of this review. Studies in which full text were unavailable were excluded.
Data Extraction and Quality Assessment
The data extracted from each report included the study type, sample characteristics, type and dosage of intervention, primary outcome measures, side effect and adverse events. Studies were evaluated for methodological quality using the Cochrane risk of bias tool,12) on sequence generation, allocation concealment, blinding, incomplete data and selective outcome reporting. The ratings were high, low or unclear risk of bias (Table 213–36)). The assessment of methodological quality was performed by two independent raters. Discrepancies were resolved by mutual discussion.
Table 2.
Cochrane risk of bias tool ratings of included studies
| Study | Cochrane risk of bias tool | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Random sequence generation | Allocation concealment | Blinding of participant/personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Overall | |
| Psychiatric condition | |||||||
| Anorexia Nervosa | |||||||
| Gross et al. (1983)13) | ? | ? | ? | ? | ? | − | − |
| Andries et al. (2014)14) | + | + | + | + | + | + | + |
| Anxiety | |||||||
| Fabre et al. (1981)15) | ? | − | − | − | − | ? | − |
| Glass et al. (1981)16) | ? | ? | − | − | ? | ? | − |
| Zuardi et al. (1982)17) | ? | ? | ? | ? | + | + | ? |
| Bergamaschi et al. (2011)18) | − | − | + | ? | + | + | − |
| Crippa et al. (2011)19) | ? | ? | ? | ? | + | + | ? |
| Post-traumatic stress disorder | |||||||
| Jetly et al. (2015)20) | ? | ? | + | + | + | + | ? |
| Psychotic symptoms | |||||||
| Leweke et al. (2012)21) | + | ? | ? | ? | + | − | − |
| Neurodegenerative disorders | |||||||
| Alzheimer’s disease | |||||||
| Volicer et al. (1997)22) | ? | ? | ? | ? | ? | + | ? |
| Dementia | |||||||
| Walther et al. (2011)23) | ? | ? | ? | ? | + | ? | ? |
| van den Elsen et al. (2015)24) | + | ? | + | + | ? | + | ? |
| van den Elsen et al. (2015)25) | + | ? | + | + | ? | + | ? |
| Amyotrophic lateral sclerosis | |||||||
| Weber et al. (2010)26) | + | ? | + | + | + | + | ? |
| Movement disorders | |||||||
| Dystonia | |||||||
| Fox et al. (2002)27) | + | + | ? | ? | ? | ? | ? |
| Zadikoff et al. (2011)28) | + | ? | ? | ? | − | + | − |
| Huntington’s disease | |||||||
| Consroe et al. (1991)29) | ? | ? | + | + | ? | + | ? |
| Curtis et al. (2009)30) | ? | + | ? | ? | + | + | ? |
| López–Sendón Moreno et al. (2016)31) | + | ? | + | + | + | + | ? |
| Parkinson’s disease | |||||||
| Sieradzan et al. (2001)32) | ? | ? | ? | ? | ? | + | ? |
| Carroll et al. (2004)33) | + | ? | + | + | + | + | ? |
| Chagas et al. (2014)34) | ? | ? | + | + | + | + | ? |
| Tourette syndrome | |||||||
| Müller-Vahl et al. (2002)35) | ? | ? | + | + | + | + | ? |
| Müller-Vahl et al. (2003)36) | ? | ? | + | + | − | + | − |
+, low risk of bias; −, high risk of bias; ?, unclear risk of bias.
