Abstract
Purpose
Sjögren's syndrome is a systemic chronic autoimmune inflammatory disease that primarily targets the salivary and lacrimal glands (LGs). Currently there is no cure; therefore, cell-based regenerative therapy may be a viable option. LG inflammation is facilitated by extracellular ATP and mediated by the Pannexin-1 (Panx1) membrane channel glycoprotein. We propose that suppression of inflammation through manipulation of Panx1 activity can stimulate epithelial cell progenitor (EPCP) engraftment.
Methods
The expression of pannexins in the mouse and human LG was assayed by qRT-PCR and immunostaining. Acute LG inflammation was induced by interleukin-1α (IL1α) injection. Prior to EPCP transplantation, IL1α-injured or chronically inflamed LGs of thrombospondin-1–null mice (TSP-1−/−) were treated with the Panx1-specific blocking peptide (10panx) or the self-deliverable RNAi (sdRNAi). The efficacy of cell engraftment and the area of inflammation were analyzed by microscopy.
Results
Panx1 and Panx2 were detected in the mouse and human LGs. Panx1 and proinflammatory factors were upregulated during acute inflammation at days 1 to 3 after the IL1α injection. The analysis of EPCP engraftment demonstrated a significant and reproducible positive correlation between the 10panx peptide or Panx1 sdRNAi treatment and the number of engrafted cells. Similarly, treatment of the LG of the TSP-1−/− mouse (mouse model of chronic LG inflammation) by either Panx1 or Caspase-4 (also known as Casp11) sdRNAi showed a significant decrease in expression of proinflammatory markers and the lymphocyte infiltration.
Conclusions
Our results suggest that blocking Panx1 and/or Casp4 activities is a beneficial strategy to enhance donor cell engraftment and LG regeneration through the reduction of inflammation.
Keywords: lacrimal gland, Pannexin-1, regeneration, epithelial progenitor cells, injury-induced inflammation
The lacrimal gland (LG) is the primary contributor to the aqueous layer of the tear film in humans. LG dysfunction or degeneration may lead to aqueous tear deficiency or dry eye disease, a condition that currently has no cure. In many ocular tissues, stem/progenitor cell-based therapies have become a highly promising approach to treating diseases previously considered incurable.1–5 Similar to other exocrine glands, including the pancreas, salivary, and mammary glands, the “healthy” adult LG has a high regenerative capacity and is able to repair itself even after substantial damage.6–9 Recently, replacement of an adult mouse LG with an embryonic LG-derived epithelio-mesenchymal reaggregate or epithelial cell progenitors has been demonstrated.9,10 However, the major cause of therapeutic limitations for cell transplantation in humans results from the poor engraftment of the transplanted cells.11–13 The use of stem/progenitor cells can be instrumental in therapeutic LG regeneration14; however, in contrast to a healthy gland, the chronically inflamed “diseased” LG shows a substantial delay in regeneration.15 The reason for this is unclear and may be related to disruption of the LG stem cell niche function due to chronic inflammation. We recently reported that engraftment of epithelial cell progenitors (EPCPs) after transplantation into an acutely injured LG varies depending on the state of inflammation: fewer engrafted EPCPs were observed during inflammation than during LG regeneration.14 We decided to use this acute inflammation model to study whether reduction of inflammation would improve LG progenitor cell engraftment.
An important part of the initiation of the inflammatory process is the formation of the inflammasome.16,17 The inflammasome is a cytoplasmic macromolecular complex containing apoptosis-associated speck-like protein (ASC) and NOD-like receptor protein (NLRP) domains, caspase-1, and/or caspase-4 (also known as caspase-11) convertases in a catalytic core.18,19 The main function of this complex is to process precursors and secrete mature IL1β and IL18.20,21 A large body of experimental evidence identifies pannexin-1 (Panx1) and P2X receptors (Panx1/P2XR) as the essential upstream regulators of the inflammasome required for proteolytic activation of caspase 1 and/or Casp4(11).18,22–24 Pannexins are a family of ubiquitously expressed gap junction-like proteins that form large membrane channels, permeable to small molecules, including ATP, glutamate, NAD, etc.25–27 Panx1 channels were demonstrated to serve as ATP release channels and contribute to purinergic and adenosine signaling.28,29 There is ample evidence for a role of Panx1 in various pathologies,25,26 ischemic and traumatic brain injuries,30,31 postischemic glutamate toxicity,32 and, more recently, in inflammatory damage in the nervous system.24,33–35 At the same time, several recent reports implicate Panx1 activity in regeneration, particularly in progenitor cell maintenance, motility, and differentiation.36–38 The role of Panx1 in the LG is largely unknown.
In this study, we show that Panx1 is expressed in mouse and human LGs. Panx1 expression has been highly upregulated during LG injury/inflammation. The increase in Panx1 expression in experimentally injured LG was associated with activation of inflammasome complex genes. We recently showed poor EPCP engraftment during this stage of acute LG inflammation.14 We hypothesized that blocking the Panx1 channel, known to mediate inflammasome activation,33,39–41 may improve EPCP engraftment into an injured LG. To test this hypothesis, we deactivated the Panx1 signaling pathway using a Panx1 blocking peptide (10panx) or self-deliverable Panx1 RNAi (sdRNAi). The analysis of EPCP engraftment showed a significant and reproducible positive correlation between these treatments and the number of engrafted cells per cross section, with an average increase of engrafted cells of approximately 63% after 10panx treatment and 169% after Panx1 sdRNAi treatment relative to untreated controls. Similarly, treatment of the LGs of thrombospondin-1–null (TSP-1−/−) mice, a recently reported model of chronic LG inflammation,9,42,43 with sdRNAi to either Panx1 or Casp4(11) showed a significant decrease in mRNA level of proinflammatory genes and LG inflammation.
This study provides new insight into the role of Panx1-mediated inflammation in engraftment of LG progenitor cells and suggests that inhibition of Panx1 and/or Casp4(11) could be a beneficial therapeutic strategy to enhance donor cell engraftment in the LG.
Materials and Methods
Animals and Treatments
Because the prevalence for aqueous deficiency dry eye (ADDE) in human population is higher among women than men,44 we used female mice in all our experiments. Pax6-LacZ female mice (3 to 5 weeks old) on a C57BL/6 background45 were used to prepare EPCP cells for transplantation, as described previously.14 Wild-type C57BL/6 females were used as recipient mice. LG inflammation in recipient mice was induced by intraglandular injection of interleukin-1 α (IL1α), as previously described.6,14 Briefly, C57BL/6 female mice (10 to 12 weeks old) were anesthetized, and the exorbital LGs were injected with either saline (vehicle) or IL1α (1 μg; PeproTech, Rocky Hill, NJ, USA) in a total volume of 2 μL. The LGs from noninjected mice were used as an additional control. The LGs were harvested 1, 2, 3, 4, 5, 7, and 21 days after injection, and total RNA was extracted.
TSP-1−/− mice were originally purchased from the Jackson Laboratory (Sacramento, CA, USA; https://www.jax.org) and were bred and maintained on the C57BL/6J background at The Scripps Research Institute (TSRI) vivarium.
Mice were housed under standard conditions of temperature and humidity, with a 12-hour light/dark cycle and free access to food and water. All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were preapproved by TSRI Animal Care and Use Committee.
Immunostaining and Confocal Microscopy
Dissected LGs were fixed with 2% paraformaldehyde in PBS (pH 7.4) for 20 minutes and frozen in 2-methylbutane (isopentane; Sigma-Aldrich, St. Louis, MO, USA) cooled by liquid nitrogen, and 15-μm frozen sections were cut with a Microm HM500 cryostat (MICROM International GmbH, Dreieich, Germany). Sections were blocked with 5% goat serum in Tris-buffered saline containing 0.1% Tween 20 (TBST). The following primary antibodies were used for immunostaining: rabbit polyclonal antibody to Panx1 (Sigma-Aldrich; HPA016930), affinity-purified rabbit polyclonal antibody against the carboxyl terminus of human PANX1,46 affinity-purified rabbit Panx1 antibody CT-395 (Px-34),47 kindly provided by Dale W. Laird (University of Western Ontario, Ontario, Canada), rabbit polyclonal antibody to Panx2 (Aviva Systems Biology Corp., San Diego, CA, USA; Cat# ARP42778_T100), mouse monoclonal α-smooth muscle actin antibody (clone 1A4; cat.# A2547; Sigma-Aldrich). Appropriate secondary antibodies were obtained from Invitrogen (Waltham, MA, USA). Images were taken using a Zeiss LSM 780 laser (San Diego, CA, USA) scanning confocal microscope (LSCM). The isotype-specific immunoglobulins (normal rabbit or mouse IgGs; Sigma-Aldrich) or preimmune serum, as a substitute for the primary antibody, were used for negative controls.
