Abstract
Background
Hereditary diffuse gastric carcinoma (HDGC) accounts for 1–3% of all gastric carcinomas. Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the gastrointestinal (GI) tract but they comprise fewer than 1% of all GI malignancies. Small-cell carcinoma (SmCC) is a rare histological type of esophageal carcinoma, accounting for 0.4% to 2.8% of all esophageal tumors. Co-occurrence of SmCC with esophageal tumors caused by squamous carcinoma is also very uncommon. Although multiple primary malignancies are no longer rare in clinical practice, the simultaneous appearance of HDGC, GIST, esophageal small cell and squamous carcinoma in situ is extremely rare and very few cases have been reported.
Case presentation
We present a case of a 53 year-old woman with synchronous occurrence of four malignancies including HDGC, GIST, esophageal small cell- and local squamous carcinoma in situ. A total gastrectomy with D2 lymph node dissection and postoperative adjuvant chemotherapy with oxaliplatin and paclitaxel liposome were performed. After a 1-year follow-up, this patient was still in good condition with no evidence of recurrence.
Conclusion
This is the unique case that describes the co-existence of the aforementioned four types of neoplasm. This case demonstrates that a diagnosis of gastric cancer does not preclude the presence of other malignancies and every case should be thoroughly analyzed to avoid missing other problems, which may worsen the prognosis.
Keywords: Synchronous tumors, Gastric adenocarcinoma, Gastrointestinal stromal tumors, Esophageal small cell carcinoma, Esophageal squamous carcinoma
Background
Synchronous tumors are independent primary tumors occurring simultaneously [1]. Currently, more and more multiple primary malignant neoplasms have been found as a consequence of improvements in diagnostic methods and in the higher overall survival and life expectancy rate. However, synchronous occurrence of four malignancies is extremely rare and very few cases have been reported in English literature [2]. Here, we report a case of synchronous occurrence of HDGC, GIST, SmCC, and local esophageal squamous cell carcinoma (ESCC) in situ.
Gastric cancer (GC) is the third most common cause of cancer-related mortality worldwide [3]. There had been 246,660 new cases and 10,730 deaths in the United States alone during 2016 [4]. In China, gastric cancer is the second most common cancer and the third leading cause of cancer-related death as reported by the National Central Cancer Registry of China (NCCR) [5]. HDGC is an autosomal dominant cancer syndrome, accounting for 1–3% of all gastric carcinoma [6]. There are two established clinical diagnostic criteria for HDGC: (1) Confirmed diffuse gastric carcinoma (signet ring cell) are diagnosed in ≥2 first or second degree relatives, at least one patient <50 years of age, or (2) Documented diffuse gastric carcinoma are diagnosed in ≥3 first and second degree relatives, independent of age of onset [7]. The tumor suppressor gene CDH1 germline mutation (encoding E-cadherin) is deemed to be the most common molecular event in HDGC. However, this mutation is only detected in approximately 30–40% of cases, and in Asian countries, the proportion even smaller [8]. Recent publications have named other closely related mutations in gastric cancer with hereditary background: CTNNA1, BRCA2, ATM, MUC1, PSCA and PLCE1 [9].
Case presentation
A 53-year-old female who reported no discomfort underwent a medical examination and barium testing revealed a small filling-defect in the fundus of her stomach (Fig. 1a). Esophagogastroduodenoscopy(EGD) showed a linear ulcer in the subcardiac region and a polyp in the fundus of stomach (Fig. 1b and c). Biopsy revealed a high-grade intraepithelial neoplasia of glandular epithelium, with local intramucosal carcinoma. Her family history was significant: her mother had advanced gastric adenocarcinoma (deceased at age 50), her grandmother and an uncle had metastatic gastric carcinoma (diagnosed in their 50s, both deceased). She has no personal history of smoking or drinking alcohol. Physical examination was normal, showing good general condition with no obvious anemia or emaciation. Nothing abnormal was found in the cardiovascular or respiratory systems and there were no clinical symptoms within the abdomen. Blood chemistry and tumor markers were within normal limits. Metastatic work-up with a contrast-enhanced computed tomography (CT) scan of the chest and abdomen demonstrated the thickening lateral wall of the lesser curvature of the fundus and body of stomach. CT showed obvious strengthening of the mucosal surface of the thickening lateral wall without any lymph nodes or distant metastasis (Fig. 1d, e, and f).
Fig. 1.