RESULTS
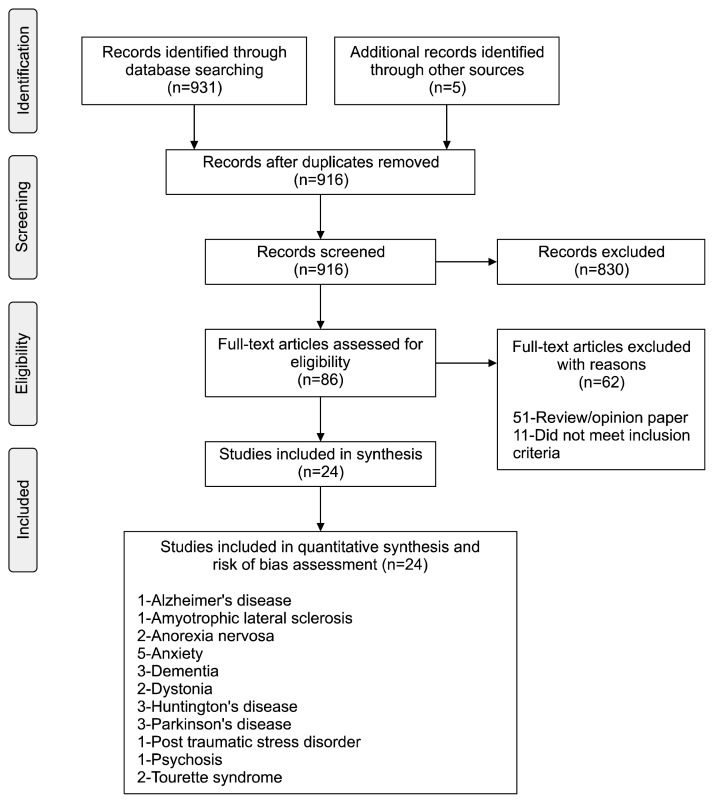
The search yielded 931 records (hits), of which 916 records remained after removing duplicates. Eighty-six records were then considered as potentially relevant after evaluation of title and abstract. The reference lists of these records were also reviewed. The full texts of these records were retrieved and reviewed based on the inclusion criteria and objectives of this review. A total of 62 records were excluded and 24 records were included in this review (Fig. 1).37) Of the 24 reports (480 participants), 18 were crossover trials, 6 were parallel trials. All of the studies were conducted in Western societies.
Fig. 1.
Flow diagram of study review process – PRISMA flow chart.37)
Psychiatric Disorders
Anorexia nervosa
Two studies (36 participants), rated as having an low and high risk of bias, evaluated cannabinoids for the treatment of anorexia nervosa.13,14) In an early crossover trial involving 11 females with anorexia nervosa, titrated THC 7.5 mg (2.5 mg, three times a day) to a maximum of 30 mg (10 mg, three times a day) showed similar weight gain to titrated diazepam 3.0 mg (1 mg, three times a day) to 15.0 mg (5 mg, three times a day). Three patients in the THC treated group were withdrawn due to severe dysphoric reactions. More recently, in two 4-week treatments separated by a 4-week washout period, dronabinol (2.5 mg, twice a day) produced significant weight gain of 0.73 kg (p< 0.01), compared to placebo.14)
Anxiety
The anti-anxiety efficacy of cannabinoids was assessed in 5 studies15–19) involving a total of 38 patients and 44 healthy volunteers (Table 3). Three studies were rated as high risk of bias and 2 as unclear risk of bias. Two early studies indicated equivocal anti-anxiety effects of nabilone.15,16) Specifically, in a double-blind study involving 20 patients, compared to placebo, 1 mg nabilone administered twice daily for 28 days significantly improved anxiety measured by the Hamiliton Rating Scale for Anxiety.15) However, this was not observed in another study involving 8 symptomatic volunteers.16) In another crossover trial involving 8 healthy volunteers with a history of cannabis use, cannabidiol attenuated anxiety induced by THC.17) More recently, in a parallel study, 24 generalized social anxiety disorder patients were randomized to receive either a single dose of 600 mg cannabidiol or placebo, and were also subjected to a simulated public speaking test.18) Pre-treatment of cannabidiol significantly reduced anxiety measured by the visual analogue mood scale. In a another crossover trial involving 10 male patients with generalized social anxiety disorder, a single dose of 400 mg cannabidiol was associated with a significant decrease in subjective anxiety measured by the visual analogue mood scale (p<0.001).19)
Table 3.