Immunohistochemistry on Human LG Paraffin Sections
Human LGs from three donors were obtained from Advanced Tissue Services (Phoenix, AZ, USA). The LG were removed ≤24 hours after death. Tissues were preserved immediately in RNAlater and shipped at 4°C overnight. All donors were females, and their ages at the time of death were 62, 84, and 90 years. The LGs were embedded in paraffin, and 5-μm sections were prepared. Endogenous peroxidase activity on rehydrated sections was blocked by treating slides with 3% hydrogen peroxide in absolute methanol for 30 minutes. Antigen retrieval was performed for 40 minutes using 0.01 M citrate (pH 6.39) in a humidified heated chamber. Sections were blocked with 5 g/L casein (Sigma Aldrich) in PBS containing 0.5 g/L thimerosal (Sigma-Aldrich; cat# T5125-25G) for 30 minutes, incubated with primary antibodies, and diluted in casein buffer 1:50 overnight at 4°C. Biotinylated goat anti-rabbit IgG antibodies (Vector Labs, Burlingame, CA, USA) were used at a 1:300 dilution. Visualization was achieved using biotin/avidin-peroxidase (Vector Labs) and Nova Red (Vector Labs). Counterstaining was made with Gill's hematoxylin (Fisher Scientific, San Diego, CA, USA; CS400).
LG Cell Dissociation and Fluorescence Activated Cell Sorting
To obtain sufficient cells for flow cytometric analysis and fluorescence activated cell sorting (FACS), we pooled LGs from 6 to 12 mice. The mice were euthanized, and the skin was sterilized with 70% ethanol before surgically exposing the LG. The LG capsule was removed with tweezers, and a cell suspension was prepared as described by Gromova et al.14 To remove red blood cells, 25 mL cold red blood cell lysis buffer (RBCLB: 0.2% wt/vol Tris, pH 7.5, 0.747% wt/vol NH4Cl) was added to each tube of LG cells suspended in growth media. Purified LG cells were collected by centrifugation at 1000g, resuspended in 100 μg/mL DNAse and 5 mM MgCl2 in Hank's salt (HBSS) (DNAse Sigma D-4513; HBSS, Sigma H-6648), and incubated for 15 to 30 minutes at room temperature. The cells were pelleted at 1000g, washed in HBSS, resuspended in staining buffer PBE (1× PBS, 0.5% BSA, and 1 mM EDTA), and counted. For FACS analysis, approximately 0.5 × 106 cells were pelleted at 1200g for 10 minutes at 4°C and resuspended in 100 μL staining buffer containing the appropriate conjugated antibody. A panel of surface markers for isolation of LG progenitor cells was based on existing publications48–54 and tested in our recent study.9
The following antibodies were used: R-phycoerythrin (PE) rat anti-mouse Ly-6A/E (Sca1, #553336; BD Pharmigen, San Jose, CA, USA); FITC rat anti-mouse CD34 (#553733; BD Pharmigen); anti-mouse CD117 (c-Kit) Adenomatous polyposis coli (APC)-eFluor 780 (#47-1171-80; eBiosciences, Waltham, MA, USA); and anti-mouse CD326 (EpCAM) APC (#17-5791-80; eBiosciences). The cells were stained on ice for 1 hour with gentle vortexing every 15 to 20 minutes, pelleted as above, and resuspended in 1 mL cold PBE in FACS tubes. Flow cytometric analysis and FACS were performed at the TSRI Flow Cytometry Core Facility using Digital LSRII and FACS Vantage DiVa instruments. Data analyses were performed using FlowJo software. Cells were sorted as described previously.14 Control samples labeled with isotype control antibodies and with a single primary antibody were used to determine the background noise due to nonspecific antibody binding and to establish proper compensation for optimum separation between signals.
Self-Deliverable RNAi Injections
Modified RNAi compounds (sdRNAi) (Supplementary Fig. S1) silencing the expression of Pannexin 1 (Px23, cat. 40423) and Caspase 4 (Cs12, cat. 30412) were developed in collaboration with Advirna, LLC (Cambridge, MA, USA). Px23 and Cs12 duplexes with the highest efficacy were selected based on in vitro screening using the luciferase reporter assay (Supplementary Methods, Supplementary Fig. S2, and Supplementary Table S1). Prior to the experiments on LG, the Px23 and Cs12 compounds were tested in cell base and in vivo assays (Supplementary Fig. S3). In in vivo experiments female mice were anesthetized, and 2 to 3 μL solution of sdRNAi (diluted with saline to 250 nM) was injected into several lobes of the LG on the right side of the mouse. The left LG was injected with control sdRNAi or vehicle. As additional controls, we used LGs from noninjected mice or mice injected with only control sdRNAi. In addition to intraglandular injection, each sdRNA was premixed with 30 μL pluronic gel 30% Pluronic F-127 gel (Sigma-Aldrich; cat. P2443-250G) to a final concentration of 250 nM and injected under the LG capsule. The pluronic gel solution in PBS was prepared in advance and kept at 4°C. The left LG was injected with vehicle (pluronic gel) and used as a control. On contact with tissues, the pluronic gel solidifies and stays in contact with tissue for 8 to 12 hours.55 Injections were performed once a week for 4 to 5 weeks, and the mice were euthanized 2 weeks after the last injection.
Cell Transplantation Experiments
Donor EPCPs were obtained by cell sorting from uninjured LGs (see above) of 3- to 4-week-old Pax6-LacZ mice, as described previously.14 Cells were pelleted and suspended in minimum essential medium α (α-MEM) medium (Corning, Manassas, VA, USA). Control LGs of 2- to 3-month-old wild-type (WT) recipient mice were injured by a single injection of IL1α,14,56 and EPCP transplantation was performed 1 day later (during the inflammation stage). Experimental LGs were injected with IL1α + specific mimetic peptide inhibitor of Panx1 10panx (100 μm, sequence WRQAAFVDSY)57–59 or Panx1 sdRNAi (diluted with saline to 250 nM), and sorted EPCP cells were transplanted 1 day after the IL1α and inhibitor treatment. As an additional control, we used LGs injected with vehicle (saline or with control sdRNAi). Transplantation was repeated for a total of three independent experiments. Analysis of EPCP engraftment was carried out 40 days after transplantation as we described previously.9 Briefly, engraftment was assessed as the percent of LacZ+ donor-derived cells in each injected LG lobe. Cells were counted in 200-μm2 fields, with approximately 5 to 6 fields per section and 10 to 15 sections per LG. Four LGs were assessed in each of three independent experiments. LacZ+ cells per field were enumerated blindly.
X-Gal Staining
Fixed tissue was rinsed three times in 0.1 M PBS, pH 7.4, for 20 to 30 minutes and stained overnight at room temperature in the dark in a X-gal staining solution. Solution was comprised of 5 mM K3[Fe(CN)6], 5 mM K4[Fe(CN)6], 0.1 mM MgCl2, 0.02% NP40, 0.02% deoxycholate, and 1 mg/mL of X-gal in 0.1 M PBS, pH 7.4. A 50 mM stock solution of X-gal (Sigma #B4252) in N,N–dimethylformamide (DMF) or dimethylsulfoxide (DMSO) was prepared in advance and stored at −80°C.
RNA Extraction and Real-Time RT PCR
RNA was isolated using a RNeasy Mini kit (cat. 74104; SABiosciences, Qiagen, Valencia, CA, USA); cDNA was made using a RT2 First Strand Kit (cat. 330401; SABiosciences, Qiagen). To compare expression of Panx1, 2, and 3 in the LGs at mouse postnatal day 1 (P1) with their expression in the adult LG at P60, we used sets of primers reported previously60 and housekeeping genes β-actin (forward: 5′-GATCATTGCTCCTCCTGAGC-3′, reverse: 5′-CCGGACTCATCGTACTCCTG-3′) and GAPDH (forward: 5′-GAACGGATTTGGCCGTATT-3′, reverse: 5′-TTGCCGTGAGTGGAGTCATA-3′). The qRT-PCR amplification products were examined by melting curve analysis. Expression of the housekeeping genes was used to normalize the level of target gene expression.