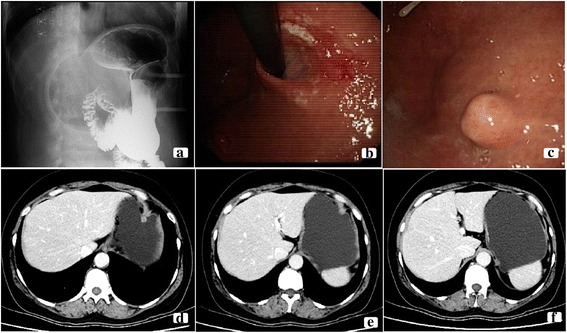
Imaging and endoscopic finds. a Barium swallow showing a small filling defect in the fundus of stomach. b Endoscopic examination showing a linear ulcer in the subcardiac region. c Endoscopic examination showing a polyp in the fundus of stomach. d, e, and f Enhanced abdominal CT scan showing obvious strengthening of the mucosal surface by thickening of the lateral wall of the lesser curvature of the fundus and body of stomach
A standard radical total gastrectomy with D2 lymph node dissection was performed on August 20, 2015. The histopathological examination revealed the following: a. well-differentiated gastric adenocarcinoma, invading the muscularis mucosa, with no carcinoma at the omentum; b. small cell carcinoma of esophagus, invading the submucosa, with local squamous cell carcinoma in situ; c. GIST with very low risk; d. no lymph node metastasis (0/18). The patient was discharged 3 weeks after the surgery and then underwent 6 courses of adjuvant chemotherapy consisting of oxaliplatin (130 mg/m2, intravenous) and paclitaxel liposome (75 mg/m2, intravenous).
Pathological findings
Microscopic findings
The gastric cancer cells presented adenocarcinoma features, which were in adenoid arrangement with obvious atypia and karyokinesis (Fig. 2a and b). The submucosal gray mass of the stomach consisted of spindle cell proliferation with uniform tapering nuclei and indistinct syncytial cytoplasm (Fig. 2c and d). The esophageal lesions were characterized by small, round, or ovoid shape, scarce cytoplasm, and an inconspicuous or absent nucleolus (Fig. 2e and f). In local mucosa of the esophagus showed heterogenic squamous epithelium spread the whole epithelial cell stroma, with no breakage of the basement membrane (Fig. 2g and h). The distance of proximal resection margin is 3 cm up from the cardiac area of the stomach, and the distal resection margin reached the duodenal bulb.
Fig. 2.
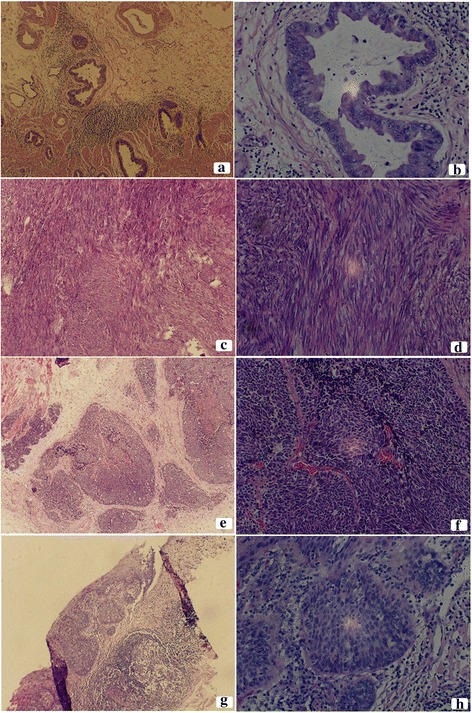
Photomicrograph showing the cytomorphological finds of the surgical resected specimen. a Gastric adenocarcinoma (H&E,×10) and b gastric adenocarcinoma (H&E,×40), (C) GIST (H&E,×10) and d GIST (H&E,×40), e esophageal small cell carcinoma (H&E,×10) and f esophageal small cell carcinoma (H&E,×40), g esophageal squamous cell carcinoma (H&E,×10) and h esophageal squamous cell carcinoma (H&E,×40)
Immunohistochemistry
The cells of GIST were stained positive for CD34, Dog-1, Ki-67 (2%), CD117, and negative for Desmin, SMA and S-1 (Fig. 3).
Fig. 3.

Photomicrograph showing immunohistochemical stains of GIST (IHC × 10). Positive for CD34, Dog-1, Ki-67(2%), CD117, and negative for Desmin, SMA and S-1
The cells of SmCC were stained positive for CK8/18 (focal+), CD (56), Syn, TTF-1 and AE1/AE3, and negative for CK5/6, p40, CD34, Dog-1 (Fig. 4).
Fig. 4.