Clinical trials of cannabis and its derivatives for psychiatric conditions
| Study | Design/duration | Condition | Sample characteristics* | Intervention (No. of patients) | Outcome | Side effects/adverse events | Results | Cochrane risk of bias |
|---|---|---|---|---|---|---|---|---|
| Anorexia nervosa | ||||||||
| Gross et al. (1983)13) | Double blind Crossover | Anorexia nervosa |
|
|
Primary:
|
3 withdrawn due to severe dysphoria |
|
High |
| Andries et al. (2014)14) | Double blind Crossover | Anorexia nervosa |
|
|
Primary:
|
No severe adverse event |
|
Low |
| Anxiety | ||||||||
| Fabre et al. (1981)15) | Double blind Parallel (28 days) | Anxiety |
|
|
|
Dry mouth Dry eyes Drowsiness |
|
High |
| Glass et al. (1981)16) | Single blind Crossover | Generalized anxiety disorder |
|
|
|
Light-headedness Headache Nausea |
|
High |
| Zuardi et al. (1982)17) | Double blind Crossover | Anxiety induced by THC in healthy volunteers |
|
|
|
Sleepiness |
|
Unclear |
| Bergamaschi et al. (2011)18) | Double blind Parallel | Anxiety induced by simulated public speaking in social phobia patients |
|
|
|
No information |
|
High |
| Crippa et al. (2011)19) | Double blind Crossover | Generalized social anxiety disorder |
|
|
|
No information |
|
Unclear |
| Post–traumatic stress disorder (PTSD) | ||||||||
| Jetly et al. (2015)20) | Double blind Crossover | PTSD associated nightmares |
|
|
|
Dry mouth Headache |
Significant improvement in:
|
Unclear |
| Psychotic symptoms | ||||||||
| Leweke et al. (2012)21) | Double blind Parallel | Schizophrenia patients |
|
|
|
No information |
|
High |
THC, tetrahydrocannabinol; EDI-2, Eating Disorder Inventory-2; POMS, Profile of Mood States; CBD, cannabidiol; STAI, State-Trait Anxiety Inventory; ARCI-Ma, Addiction Research Center Inventory for marihuana effects; VAMS, visual analogue mood scale; SSPS-N, Negative Self-statement scale; SPECT, single-photon emission computed tomography; ECD, ethylene cysteine dimer; CAPS, Clinicians Administered PTSD scale; CGI-C, Clinical Global Impression of change; WBQ, General Well-Being Questionnaire; PANSS, Positive and Negative Syndrome Scale; BPRS, Brief Psychiatric Rating Scale.
•Total (completed)/•male:female/•age.
Post-traumatic stress disorder (PTSD)
In a first randomized controlled crossover trial on PTSD, 10 males with PTSD associated nightmares were administered with titrated 0.5 to 3.0 mg nabilone or placebo, in two 7-week treatment periods, separated by a 2-week washout period.20) Compared to placebo, nabilone significantly (p=0.03) reduced nightmares as measured by the Clinicians-administered PSTD scale. Furthermore, the Clinical Global Impression of Change (CGI-C) indicated a greater global improvement in nabilone (1.9±1.1, i.e. much improved) than placebo group (3.2±1.2, i.e. minimally improved). This study was rated as having an unclear risk of bias.
Psychotic symptoms
To date, only one published trial investigated the antipsychotic properties of cannabidiol in patients with schizophrenia.21) This study was rated as high risk of bias. In this 4-week parallel, active-controlled trial, 42 patients with schizophrenia were randomized to receive either cannabidiol or amisulpride (up titration of 200 mg per day each, to a daily dose of 200 mg four times daily).21) While significant clinical improvements were observed in both treatments as indexed by the Brief Psychiatric Rating Scale and the Positive and Negative Syndrome Scale, no statistical significant difference was reported between groups. However, cannabidiol treatment displayed a superior side-effect profile, compared to amisulpride treatment. Specifically, cannabidiol was associated with significantly smaller weight gain, lower prolactin levels and lesser extrapyramidal symptoms.
Neurodegenerative Disorders
Alzheimer’s disease
One trial on Alzheimer’s disease, rated as unclear risk of bias, examined the use of dronabinol for managing Alzheimer’s disease (Table 4).22) In a 6-week crossover trial, 2.5 mg dronabinol appeared to reduce disturbed behaviors in 12 patients, as measured by the Cohen-Mansfield Agitation Inventory (p=0.05).22)
Table 4.