In all other experiments, primers to Panx1 (NM_019482, cat. PPM31514B), Panx2 (NM_001002005, cat. PPM59262A), IL1β (Il1b: NM_008361, cat. PPM03109F), Il6 (NM_031168, cat. PPM03015A), Il18 (NM_008360, cat. PPM03112B), Nlrp3 (NM_145827, cat. PPM29506F), CCL2 (NM_011333, cat. PPM03151G), Ccl7 (NM_013654, cat. PPM02955B), Ccl12 (NM_011331, PPM02977E), P2RX7 (NM_011027, cat. PPM04252A), P2RY2 (NM_008773, cat. PPM04914A), Col1a1 (NM_007742, cat. PPM36469A), Casp8 (NM_009812, cat. PPM02923F), Casp1 (NM_009807, cat. PPM02921E), Casp3 (NM_009810, cat. PPM02922F) Casp4 (NM_007609, cat. PPM03075C), and housekeeping genes PrS13 (forward: 5′-TCCCTCCCAGATAGGTGTAATCC-3′ and reverse: 5′-TCCTTTCTGTTCCTCTCAAGGT-3′) and PrL27 (forward: 5′-AAAGCCGTCATCGTGAAGAAC-3′ and reverse: 5′-GCTGTCACTTTCCGGGGATAG-3′), as well as RT2 SYBR Green qPCR Mastermix were purchased from Qiagen. Quantitative RT-PCR was performed on an ABI 7300 system (Life Technologies, Grand Island, NY, USA), and the data were analyzed using online normalization and analysis tools (provided in the public domain, http://sabiosciences.com/pcrarraydataanalysis.php). Three independent experiments were performed.
Statistical Analysis and Data Presentation
Statistical analyses were performed using Prism Software (GraphPad, San Diego, CA, USA). In bar graphs, data are presented as means ± SD of replicates from a representative experiment or of the normalized data from several experiments. In the latter case, mean fold changes were calculated by first determining the ratio of the test conditions over the appropriate control conditions for each individual measurement and then averaging these ratios. The Anderson-Darling normality test was performed prior to further data analyses. The unpaired two-tailed Student's t-test was used to determine significance (P < 0.05) in the differences between data sets.
Results
Panx1 and 2 Are Expressed in the Mouse and Human LG
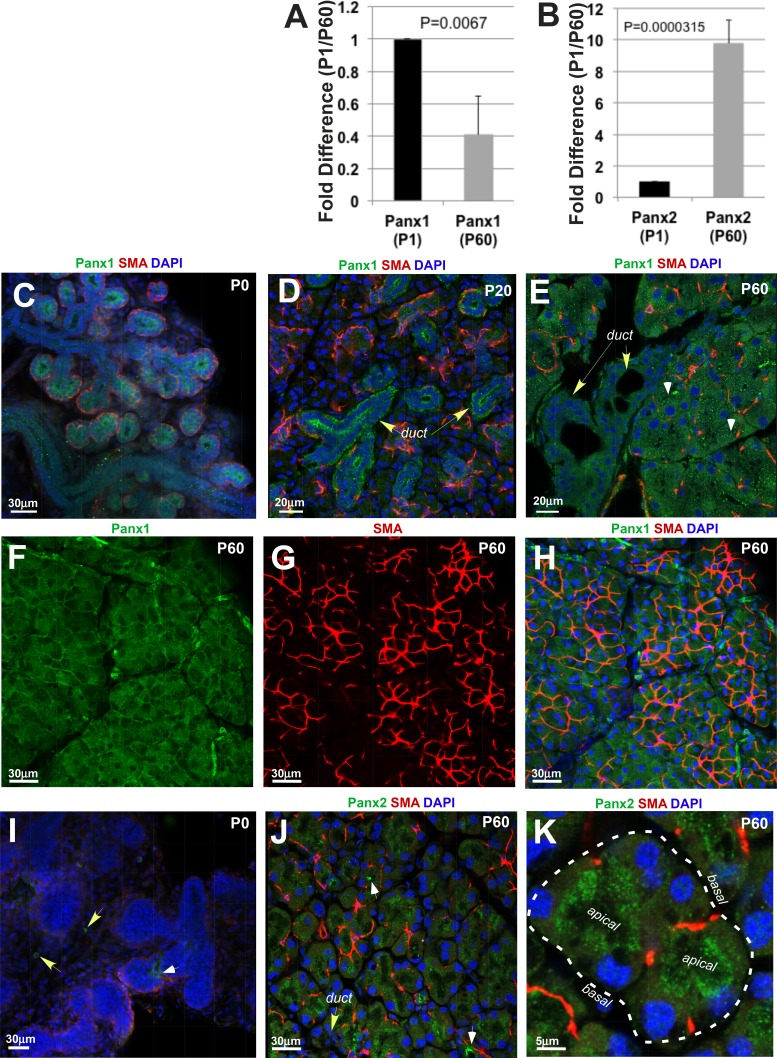
The pannexin family of channel-forming glycoproteins includes three members: Panx1, 2, and 3. The presence and distribution of Panx1, 2, and 3 proteins in the mouse LG were studied by qRT-PCR and immunohistochemistry. Analysis of Panx1, 2, and 3 mRNA expressions in the LGs of newborn pups at P1, when the gland is still undergoing substantial growth/remodeling, was compared with their expression in adult LGs (at P60), when LG morphogenesis is largely completed. Panx1 mRNA expression was found in the LGs at P1 and P60. The mean cycle threshold (Ct) values were 25.7 ± 0.5 (at P1) and 28.4 ± 0.2 (at P60). Expression levels of Panx2 were generally lower than of Panx1, with mean cycle threshold (Ct) values of 30.1 ± 0.8 (at P1) and 27.5 ± 0.8 (at P60). Expression of Panx3 mRNA in the LGs was not detectable at any stage. Moreover, Panx1 expression was 2.5 times higher in the LGs at P1 compared with adult LGs at P60 (Fig. 1A). In contrast, Panx2 expression increased 9.8-fold in mature LGs compared with its expression level at P1 (Fig. 1B). These findings suggest that Panx1 and 2 may have different roles in LG morphogenesis and homeostasis. Panx1 protein was highly expressed in the epithelial component of the LGs during embryonic and early postnatal development (E19 to P0; Fig. 1C). Later in postnatal development (P20), a punctate expression of Panx1 was found in the epithelial ducts and acini (Fig. 1D). Moreover, the highest level of Panx1 protein at P20 was detected in the LG ducts (Fig. 1D, duct, yellow arrows), whereas acinar cells expressed a lower level of the protein (Fig. 1D; acini are surrounded by myoepithelial cells [MECs], red). In adult LGs at P60, Panx1 punctate labeling was found in both ducts and acini at similar levels (Fig. 1E). Sections through the acinar compartment of the LGs showed a higher level of labeling in more apical parts of acinar cells (Fig. 1E, apical parts of acinar cells labeled with white arrowheads). The expression of Panx1 in the MECs of the LGs was lower and less conspicuous (Figs. 1F–1H, Supplementary Fig. S4, and Supplementary Movie S1). In contrast to Panx1, expression of the Panx2 protein was almost absent at early postnatal development at P1 (Fig. 1I), but Panx2 was expressed in mature LGs (Figs. 1J, 1K). The pattern of Panx2 expression was similar to that of Panx1; it was highly expressed in the apical parts of the acinar cells (Figs. 1J, 1K). It was also found in the LG ducts (Fig. 1J, yellow arrow) and blood vessels (Fig. 1J, white arrows).
Figure 1.
Panx1 and 2 expression in mouse LG. (A) Panx1 expression slightly decreases in the LG of adult (postnatal day 60 [P60]) mice compared with the LG obtained from mice at postnatal day 1 (P1). (B) Expression of Panx2 increases in adult LGs compared with LGs at P1. In A and B, histograms represent the normalized expression of Panx1 and 2 to GAPDH assessed by qRT-PCR in three independent experiments. (C–H) Panx1 expression in mouse LGs at P0 (C) during postnatal (D: P20) development and adulthood (E–H). At P and P20, Panx1 (green) is highly expressed in the developing LG ducts (C, D). Panx1 expression in the developing acini is low (D). Myoepithelial cells (red) are localized around acini, nuclei labeled with 4′,6-Diamidine-2-phenylindole (DAPI) (blue). (E, F, H) Panx1 expression in adult LG (P60). (F–H) MECs have low level of Panx1 expression. Low level of Panx1 expression in MEC is confirmed in nonmerged images (F, Panx1 expression; green; and G, SMA [marker of MECs] expression, red). (I) Low level of Panx2 expression in the epithelial components of the LG at P. Panx2 was only detected in blood vessels (white arrow) and some mesenchymal cells (yellow arrows) At P60, Panx2 (J, K) is expressed in the acinar cells. High levels of Panx2 protein is found in blood vessels (J, white arrows) and apical parts of acinar cells (K, apical; basal parts of acinar cells are labeled with puncture line). Myoepithelial cells (red). Nuclei are labeled with DAPI (blue).