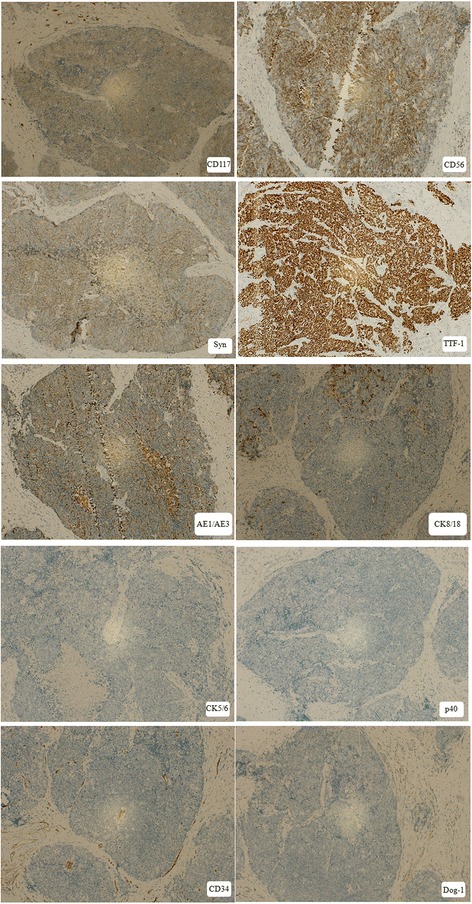
Photomicrograph showing immunohistochemical stains of SmCC (IHC × 10). Positive for CK8/18(focal+), CD (56), Syn, TTF-1 and AE1/AE3, and negative for CK5/6, p40, CD34, Dog-1
On the basis of the microscopic and immunohistochemical findings, the diagnosis was gastric adenocarcinoma with synchronous gastrointestinal stromal tumor (GIST) (very low-risk), esophageal small cell carcinoma, and local squamous cell carcinoma in situ.
Follow-up
The patient is still asymptomatic as of the writing of this report, and the results of physical examinations remained normal. Except for the presence of granuloctopenia, laboratory data were all normal at the time of discharge after her six courses of treatment (Grade 1), but there were no significant complications. One year after the start of treatment, the patient was still in good general condition and the disease was stable.
Discussion
Multiple primary cancers are frequent findings in recent years, but triple or quadruple cancers occur in less than 0.5%. Among these patients, one-third is gastric cancers. It was reported that the most common sites for synchronous gastric cancer were head and neck, esophagus, lung and kidney. Here, we reported an extremely rare case of synchronous occurrence of four malignancies, including HDGC, GIST, and esophageal small cell and local squamous carcinoma in situ. To our knowledge, this is the first case of four primary tumors occurring simultaneously in a single person’s digestive tract reported in English literature.
Although this patient was the only family member diagnosed and treated at our facility (all others were deceased), her strong family history suggests that genetic testing could give her family the information they need to take potentially life-saving measures. Consequently, SNPs rs2294008 C > T in prostate stem cell antigen (PSCA) gene were found in the current study using next-generation sequencing (NGS). This gene, which has been reported to be involved in the regulation of gastric epithelial-cell proliferation and significantly closely associated with increased risk of diffuse-type gastric cancer (DGC) with hereditary background [10]. However, no important deleterious mutations were observed in other genes identified as closely related to HDGC, such as CDH1, ATM, BRCA2, CTNNA1, MSR1, PALB2, PRSS1, SDHB, or STK11.
Very little of the mechanisms underlying the carcinogenesis of gastric cancer are fully understood. Infectious agents (i.e., Helicobacter pylori (HP), Epstein-Barr virus (EBV)), behavioral factors (i.e., alcohol consumption, cigarette smoking, nitrated foods), and genetic background were associated with the risk of developing gastric cancer. Human PSCA gene maps on chromosome 8q24.2 and encodes an 123 amino acid cell surface protein which is a member of the Ly-6/Thy-1 family having an important function on cell adhesion, proliferation, and survival [11]. rs2294008 C > T polymorphism is the most extensively studied SNP in this gene and it has been shown to be significantly closely associated with increased overall cancer risk, especially for gastric cancer [12]. The mechanism and physiological function are not fully understood, in vitro experiments have demonstrated that the PSCA rs2294008T might decrease the transcriptional activity of the host gene by recruiting transcription factor Yin Yang 1 (YY1) to its promoter and eventually predispose gastric epithelial cells to GC development [13]. In addition to PSCA, the CDH1 gene is deemed to be the most common mutation in HDGC. Unfortunately, this germline mutation was not found in this case. Considering the strong family history, relatives of this patient are strongly advised to undergo genetic testing and screening endoscopic gastric biopsy evaluations.