Clinical trials of cannabis and its derivatives for neurodegenerative conditions
| Study | Design/duration | Condition | Sample characteristics | Intervention (No. of patients) | Outcome | Side effects/adverse events | Results | Cochrane risk of bias |
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease | ||||||||
| Volicer et al. (1997)22) | Double-blind Crossover | Disturbed behavior in Alzheimer’s disease |
|
|
|
Common side effects:
|
|
Unclear |
| Dementia | ||||||||
| Walther et al. (2011)23) | Crossover | Nighttime agitation in Alzheimer’s disease |
|
|
|
No adverse event |
|
Unclear |
| van den Elsen et al. (2015)24) | Double-blind Crossover | Dementia |
|
|
|
Common side effects:
|
|
Unclear |
| van den Elsen et al. (2015)25) | Double-blind Crossover | Dementia |
|
|
|
Lack of information on common adverse event |
|
Unclear |
| Amyotrophic lateral sclerosis (ALS) | ||||||||
| Weber et al. (2010)26) | Double-blind Crossover | ALS |
|
|
Primary:
|
2 serious adverse events |
|
Unclear |
CMAI, Cohen-Mansfield Agitation Inventory; NPI, Neuropsychiatric Inventory; THC, tetrahydrocannabinol; QoL-AD, Quality of Life in Alzheimer’s Disease scale; CCGIC, Caregiver Clinical Global Impression of Change; ZBI, Zarit Burden Interview; VAS, visual analogue scale; ALSFRS-R, ALS functional rating scale revised; ALSAQ-40, ALS assessment questionnaire; SDQ24, Sleep Disorder Questionnaire.
• Total (completed)/• male:female/• age.
Dementia
Three trials on dementia (78 participants), rated as having an unclear risk of bias, showed equivocal results. In a 4-week trial, 2.5 mg dronabinol reduced night-time agitation and strengthened circadian rhythms in the 2 patient enrolled in the study.23) However, two recent trials on showed that THC capsules (0.75–1.5 mg) did not improve neuropsychiatric symptoms in patients with dementia.24,25)
Amyotrophic lateral sclerosis
The only RCT was conducted in 27 patients with ALS.26) In this crossover trial, patients were randomized to receive 2 weeks of 5 mg THC twice daily or placebo, separated by a 2-week washout period. There is a lack of treatment effect on cramp intensity and number of cramps. This study was rated as having an unclear risk of bias.
Movement Disorders
Dystonia
Two trials27,28) (24 participants) indicated lack of evidence on the use of cannabinoid for dystonia (Table 5). The studies were rated as having an unclear risk of bias and high risk of bias. In a crossover trial, 15 patients with primary dystonia received a single dose of 0.03 mg/kg nabilone or placebo.27) Although four patients reported a subjective improvement in dystonia severity, there was no significant difference between groups on the primary endpoint at 60, 120 or 180 minutes post-treatment, as indexed by the Burke-Fahn-Marsden dystonia scale. In another 8-week crossover trial, 9 female patients with cervical dystonia were randomized to receive titrated 2.5 mg dronabinol, up to 3 tabs twice a daily (15 mg/day) or placebo.28) There was no significant treatment effect of dronabinol on cervical dystonia as indexed by the Toronto Western Hospital Spasmodic Torticollis Rating Scale, or any of the secondary measures.
Table 5.
Clinical trials of cannabis and its derivatives for movement disorders
| Study | Design/duration | Condition | Sample characteristics | Intervention (No. of patients) | Outcome | Side effects/adverse events | Results | Cochrane risk of bias |
|---|---|---|---|---|---|---|---|---|
| Dystonia | ||||||||
| Fox et al. (2002)27) | Double-blind Crossover | Primary dystonia |
|
|
|
2 patients withdrawn due to postural hypotension and marked sedation |
|
Unclear |
| Zadikoff et al. (2011)28) | Double-blind Crossover | Cervical dystonia |
|
|
|
|
|
High |
| Huntington’s disease | ||||||||
| Consroe et al. (1991)29) | Double blind Crossover | Chorea severity in Huntington’s disease |
|
|
|
No information |
|
Unclear |
| Curtis et al. (2009)30) | Double-blind Crossover | Huntington’s disease |
|
|
|
Common side effects:
|
|
Unclear |
| López-Sendón Moreno et al. (2016)31) | Double-blind Crossover | Huntington’s disease |
|
|
|
Common side effects:
|
|
Unclear |
| Parkinson’s disease | ||||||||
| Sieradzan et al. (2001)32) | Double-blind Crossover | Dyskinesia in Parkinson’s disease |
|
|
|
Common side effects:
|
|
Unclear |
| Carroll et al. (2004)33) | Double-blind Crossover (4 weeks) | Dyskinesia in Parkinson’s disease |
|
|
|
|
|
Unclear |
| Chagas et al. (2014)34) | Double-blind Parallel (6 weeks) | Parkinson’s disease |
|
|
|
No reported side effects |
|
Unclear |
| Tourette syndrome | ||||||||
| Müller-Vahl et al. (2002)35) | Double-blind Crossover | Tic in Tourette syndrome |
|
|
|
Common side effects:
|
|
Unclear |
| Müller-Vahl et al. (2003)36) | Double-blind Parallel | Tourette syndrome |
|
|
|
Common side effects:
|
|
High |
TWSTRS, Toronto Western Hospital Spasmodic Torticollis Rating Scale; CBD, cannabidiol; UHDRS, Unified Huntington’s Disease Rating Scale; RDS, Rush dyskinesia disability scale; THC, tetrahydrocannabinol; UPDRS, Unified Parkinson’s Disease Rating Scale; PDQ-39, 39-item Parkinson’s disease questionnaire; TSSL, Tourette’s Syndrome Symptoms List; STSSS, Shapiro Tourette syndrome severity scale; YGTSS, Yale Global Tic Severity Scale; TS-CGI, Tourette Syndrome-Clinical Global Impression Scale.
