Abstract
Introduction
Fibrinogen A alpha chain amyloidosis is an autosomal dominant disease associated with mutations in the fibrinogen A alpha chain (FGA) gene, and it is the most common cause of hereditary renal amyloidosis in the UK. Patients typically present with kidney impairment and progress to end-stage renal disease over a median time of 4.6 years.
Methods
Six patients presented with proteinuria, hypertension, and/or lower limb edema and underwent detailed clinical and laboratory investigations.
Results
A novel FGA gene mutation was identified in each case: 2 frameshift mutations F521Sfs*27 and G519Efs*30 and 4 single base substitutions G555F, E526K, E524K, R554H. In 5 subjects, extensive amyloid deposits were found solely within the glomeruli, which stained specifically with antibodies to fibrinogen A alpha chain, and in one of these cases, we found coexistent fibrinogen A alpha chain amyloidosis and anti-glomerular basement membrane antibody disease. One patient was diagnosed with light-chain amyloidosis after a bone marrow examination revealed a small clonal plasma cell population, and laser microdissection of the amyloid deposits followed by liquid chromatography and tandem mass spectrometry identified kappa light chain as the fibril protein.
Discussion
We report 6 novel mutations in the FGA gene: 5 were associated with renal fibrinogen A alpha chain amyloidosis and 1 was found to be incidental to light-chain amyloid deposits discovered in a patient with a plasma cell dyscrasia. Clinical awareness and suspicion of hereditary amyloidosis corroborated by genetic analysis and adequate typing using combined immunohistochemistry and laser microdissection and mass spectrometry is valuable to avoid misdiagnosis, especially when a family history of amyloidosis is absent.
Keywords: anti-GBM glomerulonephritis, end-stage renal disease (ESRD), fibrinogen A alpha chain, hereditary renal amyloidosis, mass spectrometry-based proteomics
Amyloidosis is a disorder of protein folding characterized by conformational change of native globular proteins into fibrils with a beta-sheet appearance. The deposition of amyloid fibrils in an extracellular space readily leads to progressive disruption of the structure and function of affected tissues and organs.1 A growing number of proteins have been implicated in systemic amyloidosis resulting in substantial disease heterogeneity; virtually any organ or combination of organs other than the brain may be affected by amyloid.
Hereditary renal amyloidosis is associated with genetically altered proteins encoded by 7 genes: fibrinogen A alpha chain (FGA),2, 3, 4, 5, 6 apolipoprotein A1 (APOA1),7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 apolipoprotein A2 (APOA2),19 lysozyme (LYZ),20, 21 gelsolin (GSN),22, 23, 24, 25 apolipoprotein C2 (APOC2),26 and apolipoprotein C3 (APOC3).27 Although the clinical syndromes caused by mutations in these genes might vary with respect to the age at disease onset, rate of progression, prognosis, and organ involvement, patients typically present with proteinuria and/or hypertension and progress to end-stage renal disease (ESRD). Apolipoprotein A-I amyloidosis may also present with cardiomyopathy, gastrointestinal involvement, polyneuropathy, and skin and laryngeal amyloid deposition. Lysozyme amyloidosis can be associated with substantial accumulation of amyloid within the liver and gastrointestinal tract, and gelsolin amyloidosis typically causes cranial neuropathy.
The natural history of the renal decline to ESRD in hereditary fibrinogen A alpha chain (AFib) amyloidosis is approximately 4.6 years from the onset of symptoms and is much faster than in hereditary apolipoprotein A–I amyloidosis where the median time is approximately 8 years,28 but substantially slower than in untreated systemic light-chain (AL) amyloidosis. To date, 9 mutations in the fibrinogen A alpha chain gene (FGA, GenBank accession no. NW_922217) have been described and all, except the E526V variant that is reported largely in Northern Europeans, were found only in isolated kindreds from various parts of the world. Kidney biopsies from all subjects with AFib amyloidosis show a characteristic histological appearance with striking glomerular enlargement and almost complete obliteration of the normal architecture by amyloid deposition with little or no vascular or interstitial amyloid deposits.
Here we report 6 novel mutations in the FGA gene: 5 were associated with renal AFib amyloidosis and 1 was found to be incidental to AL amyloid deposits discovered in a patient with a plasma cell dyscrasia. The clinical phenotypes associated with the novel FGA mutations are described in detail.
Methods
Patients
Six unrelated patients were presented with proteinuria and were subsequently discovered to have amyloid deposits on kidney biopsy: a 51-year-old Norwegian man (patient 1), a 30-year-old North African woman (patient 2), a 43-year-old French woman (patient 3), an 80-year-old Russian man (patient 4), a 69-year-old Scottish man (patient 5), and a 68-year-old British woman (subject 6). They underwent detailed clinical evaluation as well as electrocardiography, echocardiography, blood and urine biochemistry including a search for a monoclonal protein by immunofixation electrophoresis of serum and urine, and serum free light chains. Patients 1 and 6, both of whom were assessed at the UK National Amyloidosis Centre, underwent whole-body 123I-labeled serum amyloid P component (SAP) scintigraphy.