GIST is a rare cancer but still the most common type of mesenchymal tumor in the GI tract [12]. The tumor originates from Cajal cells or interstitial pacemaker cells [13, 14]. As in this case, small GISTs are usually detected incidentally during surgery to address other diseases, and the majority show a low level of mitotic activity [15]. In previous studies, less than 20% of synchronous GISTs and other primary tumors are coincidentally diagnosed, and more than a half of these patients presented lesions in stomach [16, 17]. It has been reported that among the cancers occurring alongside non-GIST cancer, gastric adenocarcinoma accounted for 42.6%, and ESCC for 50% [15].
ESCC accounts for 90% of cases among Asian countries, while SmCC is a rare histological type of esophageal carcinoma, accounting for 0.4% to 2.8% of all esophageal carcinoma [18, 19]. Actually, the synchronous occurrence of squamous carcinoma and SmCC is also extremely uncommon. SmCC is characterized as a highly aggressive malignant by early dissemination and poor prognosis, with median survival ranging from 3.1 to 15.5 months [20–22]. The standard treatment protocol for such disease has not been established, although surgical resection, radiotherapy, and multidrug chemotherapy have been used either alone or in combination [23, 24]. At present, there are two established hypotheses regarding the histological origin of SmCC: (1) SmCC originates from the amine precursor uptake and decarboxylase (SPUD) cells of the submucosal gland or stratum basal and (2) SmCC originates from pluripotent stem cells of the endoderm [25, 26]. Because most of the stem cells may be differentiated into squamous cell carcinoma and some did differentiate into small cell carcinoma, this may be the histological basis of the coexistence of SmCC and ESCC.
This is the first documented case of the simultaneous appearance of four primary malignancies in any esophagogastric location reported in English. No convincing explanation is still given for this coexistence. Simple coincidence could be the most reasonable explanation.
Conclusion
In summary, this report describes an extremely rare case of synchronous occurrence of HDGC, GIST, and esophageal small cell and squamous carcinoma in situ. The nature of the association between them is unknown, and further research is needed to explain the simultaneous tumors, if there is such. This case demonstrates that the diagnosis of one type of cancer should not exclude the presence of other malignancies and every case should be thoroughly analyzed to avoid overlooking any relevant condition, which may worsen the prognosis.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Consent to publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Funding
This work was supported by grants from the Postdoctoral Science Foundation of China (2015 M570634 and 2016 T90682) and the Postdoctoral Science Foundation of Henan Province (2014021).
Abbreviations
- CT
Computed tomography
- DGC
Diffuse-type gastric cancer
- EBV
Epstein- Barr virus
- ESCC
Esophageal squamous cell carcinoma
- GC
Gastric cancer
- GIST
Gastrointestinal stromal tumor
- GPI
Glycosylphosphatidyl-inositol
- HDGC
Hereditary diffuse gastric cancer
- HP
Helicobacter pylori
- NCCR
National Central Cancer Registry of China
- NGS
Next-generation sequencing
- PSCA
Prostate stem cell antigen gene
- SmCC
Small cell carcinoma
- SNP
Single nucleotide polymorphism
- YY1
Yin Yang 1
Authors’ contributions
HJF compiled all information relating to the patient and wrote the manuscript. PL revised it critically. LX and YRQ were involved in data interpretation and manuscript preparation. JL reviewed the related literature and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethic Committee of the First Affiliated Hospital of Zhengzhou University.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pierko J, Lukaszewicz J, Sawicka-Pierko A, Hady HR, Dadan J. Synchronous gastric and rectal cancer in a 50 year-old man - case report. Pol Przegl Chir. 2012;84(11):582–584. doi: 10.2478/v10035-012-0096-y. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Rha SY, Kim C, Kim GM, Yoon SH, Kim KH, Kim MJ, Ahn JB, Chung HC, Roh JK, et al. Clinicopathologic features of metachronous or synchronous gastric cancer patients with three or more primary sites. Cancer Res Treat. 2010;42(4):217–224. doi: 10.4143/crt.2010.42.