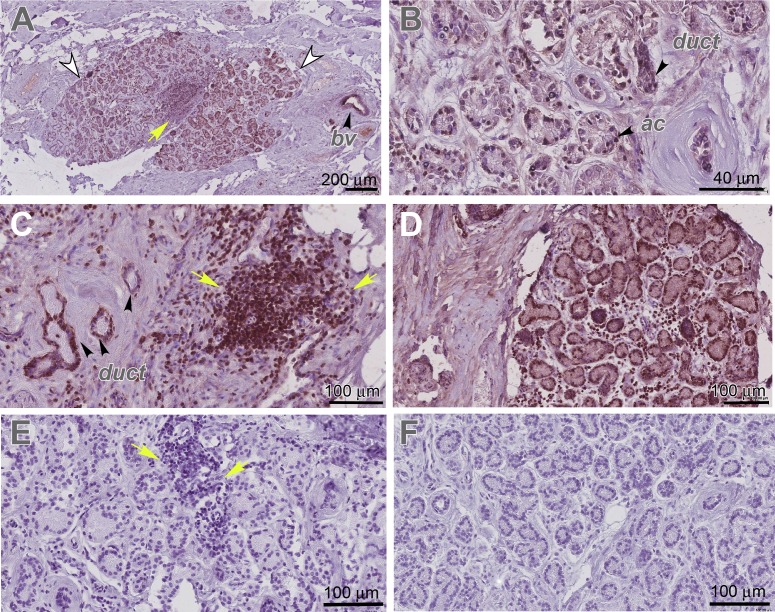
To determine whether pannexins are expressed in human LGs, we performed immunostaining of human LG paraffin sections. All human LGs were obtained from female donors of age 62, 84, and 90. To confirm a specific pattern of Panx1 expression in humans, we used three different antibodies to Panx1 (Materials and Methods). Analysis of pannexin expression in human LGs showed that all three antibodies detected Panx1 in an identical pattern to that seen in the adult mouse LGs (Fig. 1, Supplementary Fig. S5). In human LG sections, Panx1 protein was expressed within the epithelial lobes (Figs. 2A, 2B and Supplementary Figs. S5A, S5B, and S5E–S5G, LG lobes labeled with white arrowheads), whereas the surrounding tissue had low or no Panx1 expression. Similar to the mouse LGs, Panx1 was highly expressed in the epithelial compartments (ducts and acini) of the human LGs (Figs. 2B, 2C and Supplementary Figs. S5B, S5F, S5G, duct, ac). It was also found in the blood vessels (Fig. 2A; Supplementary Figs. S5A, S5E, bv). In the LGs obtained from 84- and 92-year-old donors, we found some periductal mononucleated cell infiltrates (Figs. 2A, 2C, 2E, yellow arrows), suggesting the presence of LG inflammation. In these glands, Panx1 was also detected within the infiltrating mononucleated cells (Figs. 2A, 2C). No staining was found in controls in which the tissue was incubated with preimmune serum or isotype-specific immunoglobulins (Fig. 2E and Supplementary Figs. S5C, S5D, S4H).
Figure 2.
Panx1 and 2 expression in human LG. (A–D) Panx1 (brown) is specifically expressed in the LG (in LG lobes labeled with white arrowheads) ducts (duct), acini (ac), blood vessels (bv), and infiltrating lymphocytes (I; yellow arrows) of the human LG. Panx1 expression was visualized by different antibodies to Panx1: (A, B) rabbit polyclonal antibody to Panx1 (Sigma-Aldrich; HPA016930) and (C) affinity purified rabbit Panx1 antibody CT-395 (Px-34, also see Supplementary Fig. S1). (D) Panx2 (Aviva Systems Biology, Cat# ARP42778_T100) is expressed in the LG. (E, F) Negative controls to Panx1 and 2 antibodies in which primary antibodies were substituted with the isotype-specific immunoglobulins (normal rabbit IgG, Sigma-Aldrich). (A–F) LG was obtained from a 90-year-old female donor.
Panx2 protein expression was detected in the human LGs in a similar pattern to the one described for the mouse LGs (Fig. 2D). Negative control did not have any specific staining (Fig. 2F).
Panx1 and 2 Expression During Injury-Induced LG Inflammation and Regeneration
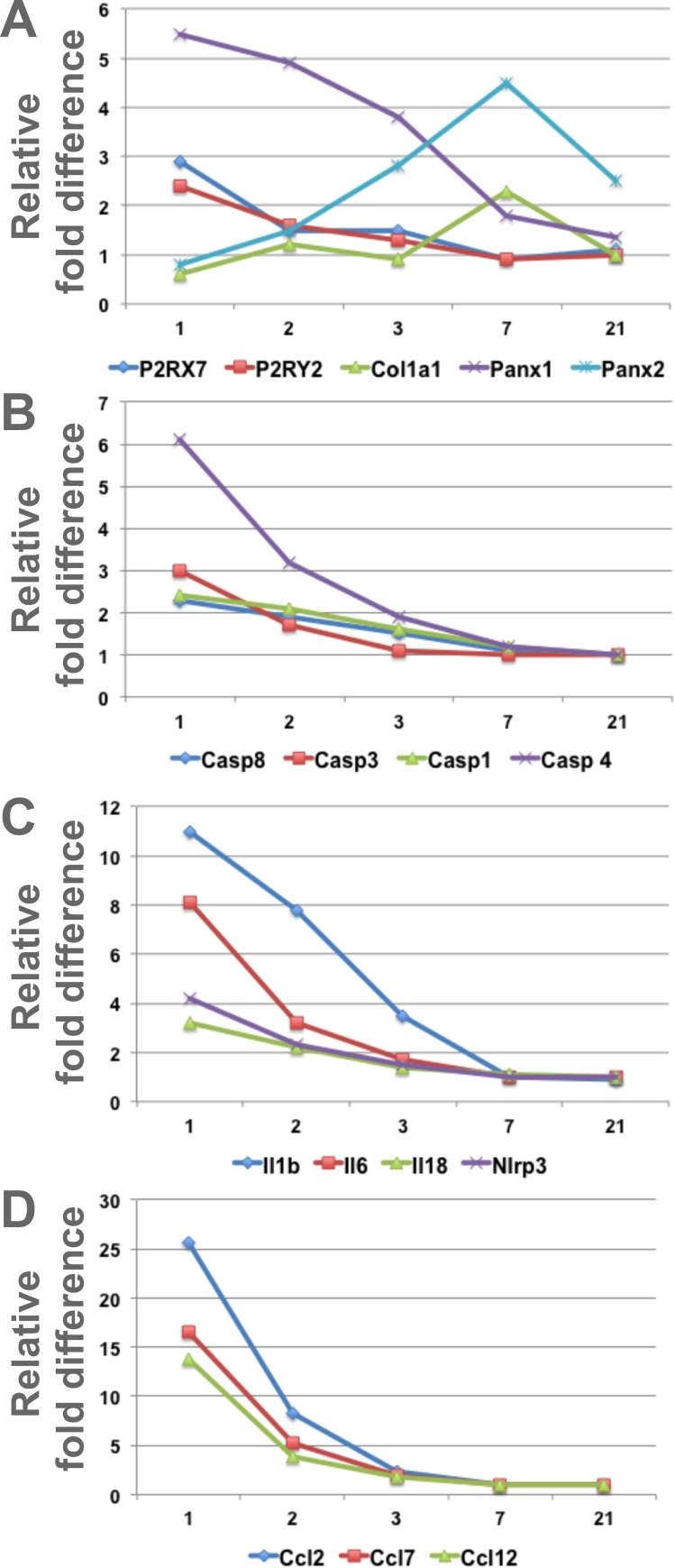
To investigate the role of Panx1 and 2 during LG regeneration, we used an acute LG injury model.6 LG injury was induced by a single injection of IL-1α, which induces extensive inflammation and LG damage, followed by a regenerative phase that restores LG morphology and function between 7 and 21 days after injury.6 Recent studies suggest that pannexins and pannexin-mediated purinergic signaling play important roles in activation of both postinjury inflammatory response and the subsequent process of tissue regeneration.33,61 In particular, Panx1 has been associated with elevated levels of proinflammatory cytokines.62 We reasoned that Panx1 and 2, which are expressed in the epithelial component of the gland, play a role in the processes of inflammation or active cellular remodeling during regeneration. LGs of 2-month-old WT mice were injected with IL1α or saline (vehicle control) and collected at days 1, 2, 3, 7, and 21 after injection for RNA extraction. Levels of the Panx1 and Panx2 and PrL27 (noncoding RNA, poly[A]-bearing) and PrS13 (16S ribosomal RNA gene) housekeeping mRNAs were determined by qRT-PCR (Table; Fig. 3A). We found that Panx1 mRNA expression was increased six- to eightfold on days 1 to 3 after LG injury when inflammation is at its highest peak, whereas Panx2 mRNA expression was increased later between days 3 and 21 after LG injury, reaching a peak on day 7 during the LG differentiation phase (Fig. 3A; Table). Thus, Panx1 may be involved in regulation of inflammation and Panx2 may have a role in LG differentiation and maturation.