Informed consent was obtained from all patients and clinical care was in accordance with the Declaration of Helsinki.
Histology and Immunohistochemistry
Renal biopsies from patients 1 and 2 were initially examined at the Oslo University Hospital and subsequently restained at the UK National Amyloidosis Centre. Biopsies from patients 3 to 6 were examined at the UK National Amyloidosis Centre. Briefly, 6-μm-thick sections from formalin fixed paraffin-embedded renal biopsies were stained for amyloid with Congo red and viewed under crossed polarized light. Immunohistochemical staining of the amyloid deposits was performed using a panel of monospecific antibodies specified in Table 1, and as previously described.29 Specificity of staining was confirmed by prior absorption of the antiserum with pure antigen in each case, and positive and negative controls were included in each run.
Table 1.
Commercial antisera used in immunohistochemistry
| Antibody to identify | Raised in | Working dilution | Source |
|---|---|---|---|
| P-component | Rabbit | 1:200 | DAKO |
| AA (REU 86.2) | Mouse | 1:100 | Euro Diagnostica |
| Kappa | Rabbit | 1:20,000 | DAKO |
| Lambda | Rabbit | 1:20,000 | DAKO |
| Lysozyme | Rabbit | 1:1000 | DAKO |
| Fibronogen alpha chain | Sheep | 1:300 | Cambichem |
| apoA1 | Goat | 1:4000 | Genzyme |
| Lect2 | Goat | 1:600 | R&D Systems |
Laser Microdissection and Tandem Mass Spectrometry
Laser microdissection (LMD) was performed on Congo red-positive glomeruli as previously described.30 The samples were digested and analyzed by liquid chromatography and tandem mass spectrometry (LC-MS/MS) on a Thermo Velos Orbitrap instrument or using an EASY-nanoLC (Proxeon; Thermo-Scientific, Hemel Hempstead, UK) coupled to HCTultra ETDII (Bruker Daltonics, Bruker UK Limited, Coventry, UK). MS data files were analyzed using Mascot31 (www.martixscience.com) to search either the SwissProt or the National Center for Biotechnology Information database. All positive samples were further examined using an expanded Swissprot database containing the 9 previously reported FGA variants.
Genetic Investigations
Genomic DNA was extracted from whole blood treated with ethylenediamine tetraacetic acid as previously described.32 Exon 5 of the fibrinogen A alpha chain gene (FGA, GenBank accession no. NW_922217) was amplified by polymerase chain reaction assay and analyzed by automated sequencing with the polymerase chain reaction primers: forward 5′-GCTCTGTATCTGGTAGTACT-3′ and reverse 5′-ATCGGCTTCACTTCCGGC-3′, and the sequencing primer: 5′-TGGGGCACATTTGAAGAG-3′ and other solutions and cycling conditions that have previously been described.33 The electropherograms of the FGA gene were analyzed on the ABI 3130xl Genetic Analyzer using Sequencing Analysis Software version 5.4.
Amplified DNA from patients 1 and 2 was cloned to determine whether a single or both alleles of the FGA gene were mutated. Briefly, purified polymerase chain reaction products were ligated to the pGEM-T Easy Vector System (Promega) and transformed into Escherichia coli NEB 5-alpha (New England Biolabs) according to the manufacturer’s instructions. Plasmid DNA was extracted from 24 independent clones using a QIAprep Spin MiniPrep Kit (Qiagen) and the inserted fragment was excised by restriction with EcoRI, gel purified and directly sequenced.
To exclude other hereditary renal amyloidoses, the APOA1, APOA2, and LYZ genes were sequenced in all 6 patients with the use of primers and reagents described elsewhere.7, 19, 20
Results
Clinical Findings
Clinical symptoms and laboratory findings in the 6 patients with renal impairement are shown in Table 2. Patient 1 presented with a lower limb edema and hypertension. Blood pressure was 149/106 mm Hg and the remaining cardiorespiratory examination was within normal limits. Investigations showed normal blood count and clotting profile. The patient had nephrotic-range proteinuria of 7.4 g per 24 hours, urine albumin-to-creatinine ratio 556 mg/mmol, serum creatinine 134 μmol/l, and estimated glomerular filtration rate (eGFR) 50 ml/min per 1.73 m2. There was no evidence of a monoclonal protein and no evidence of cardiac amyloid infiltration. SAP scintigraphy showed isolated, but intense renal uptake. His renal function had gradually declined, and 4 years after diagnosis, his creatinine was 323 μmol/l with eGFR 18 ml/min per 1.73 m2 and urine albumin-to-creatinine ratio 316 mg/mmol.