• Total (completed)/• male:female/• age.
Huntington’s disease
The efficacy of cannabinoids for Huntington’s disease was assessed in 3 trials (84 participants).29–31) All studies were rated as having an unclear risk of bias. A 6-week crossover trial evaluated cannabidiol (a total of 10 mg/kg over two doses daily) for chorea in 15 patients with Huntington’s disease. There was no significant difference between placebo and cannabidiol on chorea severity measured by the Marsden and Quinn’s Chorea Severity Scale. Conversely, in another 10-week placebo-controlled crossover trial, nabilone (1 or 2 mg) showed significant treatment effect as measured by the total motor and chorea score on the Unified Huntington’s Disease Rating Scale (UHDRS).30) More recently, no significant treatment effect was reported on the UHDRS, in a sample of 25 patients who receive nabiximols (up to 12 sprays/day) in a crossover trial.31)
Parkinson’s disease
Three studies (49 participants) examined the use of cannabinoids for Parkinson’s disease.32–34) All studies were rated as having an unclear risk of bias. In an early crossover trial involving 9 Parkinson’s disease patients with dyskinesia, 0.03 mg/kg nabilone significantly improved dyskinesia as indexed by the Rush dyskinesia disability scale. Conversely, in a 4-week dose escalation crossover trial, 19 Parkinson’s disease patients with levodopa-induced dyskinesia were administered with titrated cannador up to 0.25 mg/kg THC or placebo.33) Cannador failed to show any significant treatment effect on the primary outcome, the Unified Parkinson’s Disease Rating Scale (UPDRS) dyskinesia items, as well as the secondary measures such as motor symptoms and quality of life (39-item Parkinson’s disease questionnaire, PDQ-39). More recently, in a placebo-controlled trial, 21 patients with Parkinson’s disease were randomized to receive cannabidiol (75 mg/day or 300 mg/day) or placebo for 6 weeks. There was no statistical significant difference between the groups on the UPDRS. However, a significant improvement was reported for PDQ-39, particularly the activities of daily living and stigma subscale for the 300 mg/day cannabidiol group.34)
Tourette syndrome
Only two controlled trials (36 participants) evaluated the efficacy of cannabinoid for Tourette syndrome.35,36) The studies were rated as having a high and unclear risk of bias. In a placebo-controlled crossover trial, 12 patients with Tourette syndrome received a single dose of THC 5 to 10 mg (dose based on body weight). Using the Tourette Syndrome Symptom List, there was a significant treatment effect of THC on the subscale of tics (p=0.015) and obsessive-compulsive behavior (p=0.041). Mild adverse reactions such as dizziness, headache and mood changes were reported in 5 patients. In another 6-week trial from the same research group, 24 patients with Tourette syndrome were given oral THC up to 10 mg per day.36) Similarly, THC significantly reduced tic compared to placebo.
DISCUSSION
There is a lack of evidence on the therapeutic effects of cannabinoids for ALS and dystonia. Although results were inconsistent, there appears to be some low quality evidence of cannabinoids for anorexia nervosa, anxiety, PTSD, psychotic symptoms, agitation in Alzheimer’s disease and dementia, Huntington’s disease, and Tourette syndrome, and dyskinesia in Parkinson’s disease. However, concrete conclusion of its efficacy could not be made due to the unclear risk of bias presented by these trials, as rated on the Cochrane risk of bias tool. Methodological issues such as inadequate description of allocation concealment and blinding, varying cannabinoid formulations and doses, and small sample sizes limit its potential clinical utility.