Table.
Changes in Gene Expression of Proinflammatory and Differentiation Markers After IL-1α LG Injection
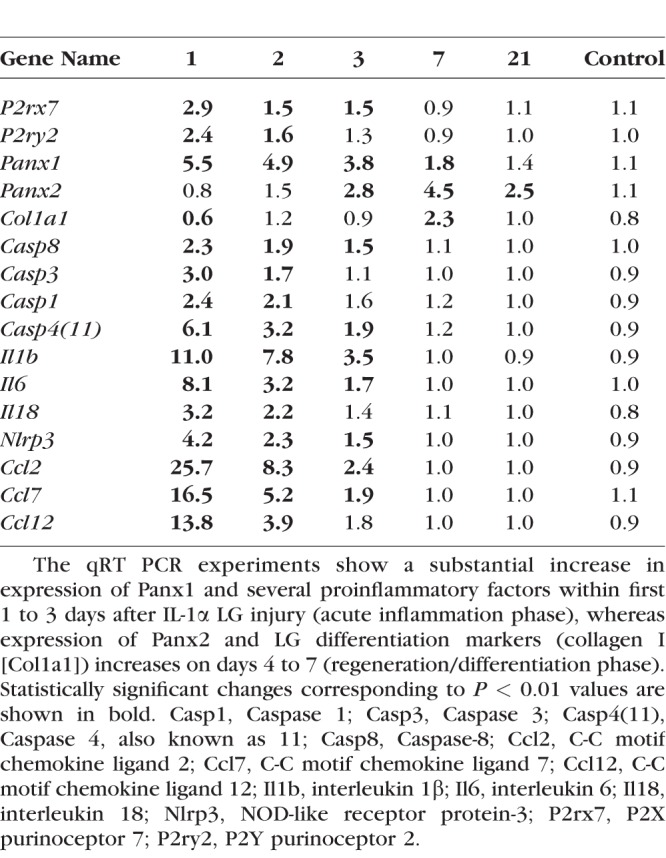
Figure 3.
Expression of Panx1, Panx2, and proinflammatory and differentiation factor mRNAs after LG injury and during LG regeneration. Graphic representation of the Table. LGs were injected with IL-1 or saline (vehicle control) and collected at days 1, 2, 3, 7, and 21 after injection for RNA extraction. Levels of mRNAs were determined by qRT-PCR. (A) Expressions of Panx1 and purinergic receptors P2RX7 and P2RY2 were upregulated during first 1 to 3 days after the LG injury (inflammation stage), whereas expressions of Panx2 and collagens I and XVI (A: Col16a1 and Col1a1) were increased during the regeneration phase, on days 5 to 7 after IL1β injection. Expressions of several inflammasome related factors (B, C: NLRP3, Casp1, Casp4 [11] and Casp8); proinflammatory cytokines (C: IL1b, IL6, IL18), and IL1 family member 9 (C: Il1f9, proinflammatory stimulator of chemokine); and specific proinflammatory chemokines (D: Ccl2, Ccl6, Ccl7, and Ccl12) were significantly increased at days 1 to 3 after LG injury.
Expression of Inflammatory Factors After Acute LG Injury
ATP is a major indicator of stress in many tissues, released from both dying and viable cells when challenged by cytokines63 or mechanical stress.64 Extracellular ATP is a very potent proinflammatory factor, which induces inflammasome activation via the Panx1–P2RX7 complex.18,22–24 To determine whether acute LG injury affects inflammasome-related gene expression, levels of their mRNAs were determined by qRT-PCR. The LGs of female BALB/c mice (three mice per treatment group) were injected with either saline (control) or with IL1α, and the LGs were harvested 1, 2, 3, 4, 5, 7, and 21 days after injection (Table; Fig. 3). Our analysis showed that mRNA expression of P2RX7 and P2RY2 was significantly upregulated during LG inflammation (first 2 to 3 days after the injury), whereas expression of collagen I (Col1a1, fibril-forming collagen), which is implicated in tissue remodeling/regeneration and maintenance of the integrity of extracellular matrix,65–67 was increased during the phase of LG regeneration, days 5 to 7 (Fig. 3A). Expressions of several inflammasome related (NLRP3, Casp1, Casp4[11], and Casp8) factors were significantly upregulated at days 1 to 3 after injury (Table; Figs. 3B, 3C). Expressions of major proinflammatory cytokines such as IL1β, IL6, and IL18 were upregulated substantially during LG inflammation stage. We also found increases in the expression of the interleukin-1 family member 9, which is known to act as a proinflammatory stimulator of chemokine production,68 and the upregulation of specific proinflammatory chemokines Ccl2, Ccl6, Ccl7, and Ccl12 (Table; Figs. 3C, 3D).
Blocking the Panx1 Function Improves Engraftment of EPCPs Into Injured LG
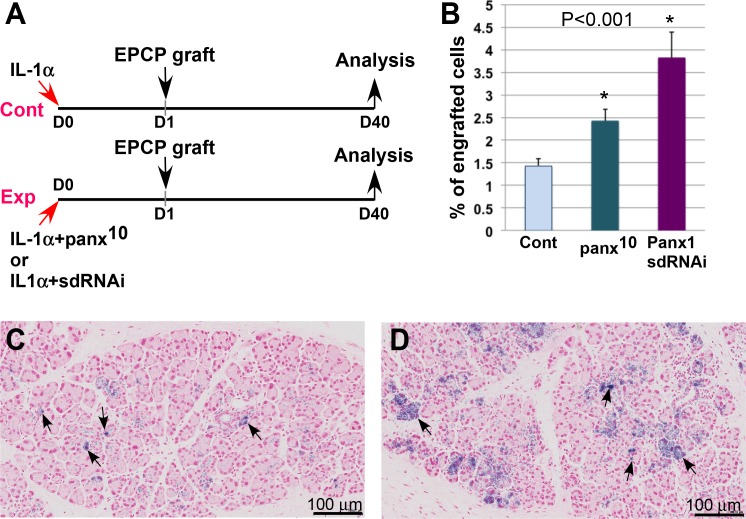
We recently demonstrated that transplantation of EPCPs isolated from WT LGs could mediate the structural and functional recovery of injured LGs and LGs of TSP1−/− mice (a new mouse model of human Sjögren's syndrome).14 Moreover, we reported that efficacy of EPCP transplantation into injured LGs varied depended on how much time passed after the injury by IL1α injection. We demonstrated that cells injected into the gland during the regeneration phase (3 days after injury) engrafted more efficiently than cells injected during the acute inflammation phase (1 day after injury).14 Here we show that the increase in Panx1 expression correlates with the activation of acute inflammatory responses induced by the IL1α injection. Panx1 is also expressed in immune cells such as monocyte and macrophages and mediates IL1β processing and release from macrophages in response to P2X7R activation58 and promotion of inflammation. Thus, blocking Panx1 during LG inflammation may improve cell engraftment efficacy. To test this hypothesis, we used an interfering peptide that targets Panx1 function, 10panx (a selective Panx1 blocker)57–59 or sdRNAi, a novel class of nontoxic stable RNAi compounds capable of efficient cellular uptake in vitro and in vivo without the use of transfection reagents for cell penetration.69,70 The compounds were designed in silico and initially tested in cell culture or in vivo by passive transfection: adding sdRNA directly to the cell culture medium or by injecting sdRNA into tissue (Supplementary Methods, Supplementary Table S1, Supplementary Figs. S3, S4). The LG of a recipient mouse was injected with IL1α alone (control), whereas the gland on the other side was injected with IL1α plus 10panx inhibitor peptide or Panx1 IL1a plus sdRNAi (experimental) (Fig. 4A). One day after injury, EPCPs isolated from Pax6-LacZ mice45,71 were injected into the injured LGs, as we described previously14 (Fig. 4A). Analysis of cell engraftment was carried out on the 40th day after cell transplantation (Fig. 4A). The percentage of engrafted LacZ+ cells was quantified in serial LG sections stained with X-gal and Fast red to visualize transplanted cells and nuclei, respectively. In both cases, EPCPs injected into the LG were found in the ductal and acinar epithelial cell compartments; however, the efficacy of cell engraftment was significantly higher in 10panx peptide or Panx1 sdRNAi treated glands. Approximately 63% more engrafted cells were found after 10panx peptide and 169% more engrafted cells were found after sdRNAi administration (Figs. 4B–4D). Control LGs injected with the vehicle did not show any LacZ+ cells (data not shown).