Table 2.
Clinical symptoms and laboratory findings in the 6 patients with renal impairement
| Patient number | Sex/age at presentation/family history | Ethnicity | Clinical symptoms | Baseline proteinuria (g/24 h)/serum creatinine (μmol/l)/eGFR (ml/min per 1.73 m2) | Histology findings on a renal biopsy in patients 1–5 and in patient 6 on both renal and bone marrow trephine (bmt) biopsies | FGA gene mutation |
|---|---|---|---|---|---|---|
| 1 | Male/48/no | Norwegian | Lower limb edema, hypertension, nephrotic-range proteinuria, gradual decline of renal function | 7.4/134/50 | Amyloid deposits localized uniquely within the glomeruli | G5555F (p.G574F) c.1720_1721delGGinsTT |
| 2 | Female/28/no | North African | Proteinuria diagnosed in early pregnancy progressed to nephrotic range. Slow decline in renal function resulted in hemodialysis | 1.8/74/>60 | Amyloid deposits localized uniquely within the glomeruli | F521Sfs*27 (p.F540Sfs*27) c.1619_1622delTTGT |
| 3 | Female/41/yes on maternal side | French | Progressive nephrotic syndrome, lower limb edema, high blood pressure | 5.3/141/35 | Amyloid deposits localized uniquely within the glomeruli | G519Efs*30 (p.G538E*30) c.1611delA |
| 4 | Male/70/no | Russian | 10-yr history of renal insufficiency, dialysis | 6.3/438/12 | Amyloid deposits localized uniquely within the glomeruli | E526K (p.E545K) c.1627G>A |
| 5 | Male/69/no | Scottish | Hemoproteinuria and advanced CKD after a short history of general malaise, high titer anti-GBM antibodies, rapid decline in renal function, dialysis | 3.2/400/<15 | Amyloid deposits localized uniquely within the glomeruli; in addition, linear deposition of anti-GBM antibodies was detected | E524K (p.E543K) c.1633G>A |
| 6 | Female/68/no | British | Heavy proteinuria, history of recurrent urinary tract infection, renal dysfunction | 4.3/49/>90 | Amyloid deposits in the glomeruli and in the vessels. In addition, bmt revealed amyloid with no immunospecific staining | R554H (p.R573H) c.1718G>A (nonamyloidogenic) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GBM, glomerular basement membrane.
Patient 2 presented with proteinuria diagnosed in early pregnancy, which progressed to nephrotic range in the third trimester and persisted after delivery. The patient had previously been treated for pulmonary tuberculosis. She was normotensive. Initial investigations revealed normal blood count and clotting profile, proteinuria of 1.8 g per 24 hours, urine albumin-to-creatinine ratio 117 mg/mmol, serum creatinine 74 μmol/l, and eGFR > 60 ml/min per 1.73 m2. Electrocardiography and echocardigraphy were normal, and there was no evidence of a monoclonal protein. Her renal function slowly declined over the following 3 years, with a more rapid decline during and after her next pregnancy, when she developed nephrotic-range proteinuria and exacerbation of hypertension. Four years after diagnosis, she started hemodialysis.
Patient 3 presented with nephrotic syndrome. On direct questioning, the patient revealed a 2-year history of hypertension and an extensive family history of renal disease. Her mother and maternal grandmother were both diagnosed with proteinuric nephropathy of unknown origin and died at the age of 45 and 57 years, respectively. The mother underwent renal transplantation at the age of 43 years, but died with a functioning renal allograft from sepsis 2 years later. The patient’s physical examination showed a blood pressure of 180/80 mm Hg and pitting lower limb edema. Investigations revealed serum creatinine of 141 μmol/l, eGFR 35 ml/min per 1.73 m2, proteinuria 5.3 g per 24 hours, and serum albumin 36 g/l. There was no evidence of a monoclonal protein in serum or urine. Abdominal ultrasound showed normal sized hyperechoic kidneys and no other abdominal organomegaly. Echocardiography revealed no signs of cardiac amyloidosis.
Patient 4 presented with proteinuria and a 10-year history of renal insufficiency. He was normotensive and physical examination was unremarkable except for edema of the lower extremities. There was no evidence of a monoclonal protein in serum or urine. Baseline investigations showed normal clotting profile, proteinuria of 6.3 g per 24 hours, creatinine 438 μmol/l, and eGFR 12 ml/min per 1.73 m2, and there was no evidence of cardiac amyloidosis. Six months after diagnosis, the patient commenced dialysis.