Consistent with previous case studies38,39) and experimentally-controlled studies,40) the only RCT on cannabidiol and psychosis showed promising results on the antipsychotic potential of cannabidiol.21) Specifically, clinical symptoms negatively correlated with anandamide, an endogenous cannabinoid. It has been hypothesized that cannabidiol enhances anandamide signaling by indirectly blocking enzyme fatty acid amide hydrolase, resulting in an inhibition of anandamide degradation. Although the biological pathways of cannabidiol and anandamide is still unclear, and various potential mechanisms of action have been proposed,10) the protective role of anandamine for psychotic symptoms could potentially be a new viable antipsychotic mechanism. Nonetheless, more adequately powered clinical trials evaluating the effect of varying doses, and long term safety and efficacy are needed to supplement current findings.
For trials involving movement and neurodegenerative disorder, the limited number of trials, lack of quantitative data and underpowered samples inhibits reliable conclusion from being made. Nonetheless, the expression of endocannabinoid receptors (CB1 and CB2) in the basal ganglia and the immune systems could indicate the protective role of cannabinoids for movement and neurodegenerative disorder. This warrants future studies, in vivo and animal models, to clarify the biological mechanisms underpinning the modulatory role of cannabinoids.
Cannabinoids appear to be well-tolerated in these trials. The common short-term effects included dry mouth, dizziness, tiredness, and headache. Indeed, reviews that discussed the adverse effect of cannabis administration have reported that cannabis or cannabinoid administration was associated with a greater risk of non-serious adverse events.3,41) This illuminates the need to conduct trials that compare the effects and efficacy of cannabinoids with existing treatment. This would provide a clear cost-benefit evaluation of medical cannabis.
Overall, there are few RCTs that evaluated the efficacy of cannabis for psychiatric, neurodegenerative and movement disorders. While inconsistency in results may be attributed to different outcome measures used, varying doses and formulations, it raises the question on the mechanism underlying the therapeutic benefits of cannabinoids across indications with different pathophysiology (i.e., psychiatric, neurodegenerative and somatic conditions). Clarification of the cellular pathways and mechanisms of cannabinoids for various indications could reveal the cascading effect of cannabinoids and its interactions with pathways associated with these indications.
CONCLUSION
While there are trials that suggest potential benefit of cannabinoids for anorexia nervosa, anxiety, PTSD, psychotic symptoms agitation in Alzheimer’s disease and dementia, Huntington’s disease, and Tourette syndrome, and dyskinesia in Parkinson’s disease, insufficient conclusion could be made due to the low quality of evidence as indexed by the Cochrane risk of bias, and underpowered samples. An improved knowledge of the precise mechanism of cannabinoids at the cellular level could provide insights on the therapeutic benefits of cannabinoids for movement, psychiatric and neurodegenerative disorder. This could facilitate development of cannabinoid formulations and the conduct of clinical trials on these indications.
Acknowledgments
This research is supported by the Singapore Ministry of Health’s National Medical Research Council under the Centre Grant Programme (Grant No.: NMRC/CG/004/2013). Dr. Jimmy Lee is supported by the National Healthcare Group’s Clinician Scientist Career Scheme.