Figure 4.
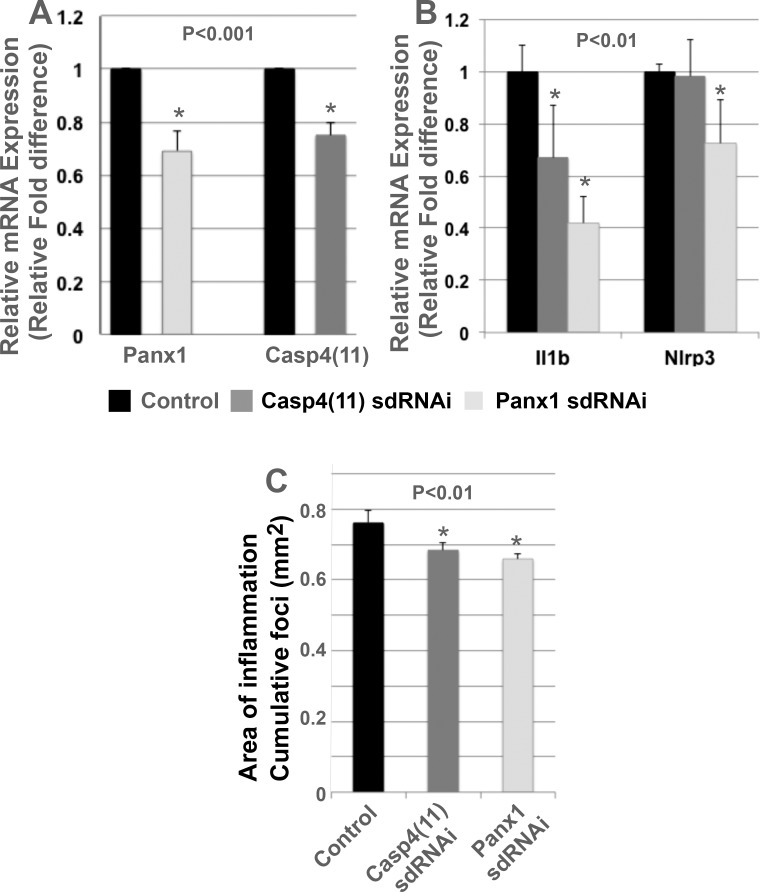
Pharmacologic blockade of Panx1 increases engraftment of transplanted EPCPs into the injured host LG. (A) Experimental paradigm. Recipient LGs were injected with IL1α alone or combined with a mimetic peptide inhibitor of Panx1 10panx (100 mM) or Panx1 sdRNAi and donor cells, sorted from R26-LacZ mouse, and were transplanted 1 day after injury and analyzed 40 days after transplantation. (B) Blocking Panx1 channels by 10panx or Panx1 sdRNAi increased the engraftment of EPCP cells relative to control LGs. Engraftment of transplanted EPCPs into control (C) or sdRNAi-treated LGs (D) (black arrows, LacZ+ engrafted cells. In (B) *P < 0.01.
Blocking Panx1 or Casp4(11) in the LGs of TSP-1−/− Mice Decreases Inflammation
Next, we tested whether silencing of Panx1 or Casp4(11) genes in the LG of the thrombospondin-1-null (TSP-1−/−) mouse, a recently reported mouse model of ADDE, would decrease LG inflammation. Although born with no apparent abnormalities of the LG, TSP-1−/− mice develop a severe inflammation of the LG with a significant loss of LG secretory function around 24 weeks of age. Thus, the TSP1−/− mouse fully mimics the development of chronic autoimmune dry eye disease in humans.42,43
Modified RNAi compounds (sdRNA) for silencing the expression of Panx1 and Casp4(11) were developed. Duplexes with the highest efficacy were selected (Supplementary Fig. S2) and tested in cell base and in in vivo assays (Supplementary Fig. S3) prior to LG application. We used the most efficient Panx1 (Px23) or Casp4(11) (Cs12) sdRNAi compounds for intraglandular injections. As demonstrated previously, inflammation in TSP1−/− LGs starts at approximately 10 to 12 weeks as periductal lymphocytic infiltrates14,43 and could be found in periacinar regions at 3 to 4 months of age.42,43 Thus, injections were started when TSP-1−/− mice were around 4 months old and performed once a week for at least 5 weeks. We injected the right LGs of female TSP-1−/− mice with a Panx1 or Casp4(11) sdRNAi, whereas the LGs on the other side were injected with a control sdRNAi or vehicle. Each sdRNAi was also mixed with a pluronic gel and injected under the LG capsule (a thin film surrounding the gland). Pluronic gel is liquid at low temperatures, 0–4°C, but sets at physiologic temperature, remaining in place for 8 to 12 hours.55,72 The gel has the additional advantage of being a mild surfactant and provides local controlled sdRNAi release for an extended time. As we and others showed previously these features of the pluronic gel appeared to markedly expedite penetration of oligonucleotides into the cells.72,73
Mice were euthanized at the end of the experiment (2 weeks after the last injection), and the LGs were processed for RNA isolation and histology. Both Panx1 and Casp4(11) sdRNAi treatments resulted in significant reduction of Panx1 and Casp4(11) mRNA expression (Fig. 5A). Analysis of IL1β and Nlrp3 (inflammation markers) mRNA expression by qRT-PCR showed that downregulation of Panx1 mRNA levels resulted in a significant decrease of expression of both IL1β and Nlrp3, whereas blocking Casp4(11) reduced only IL1β but not Nlrp3 mRNA level (Fig. 5B).
Figure 5.
Blocking Panx1 or Casp4(11) with sdRNAi in TSP1−/− LGs decreases LG inflammation. Two to 3 μL solution of Panx1 or Casp4(11) sdRNAi (at a final concentration of 250 nM) was injected into the LGs on the right side of 4-month-old TSP-1−/− mice. The left LG was injected with a control sdRNAi or vehicle (saline). Mice were injected once a week for the following 5 weeks and euthanized 2 weeks after the last injection. LGs were processed for qRT-PCR or histology. (A) LG treated with specific sdRNAi (to Panx1 or Casp4[11]) showed significant reduction in Panx1 or Casp4(11) mRNA expression compared with LG treated with control sdRNAi. (B) Decrease of Panx1 mRNA level resulted in a significant reduction of expression of both IL1β and Nlrp3, whereas blocking Casp4(11) significantly reduced only IL1β but not Nlrp3 expression. (C) Measurement of the cumulative area of inflammation (summed area of infiltration foci per field, measured in mm2) showed that Panx1 or Casp4(11) sdRNAi treatments of the LG of TSP1−/− mice significantly decreased the cumulative area of inflammation compared with controls. To quantify statistical difference, a two-tailed Student's t-test was used. Panx1 sdRNAi (P = 1.6 E−6) and Casp4 sdRNAi (P = 4.0E−5).
To further assess the level of inflammation in the LGs after sdRNAi treatment, we measured the cumulative area of inflammation (summed area of infiltration foci per field, measured in μm2) in Panx1-treated, Casp4(11)-treated, and control LGs, as described previously.14 We found that both Panx1 and Casp4(11) sdRNAi-treated LGs had a slight but a statistically significant reduction of cumulative area of inflammation (Fig. 5C).