Patient 5 presented with hemoproteinuria and advanced chronic kidney disease after a short history of general malaise. Baseline investigations showed normal full blood count and clotting screen, eGFR <15 ml/min per 1.73 m2, and normal C reactive protein. He was noted to have high titer anti-glomerular basement membrane (anti-GBM) antibodies (>500) and after a kidney biopsy was diagnosed with anti-GBM antibody disease, for which he received plasma exchange, steroids, and cyclophosphamide. He was dialysed during his therapy and did not regain dialysis independence.
Patient 6 was found incidentally to have proteinuria. She denied symptoms of peripheral edema, and did not have symptoms of cardiac or other organ dysfunction. She was normotensive. Baseline investigations showed normal blood count and clotting profile, proteinuria of 4.3 g per 24 hours, creatinine 49 μmol/l, eGFR >90 ml/min per 1.73 m2, and normal serum free light chains: kappa 19.1 mg/l, lambda 8.3 mg/l, K/L ratio 2.30. There was a 4 g/l IgG kappa paraproteinemia, and bone marrow examination revealed a low-level CD56 positive plasma cell population (<10%). There was no evidence of cardiac amyloidosis. SAP scintigraphy showed a large amyloid load in the liver, spleen, kidneys, adrenals, and avid uptake in the bones.
Histology and Immunohistochemistry
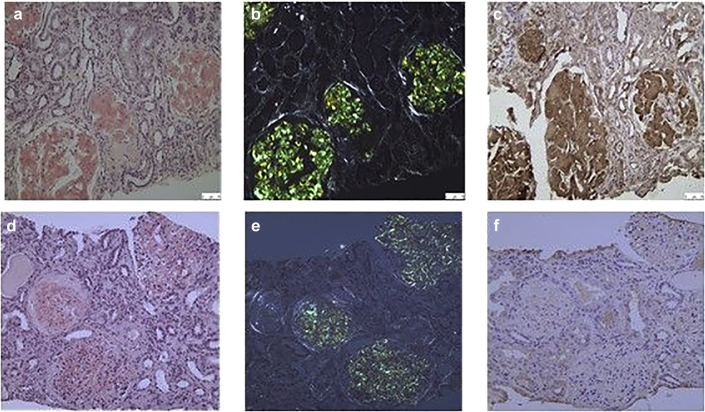
Extensive amyloid deposits were identified on the kidney biopsies of patients 1 to 4 by their pathognomonic green birefringence when stained with Congo red and viewed under crossed polarized light (Figure 1a and b). The deposits were localized exclusively in the glomeruli resulting in striking glomerular enlargement with almost complete obliteration of the normal glomerular architecture. There was no amyloid within the tubules, interstitium, or vessels. The amyloid stained specifically with antibodies to fibrinogen A alpha chain (Figure 1c), and staining was completely abolished by prior absorption of the antiserum with an excess of pure human fibrinogen A alpha chain. The antibody that was used could detect the wild-type (WT) and mutant AFib peptide, the latter resulting from either a single amino acid substitution or the frameshift mutations. There was no staining of the amyloid with the remaining antibodies listed in the Methods. In patient 5, in addition to extensive amyloid, localized uniquely within the glomeruli that stained specifically with antibodies to fibrinogen A alpha chain, the kidney biopsy showed crescentic glomerulonephritis with the involvement of 7 of 15 glomeruli. Immunofluorescence microscopy showed linear deposition of anti-GBM antibodies.
Figure 1.
Renal biopsy from patient 3 with a novel FGA mutation. Striking glomerular enlargement and almost complete obliteration of the normal architecture by amyloid deposition is shown when (a) stained with Congo red and viewed using brightfield illumination, (b) stained with Congo red and viewed under crossed polarized light, and (c) immunostained with antibodies to fibrinogen A alpha chain. For comparison, a renal biopsy from a patient with kappa light-chain (AL) amyloidosis is shown; (d), (e) extensive amyloid is visible within glomeruli, (f) but there is no staining above the background of the amyloid deposits with an antibody against fibrinogen A alpha chain.
In patient 6, the kidney biopsy revealed Congo red-positive staining in the glomeruli and the vessels, but the immunohistochemistry did not show any immunospecific staining and was therefore inconclusive. Congo red staining of the bone marrow trephine in this patient also revealed amyloid with no immunospecific staining.