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, XQ Y, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton LE, Jones K, Church N, Medlicott S. Synchronous appendiceal and intramucosal gastric signet ring cell carcinomas in an individual with CDH1-associated hereditary diffuse gastric carcinoma: a case report of a novel association and review of the literature. BMC Gastroenterol. 2013;13:114. doi: 10.1186/1471-230X-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47(7):436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan J, Li Y, Tian T, Li N, Zhu Y, Zou J, Gao J, Shen L. Risk prediction for early-onset gastric carcinoma: a case-control study of polygenic gastric cancer in Han Chinese with hereditary background. Oncotarget. 2016;7(23):33608–33615. doi: 10.18632/oncotarget.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Study Group of Millennium Genome Project for C, Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F et al: Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet 2008, 40(6):730-740. [DOI] [PubMed]
- 10.Eshel R, Zanin A, Kapon D, Sagi-Assif O, Brakenhoff R, van Dongen G, Witz IP. Human Ly-6 antigen E48 (Ly-6D) regulates important interaction parameters between endothelial cells and head-and-neck squamous carcinoma cells. Int J Cancer. 2002;98(6):803–810. doi: 10.1002/ijc.10301. [DOI] [PubMed] [Google Scholar]
- 11.Saeki N, Ono H, Yanagihara K, Aoyagi K, Sasaki H, Sakamoto H, Yoshida T. rs2294008T, a risk allele for gastric and gallbladder cancers, suppresses the PSCA promoter by recruiting the transcription factor YY1. Genes Cells. 2015;20(5):382–391. doi: 10.1111/gtc.12228. [DOI] [PubMed] [Google Scholar]
- 12.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7(6):507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30(10):1213–1220. doi: 10.1016/S0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 14.van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer. 2005;104(9):1781–1788. doi: 10.1002/cncr.21419. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ, Yang Z, Hao LS, Xia L, Jia QB, XT W. Synchronous incidental gastrointestinal stromal and epithelial malignant tumors. World J Gastroenterol. 2009;15(16):2027–2031. doi: 10.3748/wjg.15.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding J, Sun P, Cai XY, Fei SH, Wu J, Qi YK, Liu ZB, Yuan L, He YJ, Song H, et al. Synchronous poorly-differentiated neuroendocrine carcinoma and gastrointestinal stromal tumor of the stomach: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA. Int J Clin Exp Pathol. 2014;7(12):9076–9080. [PMC free article] [PubMed] [Google Scholar]
- 17.Nemes C, Rogojan L, Surdea-Blaga T, Seicean A, Dumitrascu DL, Ciuce C. Gastrointestinal stromal tumor (GIST) associated with synchronous colon adenocarcinoma - a case report. J Gastrointestin Liver Dis. 2012;21(1):101–103. [PubMed] [Google Scholar]
- 18.Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007;34(1):43–50. doi: 10.1053/j.seminoncol.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 20.Casas F, Ferrer F, Farrus B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80(8):1366–1372. doi: 10.1002/(SICI)1097-0142(19971015)80:8<1366::AID-CNCR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Law SY, Fok M, Lam KY, Loke SL, Ma LT, Wong J. Small cell carcinoma of the esophagus. Cancer. 1994;73(12):2894–2899. doi: 10.1002/1097-0142(19940615)73:12<2894::AID-CNCR2820731204>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Huncharek M, Muscat J. Small cell carcinoma of the esophagus. The Massachusetts General Hospital experience, 1978 to 1993. Chest. 1995;107(1):179–181. doi: 10.1378/chest.107.1.179. [DOI] [PubMed] [Google Scholar]
- 23.Yau KK, Siu WT, Wong DC, Chau CH, Li AC, Law BK, Li MK. Non-operative management of small cell carcinoma of esophagus. Dis Esophagus. 2007;20(6):487–490. doi: 10.1111/j.1442-2050.2007.00635.x. [DOI] [PubMed] [Google Scholar]
- 24.Lv J, Liang J, Wang J, Wang L, He J, Xiao Z, Yin W. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008;3(12):1460–1465. doi: 10.1097/JTO.0b013e31818e1247. [DOI] [PubMed] [Google Scholar]
- 25.Doherty MA, McIntyre M, Arnott SJ. Oat cell carcinoma of esophagus: a report of six British patients with a review of the literature. Int J Radiat Oncol Biol Phys. 1984;10(1):147–152. doi: 10.1016/0360-3016(84)90421-8. [DOI] [PubMed] [Google Scholar]
- 26.Lee SS, Ha HK, Kim AY, Kim TK, Kim PN, Yu E, Lee MG, Myung SJ, Jung HY, Kim JH, et al. Primary extrapulmonary small cell carcinoma involving the stomach or duodenum or both: findings on CT and barium studies. AJR Am J Roentgenol. 2003;180(5):1325–1329. doi: 10.2214/ajr.180.5.1801325. [DOI] [PubMed] [Google Scholar]