Combined these data indicates that manipulation of Panx1 signaling may reduce LG inflammation.
Discussion
Endogenous epithelial progenitor cell transplantation is a promising tool for regeneration of the damaged epithelial components of the LG in cases of severe dry eye disease. In our previous work, we showed that EPCP transplantation could improve the structure and function of chronically inflamed LGs of TSP1−/− mice.14 Our experiments also suggested that endogenous stem cell function and engraftment of transplanted cells depends on LG inflammation level.14,74 These data imply that using anti-inflammatory approaches may have important therapeutic implications in ongoing dry eye disease or as a therapy combined with stem/progenitor cell transplantation. The acute LG injury model reported previously15 was useful to study cell engraftment. Although this model has numerous clinical limitations compared with chronic models of LG inflammation for long-term studies, such as concise period of inflammation and fast and robust recovery of LG after acute injury, it also had certain benefits for our study. The inflammation process in this model is very local (observed only within injured LG), its timing is well defined and the mechanism of this acute inflammation is very well studied.6,15,75–77 In addition the role of Panx1 in acute inflammation has been reported.78,79 In current study, we demonstrated that Panx1 is highly expressed in the epithelial components of the mouse and human LGs and that progenitor cell engraftment into injured LGs was significantly improved by the blocking of Panx1 function. These findings suggest that stem/progenitor cells function more efficiently when inflammation is downregulated and the regeneration process is induced.14 Moreover, these findings support a consensus view that Panx1 is an important mediator of inflammation.78,79 Our model suggests that transplantation of progenitor cells at high level of inflammation results in their poor survival (Fig. 6A), whereas blocking Panx1 in the LG decreases the level of inflammation and promotes progenitor cell survival/function within the LG (Fig. 6B). This finding also indicates that persistent LG inflammation sustains dysfunction of the endogenous LG stem cell niche and challenges the potential efficacy of stem cell therapies aimed at mobilizing endogenous and/or transplanted progenitor cells. Although the process of acute inflammation is an important step toward its resolution,33 a delay in resolution may lead to chronic inflammation and progressive cell death. Although our previous study showed that progenitor cell transplantations could temporarily improve recovery and function of the chronically inflamed LG, we observed some variations in stem cell survival/engraftment depending on severity of inflammation; thus, the longer-term studies are necessary to analyze function of transplanted cells and to determine the mechanisms/treatments that would increase long-term disease remission. Our study also shows that “local” blockage of Panx1 or Casp4(11) could decrease expression of proinflammatory factors and to some extent improve LG structure reducing size of infiltration foci during chronic inflammation. Because we still did not observe any statistically significant changes in tear production in treated TSP1−/− mice, future studies need to use general anti-inflammatory treatments or a complete “reset” of the immune system80 to stop it attacking the body.
Figure 6.
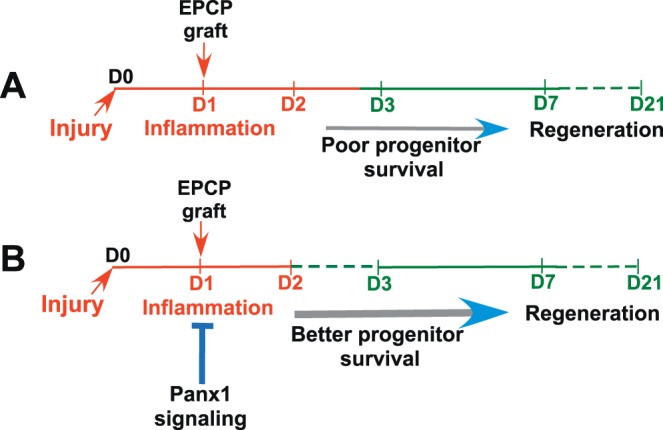
Blocking Panx1 improves donor LG progenitor cell engraftment by decreasing the inflammation and promoting transplanted cell survival. (A) Cell transplantation during a high level of inflammation results in poor transplanted cell survival, whereas (B) blocking Panx1 in the LG decreases the level of inflammation and promotes progenitor cell survival/function within the LG. The diagram represents our understanding of how Panx1 inhibition may control inflammation and therefore progenitor cell engraftment.
Our previous14,74 and current studies support the idea that persistent chronic LG inflammation mediates changes in the stem/progenitor cell survival/function that recovers when inflammation abates.
These findings have implications for therapeutic strategies targeting Panx1 signaling pathways in the LG prior to or at the time of progenitor cell transplantation. The effectiveness of this strategy will depend on successful suppression of inflammation, as well as on the long-term plasticity of the transplanted progenitor cells. Studies of Panx1 function in human cells are needed to develop new therapeutic strategies for human dry eye disease.
Supplementary Material
Acknowledgments
The authors thank Dale W. Laird, University of Western Ontario, Canada, for providing the Panx1 antibody.
Supported by National Institutes of Health, National Eye Institute Grants 1R01EY026202 (to HPM), R01-EY12383 (to DZ and HPM), and 2R01EY012383 (to VS) and Small Business Innovation Research (SBIR) Grant 5R44HG006788 from National Human Genome Research Institute (NHGRI) (to AW).
Disclosure: L.V. Basova, None; X. Tang, None; T. Umazume, None; A. Gromova, None; T. Zyrianova, None; T. Shmushkovich, None; A. Wolfson, None; D. Hawley, None; D. Zoukhri, None; V.I. Shestopalov, None; H.P. Makarenkova, None
References
- 1.Zarbin M. Cell-based therapy for degenerative retinal disease. Trends Mol Med. 2016;22:115–134. doi: 10.1016/j.molmed.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Sachdeva MM, Eliott D. Stem cell-based therapy for diseases of the retinal pigment epithelium: from bench to bedside. Semin Ophthalmol. 2016;31:25–29. doi: 10.3109/08820538.2015.1115253. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Zhang YA. Cell therapy for macular degeneration: first phase I/II pluripotent stem cell-based clinical trial shows promise. Sci China Life Sci. 2015;58:119–120. doi: 10.1007/s11427-014-4791-2. [DOI] [PubMed] [Google Scholar]
- 4.Okumura N, Kakutani K, Inoue R, et al. Generation and feasibility assessment of a new vehicle for cell-based therapy for treating corneal endothelial dysfunction. PLoS One. 2016;11:e0158427. doi: 10.1371/journal.pone.0158427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehic A, Utheim OA, Ommundsen K, Utheim TP. Pre-clinical cell-based therapy for limbal stem cell deficiency. J Funct Biomater. 2015;6:863–888. doi: 10.3390/jfb6030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49:4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. 2010;8:60–69. doi: 10.1016/s1542-0124(12)70070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirayama M, Ogawa M, Oshima M, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497. doi: 10.1038/ncomms3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromova A, Voronov DA, Yoshida M, et al. Lacrimal gland repair using progenitor cells. Stem Cells Transl Med. 2017;6:88–98. doi: 10.5966/sctm.2016-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama M, Tsubota K, Tsuji T. Bioengineered lacrimal gland organ regeneration in vivo. J Funct Biomater. 2015;6:634–649. doi: 10.3390/jfb6030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902. doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes. 2014;7:211–223. doi: 10.2147/DMSO.S50789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gromova A, Voronov DA, Yoshida M, et al. Lacrimal gland repair using progenitor cells. Stem Cells Transl Med. 2017;6:88–98. doi: 10.5966/sctm.2016-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma D, Kanneganti TD. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 20.de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol. 2008;83:507–511. doi: 10.1189/jlb.0607362. [DOI] [PubMed] [Google Scholar]
- 22.Hung SC, Choi CH, Said-Sadier N, et al. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riteau N, Gasse P, Fauconnier L, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 24.Silverman WR, de Rivero Vaccari JP, Locovei S, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranova A, Ivanov D, Petrash N, et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 27.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prochnow N, Abdulazim A, Kurtenbach S, et al. Pannexin1 stabilizes synaptic plasticity and is needed for learning. PLoS One. 2012;7:e51767. doi: 10.1371/journal.pone.0051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanderson J, Dartt DA, Trinkaus-Randall V, et al. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Muller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014;127:270–279. doi: 10.1016/j.exer.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Deng T, Sun Y, Liu K, Yang Y, Zheng X. Role for nitric oxide in permeability of hippocampal neuronal hemichannels during oxygen glucose deprivation. J Neurosci Res. 2008;86:2281–2291. doi: 10.1002/jnr.21675. [DOI] [PubMed] [Google Scholar]