FGA Gene Analysis
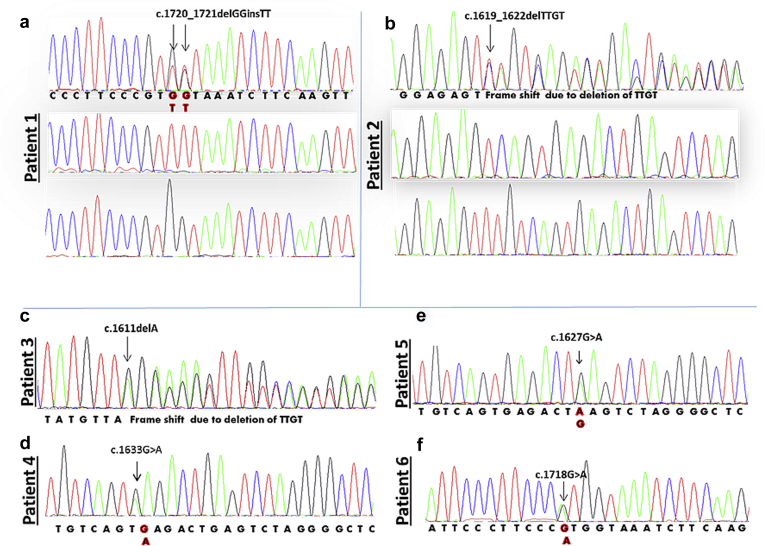
A novel FGA gene mutation was identified in each case (Figure 2). Patient 1 had 2 consecutive guanine bases deleted and 2 thymine bases inserted in their position. Cloning of the polymerase chain reaction products revealed that a single allele was affected (c.1720_1721delGGinsTT), resulting in a substitution of glycine with phenylalanine at position 555 of the mature protein (p.G574F; this nomenclature includes 19-amino-acid signal peptide) (Figure 2a). Two novel frameshift mutations F521Sfs*27 (p.F540Sfs*27), resulting from deletion of 4 consecutive nucleotides (c.1619_1622delTTGT), and G519Efs*30 (p.G538E*30), resulting from a deletion of an adenine nucleotide (c.1611delA), were found in patients 2 and 3, respectively (Figure 2b and c). The new reading frame created by each deletion predicted the premature termination of the protein 27 and 30 amino acids downstream from the site of mutation. Three single base substitutions (c.1627G>A), (c.1633G>A), and (c.1718G>A) were identified in patients 4, 5, and 6, respectively, replacing glutamic acid with lysine at codons 526 (p.E545K) and 524 (p.E543K) and arginine with histidine at position 554 (p.R573H) (Figure 2d–f). There was no mutation in other genes known to be associated with renal amyloidosis including APOA1, APOA2, and LYZ.
Figure 2.
Partial DNA sequence of exon 5 of the FGA gene showing 6 novel variants. (a) Results from patient 1. Top panel shows that 2 consecutive nucleotides GG are replaced by TT; cloning revealed that a single allele was mutated (c.1720_1721delGGinsTT) and the other allele was wild type, shown in the middle and bottom panels, respectively. (b) Results from patient 2. Overlapping nucleotides on the electropherogram indicate a frameshift mutation; cloning revealed that a single allele had 4 nucleotides deleted (c.1619_1622delTTGT), and the other allele was wild type, shown in the middle and bottom panels, respectively. (c) A deletion of an adenine nucleotide (c.1611delA) found in patient 3 resulted in a frameshift mutation. (d–f) A single base substitution: (c.1633G>A), (c.1627G>A), and (c.1718G>A) were identified in patients 4, 5, and 6, respectively.
Laser Microdissection and Mass Spectrometry
LMD followed by LC-MS/MS proteomics analysis was undertaken on samples from patients 1 and 2 in Oslo and patients 1, 3, 4, 5, and 6 in London. Amyloid signature proteins (SAP, ApoE, or ApoA4) were detected in the amyloid deposits of all patients. Fibrinogen A alpha chain was identified in patients 1 to 5 confirming AFib amyloidosis. In patient 6, only kappa light chains were evident and fibrinogen was absent; this patient was diagnosed with AL amyloidosis.
Data from patients 1, 4, and 5 were further analyzed using the extended FibA database. It was not possible to confirm the presence of the variant G555F (p.G574F) in patient 1 because the putative variant tryptic peptide could not be observed due to its very low molecular mass; however, the peptide coverage was entirely consistent with that observed in other cases of AFib amyloidosis other than the absence of the corresponding WT peptide. Variant E526K (p.E545K) was present in amyloid from patient 4 with 2 peptides identified, p.528-545 and p.546-558, covering the new tryptic cleavage site. Variant E524K (p.E543K) was identified in patient 5 by the presence of a new tryptic peptide: p.528-543. Assignments were confirmed by manual sequencing of MS/MS spectra for the relevant tryptic peptides. In both cases, there was evidence for the corresponding WT peptide.
The MS/MS spectra of the frameshift variants were examined manually. Variant F521Sfs*27 (p.F540Sfs*27) was confirmed in patient 2 by the identification of a new tryptic peptide, p.543-557, with MH2+ at m/z 793.97, and good sequence coverage (shown underlined) LSLGAQNLASSQIQR (Table 3). Variant G519Efs*30 (p.G538E*30) was identified in patient 3 by the presence of 3 new tryptic peptides: FPGFFSPMLESLSVR, MH2+ m/z 907.96; LSLGAQNLASSQIQR, MH2+ m/z 793.44; and NPVLITLG, MH2+ m/z 413.76 (Table 1). WT FibA peptide fragment p.548-599 was also identified in patient 3.