REFERENCES
- 1.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147( Suppl 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 4.Ujváry I, Hanuš L. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 2016;1:90–101. doi: 10.1089/can.2015.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klumpers LE, Beumer TL, van Hasselt JG, Lipplaa A, Karger LB, Kleinloog HD, et al. Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects. Br J Clin Pharmacol. 2012;74:42–53. doi: 10.1111/j.1365-2125.2012.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed AI, van den Elsen GA, Colbers A, Kramers C, Burger DM, van der Marck MA, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl) 2015;232:2587–2595. doi: 10.1007/s00213-015-3889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagredo O, García-Arencibia M, de Lago E, Finetti S, Decio A, Fernández-Ruiz J. Cannabinoids and neuroprotection in basal ganglia disorders. Mol Neurobiol. 2007;36:82–91. doi: 10.1007/s12035-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 8.Kluger B, Triolo P, Jones W, Jankovic J. The therapeutic potential of cannabinoids for movement disorders. Mov Disord. 2015;30:313–327. doi: 10.1002/mds.26142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leweke FM, Mueller JK, Lange B, Rohleder C. Therapeutic potential of Cannabinoids in psychosis. Biol Psychiatry. 2016;79:604–612. doi: 10.1016/j.biopsych.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Rohleder C, Müller JK, Lange B, Leweke FM. Cannabidiol as a potential new type of an antipsychotic. A critical review of the evidence. Front Pharmacol. 2016;7:422. doi: 10.3389/fphar.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015;162:153–161. doi: 10.1016/j.schres.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, et al. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol. 1983;3:165–171. doi: 10.1097/00004714-198306000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014;47:18–23. doi: 10.1002/eat.22173. [DOI] [PubMed] [Google Scholar]
- 15.Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol. 1981;21(8–9 Suppl):377S–382S. doi: 10.1002/j.1552-4604.1981.tb02617.x. [DOI] [PubMed] [Google Scholar]
- 16.Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW. Single-dose study of nabilone in anxious volunteers. J Clin Pharmacol. 1981;21(8–9 Suppl):383S–396S. doi: 10.1002/j.1552-4604.1981.tb02618.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 18.Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 20.Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–588. doi: 10.1016/j.psyneuen.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12:913–919. doi: 10.1002/(SICI)1099-1166(199709)12:9<913::AID-GPS663>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Walther S, Schüpbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol. 2011;31:256–258. doi: 10.1097/JCP.0b013e31820e861c. [DOI] [PubMed] [Google Scholar]
- 24.van den Elsen GA, Ahmed AI, Verkes RJ, Kramers C, Feuth T, Rosenberg PB, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: A randomized controlled trial. Neurology. 2015;84:2338–2346. doi: 10.1212/WNL.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Elsen GA, Ahmed AI, Verkes RJ, Feuth T, van der Marck MA, Olde Rikkert MG. Tetrahydrocannabinol in behavioral disturbances in dementia: A crossover randomized controlled trial. Am J Geriatr Psychiatry. 2015;23:1214–1224. doi: 10.1016/j.jagp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81:1135–1140. doi: 10.1136/jnnp.2009.200642. [DOI] [PubMed] [Google Scholar]
- 27.Fox SH, Kellett M, Moore AP, Crossman AR, Brotchie JM. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord. 2002;17:145–149. doi: 10.1002/mds.1280. [DOI] [PubMed] [Google Scholar]
- 28.Zadikoff C, Wadia PM, Miyasaki J, Chen R, Lang AE, So J, et al. Cannabinoid, CB1 agonists in cervical dystonia: failure in a phase IIa randomized controlled trial. Basal Ganglia. 2011;1:91–95. doi: 10.1016/j.baga.2011.04.002. [DOI] [Google Scholar]
- 29.Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav. 1991;40:701–708. doi: 10.1016/0091-3057(91)90386-G. [DOI] [PubMed] [Google Scholar]
- 30.Curtis A, Mitchell I, Patel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment in Huntington’s disease. Mov Disord. 2009;24:2254–2259. doi: 10.1002/mds.22809. [DOI] [PubMed] [Google Scholar]
- 31.López-Sendón Moreno JL, García Caldentey J, Trigo Cubillo P, Ruiz Romero C, García Ribas G, Alonso Arias MA, et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J Neurol. 2016;263:1390–1400. doi: 10.1007/s00415-016-8145-9. [DOI] [PubMed] [Google Scholar]
- 32.Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology. 2001;57:2108–2111. doi: 10.1212/WNL.57.11.2108. [DOI] [PubMed] [Google Scholar]
- 33.Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63:1245–1250. doi: 10.1212/01.WNL.0000140288.48796.8E. [DOI] [PubMed] [Google Scholar]
- 34.Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–1098. doi: 10.1177/0269881114550355. [DOI] [PubMed] [Google Scholar]
- 35.Müller-Vahl KR, Schneider U, Koblenz A, Jöbges M, Kolbe H, Daldrup T, et al. Treatment of Tourette’s syndrome with Delta 9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry. 2002;35:57–61. doi: 10.1055/s-2002-25028. [DOI] [PubMed] [Google Scholar]
- 36.Müller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, et al. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry. 2003;64:459–465. doi: 10.4088/JCP.v64n0417. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20:683–686. doi: 10.1177/0269881106060967. [DOI] [PubMed] [Google Scholar]
- 39.Zuardi AW, Morais SL, Guimarães FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- 40.Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178:1669–1678. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]