- 32.Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012;32:12579–12588. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamson SE, Leitinger N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014;588:1416–1422. doi: 10.1016/j.febslet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulbransen BD, Bashashati M, Hirota SA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orellana JA, von Bernhardi R, Giaume C, Saez JC. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev Neurosci. 2012;23:163–177. doi: 10.1515/revneuro-2011-0065. [DOI] [PubMed] [Google Scholar]
- 36.Wicki-Stordeur LE, Sanchez-Arias JC, Dhaliwal J, et al. Pannexin 1 differentially affects neural precursor cell maintenance in the ventricular zone and peri-infarct cortex. J Neurosci. 2016;36:1203–1210. doi: 10.1523/JNEUROSCI.0436-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wicki-Stordeur LE, Swayne LA. Panx1 regulates neural stem and progenitor cell behaviours associated with cytoskeletal dynamics and interacts with multiple cytoskeletal elements. Cell Commun Signal. 2013;11:62. doi: 10.1186/1478-811X-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langlois S, Xiang X, Young K, Cowan BJ, Penuela S, Cowan KN. Pannexin 1 and pannexin 3 channels regulate skeletal muscle myoblast proliferation and differentiation. J Biol Chem. 2014;289:30717–30731. doi: 10.1074/jbc.M114.572131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz SE, Gonzalez-Fernandez E, Ventura JC, et al. Contribution of pannexin1 to experimental autoimmune encephalomyelitis. PLoS One. 2013;8:e66657. doi: 10.1371/journal.pone.0066657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 41.Shestopalov VI, Slepak VZ. Molecular pathways of pannexin1-mediated neurotoxicity. Front Physiol. 2014;5:23. doi: 10.3389/fphys.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shatos MA, Hodges RR, Morinaga M, et al. Alteration in cellular turnover and progenitor cell population in lacrimal glands from thrombospondin 1-/- mice, a model of dry eye. Exp Eye Res. 2016;153:27–41. doi: 10.1016/j.exer.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175:1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Makarenkova HP, Ito M, Govindarajan V, et al. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- 46.Dvoriantchikova G, Ivanov D, Pestova A, Shestopalov V. Molecular characterization of pannexins in the lens. Mol Vis. 2006;12:1417–1426. [PubMed] [Google Scholar]
- 47.Penuela S, Bhalla R, Gong XQ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 48.Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 50.Kamal NM, Salem HM, Dahmoush HM. Immunohistochemical expression of epithelial cell adhesion molecule (EpCAM) in mucoepidermoid carcinoma compared to normal salivary gland tissues. Arch Oral Biol. 2017;79:87–94. doi: 10.1016/j.archoralbio.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Whitmire JK, Eam B, Whitton JL. Mice deficient in stem cell antigen-1 (Sca1, Ly-6A/E) develop normal primary and memory CD4+ and CD8+ T-cell responses to virus infection. Eur J Immunol. 2009;39:1494–1504. doi: 10.1002/eji.200838959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao N, Lin Y, Cao H, et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014;124:3364–3377. doi: 10.1172/JCI74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimoto M, Chang H, Shiota M, et al. Two different roles of purified CD45+c-Kit+Sca-1+Lin- cells after transplantation in muscles. Stem Cells. 2005;23:610–618. doi: 10.1634/stemcells.2004-0220. [DOI] [PubMed] [Google Scholar]
- 54.Zech NH, Gunsilius E, Clausen J, et al. Expansion of mobilized peripheral blood progenitor cells under defined culture conditions rsing CD34+CD71-CD45- cells as a starting population. J Hematother Stem Cell Res. 2003;12:367–373. doi: 10.1089/152581603322286006. [DOI] [PubMed] [Google Scholar]
- 55.Becker DL, McGonnell I, Makarenkova HP, et al. Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach. Dev Genet. 1999;24:33–42. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<33::AID-DVG5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 56.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson RJ, Jackson MF, Olah ME, et al. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 58.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012;32:12579–12588. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurtenbach S, Whyte-Fagundes P, Gelis L, et al. Investigation of olfactory function in a Panx1 knock out mouse model. Front Cell Neurosci. 2014;8:266. doi: 10.3389/fncel.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makarenkova HP, Shestopalov VI. The role of pannexin hemichannels in inflammation and regeneration. Front Physiol. 2014;5:63. doi: 10.3389/fphys.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garre JM, Yang G, Bukauskas FF, Bennett MV. FGF-1 triggers pannexin-1 hemichannel opening in spinal astrocytes of rodents and promotes inflammatory responses in acute spinal cord slices. J Neurosci. 2016;36:4785–4801. doi: 10.1523/JNEUROSCI.4195-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beckel JM, Argall AJ, Lim JC, et al. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia. 2014;62:1486–1501. doi: 10.1002/glia.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kassner A, Tiedemann K, Notbohm H, et al. Molecular structure and interaction of recombinant human type XVI collagen. J Mol Biol. 2004;339:835–853. doi: 10.1016/j.jmb.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 66.Kassner A, Hansen U, Miosge N, et al. Discrete integration of collagen XVI into tissue-specific collagen fibrils or beaded microfibrils. Matrix Biol. 2003;22:131–143. doi: 10.1016/s0945-053x(03)00008-8. [DOI] [PubMed] [Google Scholar]
- 67.Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37:214–221. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 68.Ramadas RA, Ewart SL, Medoff BD, LeVine AM. Interleukin-1 family member 9 stimulates chemokine production and neutrophil influx in mouse lungs. Am J Respir Cell Mol Biol. 2011;44:134–145. doi: 10.1165/rcmb.2009-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coles AH, Osborn MF, Alterman JF, et al. A high-throughput method for direct detection of therapeutic oligonucleotide-induced gene silencing in vivo. Nucleic Acid Therapeut. 2016;26:86–92. doi: 10.1089/nat.2015.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byrne M, Tzekov R, Wang Y, et al. Novel hydrophobically modified asymmetric RNAi compounds (sd-rxRNA) demonstrate robust efficacy in the eye. J Ocul Pharmacol Ther. 2013;29:855–864. doi: 10.1089/jop.2013.0148. [DOI] [PubMed] [Google Scholar]
- 71.Williams SC, Altmann CR, Chow RL, Hemmati-Brivanlou A, Lang RA. A highly conserved lens transcriptional control element from the Pax-6 gene. Mech Dev. 1998;73:225–229. doi: 10.1016/s0925-4773(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 72.Makarenkova H, Patel K. Gap junction signalling mediated through connexin-43 is required for chick limb development. Dev Biol. 1999;207:380–392. doi: 10.1006/dbio.1998.9171. [DOI] [PubMed] [Google Scholar]
- 73.Moore K, Ghatnekar G, Gourdie RG, Potts JD. Impact of the controlled release of a connexin 43 peptide on corneal wound closure in an STZ model of type I diabetes. PLoS One. 2014;9:e86570. doi: 10.1371/journal.pone.0086570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Umazume T, Thomas WM, Campbell S, et al. Lacrimal gland inflammation deregulates extracellular matrix remodeling and alters molecular signature of epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2015;56:8392–8402. doi: 10.1167/iovs.15-17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawley D, Ding J, Thotakura S, et al. RNA-Seq and CyTOF immuno-profiling of regenerating lacrimal glands identifies a novel subset of cells expressing muscle-related proteins. PLoS One. 2017;12:e0179385. doi: 10.1371/journal.pone.0179385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voronov D, Gromova A, Liu D, et al. Transcription factors Runx1 to 3 are expressed in the lacrimal gland epithelium and are involved in regulation of gland morphogenesis and regeneration. Invest Ophthalmol Vis Sci. 2013;54:3115–3125. doi: 10.1167/iovs.13-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.You S, Avidan O, Tariq A, et al. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Invest Ophthalmol Vis Sci. 2011;53:126–135. doi: 10.1167/iovs.11-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Xing Y, Mao L, Luo Y, Kang L, Meng G. Pannexin-1 influences peritoneal cavity cell population but is not involved in NLRP3 inflammasome activation. Protein Cell. 2013;4:259–265. doi: 10.1007/s13238-013-2114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saez JC, Cisterna BA, Vargas A, Cardozo CP. Regulation of pannexin and connexin channels and their functional role in skeletal muscles. Cell Mol Life Sci. 2015;72:2929–2935. doi: 10.1007/s00018-015-1968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388:576–585. doi: 10.1016/S0140-6736(16)30169-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.