Table 3.
Identification of frameshift mutations in fibrinogen A alpha chain peptide by tandem mass spectrometry-based proteomics
| WT p. 521-583 |
DTASTGKTFP GFFSPMLGEF VSETESRGSE SGIFTNTKES SSHHPGIAEF PSRGKSSSYS KQF....... |
| p.F540Sfs*27 p.521-565 |
DTASTGKTFP GFFSPMLGES VRLSLGAQNL ASSQIQRNPV LITLG |
| p.G538Efs*30 p.521-566 |
DTASTGKTFP GFFSPMLESL SVRLSLGAQN LASSQIQRNP VLITLG |
The C-terminal fragment of wild-type (WT) FibA alpha chain (p.521-583) is compared with the C termini (p.521 end) of 2 frameshift variants, p.F540Sfs*27 (F521Sfs*27) and p.G538Efs*30 (G519Efs*30). Variant p.F540Sfs*27 was identified by the presence of a single new tryptic peptide, p.543-557 LSLGAQNLASSQIQR (underlined), whereas the p.G538Efs*30 variant was identified by the presence of 3 new tryptic peptides: TFPGFFSPMLESLSVR, LSLGAQNLASSQIQR, and NPVLITLG, covering p.528-566. The location, in the WT protein, of the 5 amyloidogenic variants identified in patients 1–5 is shown in bold: p.G538Efs*30, p.F540Sfs*27, p.E543K, p.E545K, and p.G574F.
It is not known whether the source of the WT fibrinogen in the samples from patients 3 to 5 was blood contamination of the biopsy specimens or whether WT fibrinogen was incorporated into the amyloid.
Discussion
Fibrinogen is a 340-kD plasma protein synthesized by hepatocytes and is essential for blood coagulation. It is composed of 2 sets of 3 subunits: alpha, beta, and gamma. The alpha subunit is the largest of the three and is encoded by the FGA gene, located on chromosome 4. Mutations in this gene have been linked to renal amyloidosis and to bleeding or thrombotic disorders of variable severity such as afibrinogenemia, dysfibrinogenemia, and hypofibrinogenemia.
AFib amyloidosis is an autosomal dominant disease, first described in 1993 among members of a Peruvian kindred who were heterozygous for the R554L substitution.3 Interestingly, subject 6 described in the current study also had substitution of an arginine residue at position 554 (R554H), but unlike the R554L, it was not associated with renal amyloid in our patient. The likely explanation is that both arginine and histidine amino acids are hydrophilic, and thus the substitution may not necessarily induce a significant change in the protein structure. Leucine, by contrast, is hydrophobic and a replacement of arginine with leucine might have more profound effects on the protein conformation. Bone marrow examination of patient 6 revealed low-level clonal plasma cells, and the SAP scan showed extensive bone uptake, strongly suggesting AL amyloidosis (data not shown). LMD and LC-MS/MS examination of the renal biopsy showed kappa light chain deposits that further validated the diagnosis of systemic AL amyloidosis, subsequently confirmed by regression of amyloid and reduction of proteinuria after successful chemotherapy.
To date, 9 FGA variants have been identified in different kindreds (http://www.amyloidosismutations.com), and although the precise mechanism that promotes amyloid fibril formation in this disease is not fully understood, it has been shown that all mutations are clustered within close proximity in the 5′ end of exon 5 of the FGA gene and the amyloidogenic fibril subunit consists of residues 500–580.6 Here we report 5 patients with novel FGA mutations G555F, F521Sfs*27, G519Efs*30, E526K, and E524K as the cause of their renal amyloidosis. To our knowledge, the patients with the FGA G555F and F521Sfs*27 mutations are the first cases of AFib amyloidosis described in Scandinavia. None of these patients had an inflammatory disease; the inflammatory markers C reactive protein and serum amyloid A protein were within normal range. Nor did they have evidence of a plasma cell dyscrasia, to suggest amyloid A (AA) amyloidosis or AL amyloidosis. Analysis of other genes associated with hereditary renal amyloidosis in all 5 cases revealed no mutations. Interestingly, only 1 patient had a family history of kidney disease. This is consistent with the known variable disease penetrance in AFib amyloidosis, previously highlighted among subjects with E526V variant, the most common cause of hereditary renal amyloidosis in the United Kingdom.33 In our cohort, the diagnosis of AFib amyloidosis was made on kidney biopsy using a combination of immunohistochemistry and LMD and LC-MS/MS, in conjunction with genetic analysis. The renal histological appearance in all 5 patients was identical to that associated with all previously reported amyloidogenic FGA variants with characteristic massive glomerular amyloid in the absence of extraglomerular deposits. Interestingly, the kidney biopsy of patient 5 revealed the presence of characteristic glomerular fibrinogen amyloid deposits and crescentic glomerulonephritis, the latter in association with high titer anti-GBM antibodies. This patient’s renal function declined rapidly to ESRD, suggesting a major contribution to the clinical phenotype from the anti-GBM disease. To our knowledge, he is the first case diagnosed with both the AFib amyloidosis and anti-GBM antibody disease; the nature of this association is unclear.
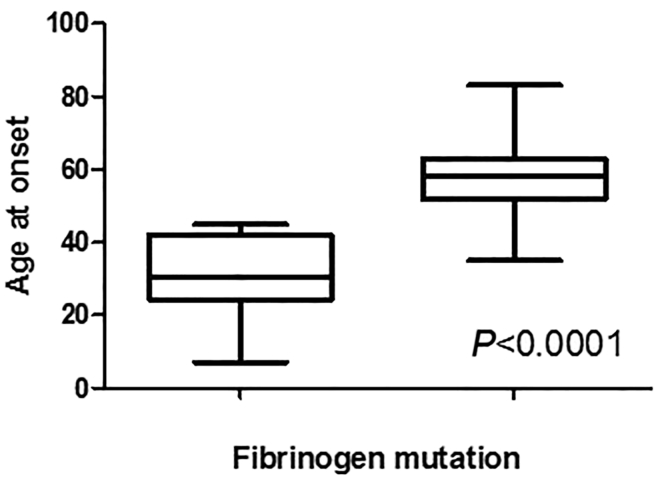
To date, 4 frameshift mutations in the FGA gene have been reported and all were associated with an early disease onset and a very aggressive phenotype quickly progressing to ESRD: M517_F521delinsQSfs*28 was identified in a Korean girl who presented at 7 years of age;34 V522Afs*27 was found in a French kindred with the index patient and his son presenting at the age of 31 and 12 years, respectively;6 E524Efs*25 was associated with a renal failure resulting in death in the fourth decade in an American kindred;5 and T525Tfs*24 was identified in a Chinese subject who presented in early thirties.33 Patients 2 and 3 described here, with the F521Sfs*27 and G519Efs*30 frameshift mutations, presented at the age of 30 and 45 years, respectively, and their renal function deteriorated more rapidly than in subjects who had a single base substitution. From this and previous reports, we have established a significant difference in the age at onset among patients who were diagnosed with frameshift mutations (median age 30 years) and those who had single amino acid substitutions (median age 59 years), P = 0.0001 (Figure 3). The sequence of all fibrinogen A alpha chain peptides arising from the frameshift mutations is a hybrid between a short fragment of normal sequence and a completely new C-terminal peptide. Remarkably, the novel C terminal has, in all cases, an almost identical amino acid sequence that terminates prematurely at position 548 instead of the 610 for the WT mature protein.6 It is notable that there was evidence of the WT protein fragment within the samples of patients 3 to 5, contrasting previous reports that identified only variant fibrinogen within the amyloid deposits. It is possible that WT fibrinogen was derived from blood contamination in these samples that is supported by the additional presence of Fib beta and gamma chains, although one cannot exclude the possibility that these particular novel variants somehow promote the incorporation of the WT protein into amyloid.
Figure 3.
Age at disease onset in patients with AFib amyloidosis. Patients with AFib amyloidosis caused by frameshift mutations (n = 6) in the FGA gene had earlier disease onset than subjects with a single amino acid substitution (n = 70) (Mann-Whitney test, P < 0.0001). AFib, fibrinogen A alpha chain.
The treatment of AFib amyloidosis includes supportive measures and for those with ESRD consideration of kidney transplantation. However, because of the recurrence of amyloid in the renal allograft, combined liver and kidney transplantation, which replaces the source of circulating amyloidogenic fibrinogen with the WT (nonamyloidogenic) protein and thus prevents ongoing amyloid deposition in the renal allograft or elsewhere, can be considered although the procedural risk is high.35 Consideration of liver and kidney transplantation may be of even more relevance in patients with AFib due to frameshift mutations, given the aggressive and early onset nature of their disease. This study highlights the importance of definitive characterization of the amyloid fibril protein in all cases of systemic amyloidosis, to avoid misdiagnosis and administration of inappropriate and potentially harmful therapy,36 while also highlighting some of the complexities in diagnosis that often require combined genetic analysis, immunohistochemistry, and LMD and LC-MS/MS.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank members of Norwegian Work group of Amyloidosis: Lorentz Brinch, Tobias Gedde-Dahl, Gunnar Husby, Fredrik Schjesvold, and Knut Sletten for contributions in amyloid typing and the Core support for the Wolfson Drug Discovery Unit (CAAPP) provided by the UK National Institute for Health Research Biomedical Research Centre and Unit Funding Scheme.
References
- 1.Pepys M.B. Amyloidosis. Annu Rev Med. 2006;57:223–241. doi: 10.1146/annurev.med.57.121304.131243. [DOI] [PubMed] [Google Scholar]
- 2.Gillmore J.D., Lachmann H.J., Rowczenio D. Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen A alpha-chain amyloidosis. J Am Soc Nephrol. 2009;20:444–451. doi: 10.1681/ASN.2008060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson M.D., Liepnieks J., Uemichi T. Hereditary renal amyloidosis associated with a mutant fibrinogen α-chain. Nat Genet. 1993;3:252–255. doi: 10.1038/ng0393-252. [DOI] [PubMed] [Google Scholar]
- 4.Uemichi T., Liepnieks J.J., Benson M.D. Hereditary renal amyloidosis with a novel variant fibrinogen. J Clin Invest. 1994;93:731–736. doi: 10.1172/JCI117027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uemichi T., Liepnieks J.J., Yamada T. A frame shift mutation in the fibrinogen A α-chain gene in a kindred with renal amyloidosis. Blood. 1996;87:4197–4203. [PubMed] [Google Scholar]
- 6.Hamidi Asl L., Liepnieks J.J., Uemichi T. Renal amyloidosis with a frame shift mutation in fibrinogen a α-chain gene producing a novel amyloid protein. Blood. 1997;90:4799–4805. [PubMed] [Google Scholar]
- 7.Rowczenio D., Dogan A., Theis J.D. Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I. Am J Pathol. 2011;179:1978–1987. doi: 10.1016/j.ajpath.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones L.A., Harding J.A., Cohen A.S., Skinner M. New USA family has apolipoprotein AI (Arg26) variant. In: Natvig J.B., Førre Ø., Husby G., editors. Amyloid and Amyloidosis 1990. Kluwer Academic Publishers; Dordrecht: 1991. pp. 385–388. [Google Scholar]
- 9.Soutar A.K., Hawkins P.N., Vigushin D.M. Apolipoprotein AI mutation Arg-60 causes autosomal dominant amyloidosis. Proc Natl Acad Sci USA. 1992;89:7389–7393. doi: 10.1073/pnas.89.16.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth D.R., Tan S.Y., Booth S.E. A new apolipoprotein AI variant, Trp50Arg, causes hereditary amyloidosis. QJM. 1995;88:695–702. [PubMed] [Google Scholar]
- 11.Booth D.R., Tan S.Y., Booth S.E. Hereditary hepatic and systemic amyloidosis caused by a new deletion/insertion mutation in the apolipoprotein AI gene. J Clin Invest. 1996;97:2714–2721. doi: 10.1172/JCI118725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persey M.R., Booth D.R., Booth S.E. Hereditary nephropathic systemic amyloidosis caused by a novel variant apolipoprotein A-I. Kidney Int. 1998;53:276–281. doi: 10.1046/j.1523-1755.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamidi Asl K., Liepnieks J.J., Nakamura M. A novel apolipoprotein A-1 variant, Arg173Pro, associated with cardiac and cutaneous amyloidosis. Biochem Biophys Res Commun. 1999;257:584–588. doi: 10.1006/bbrc.1999.0518. [DOI] [PubMed] [Google Scholar]
- 14.Hamidi Asl L., Liepnieks J.J., Hamidi Asl K. Hereditary amyloid cardiomyopathy caused by a variant apolipoprotein A1. Am J Pathol. 1999;154:221–227. doi: 10.1016/S0002-9440(10)65268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obici L., Bellotti V., Mangione P. The new apolipoprotein A-I variant Leu174 → Ser causes hereditary cardiac amyloidosis, and the amyloid fibrils are constituted by the 93-residue N-terminal polypeptide. Am J Pathol. 1999;155:695–702. doi: 10.1016/S0002-9440(10)65167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Sousa M.M., Vital C., Ostler D. Apolipoprotein AI and transthyretin as components of amyloid fibrils in a kindred with apoAI Leu178His amyloidosis. Am J Pathol. 2000;156:1911–1917. doi: 10.1016/S0002-9440(10)65064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy C.L., Wang S., Weaver K. Renal apolipoprotein A-I amyloidosis associated with a novel mutant Leu64Pro. Am J Kidney Dis. 2004;44:1103–1109. doi: 10.1053/j.ajkd.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Obici L., Palladini G., Giorgetti S. Liver biopsy discloses a new apolipoprotein A-I hereditary amyloidosis in several unrelated Italian families. Gastroenterology. 2004;126:1416–1422. doi: 10.1053/j.gastro.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Benson M.D., Liepnieks J.J., Yazaki M. A new human hereditary amyloidosis: the result of a stop-codon mutation in the apolipoprotein AII gene. Genomics. 2001;72:272–277. doi: 10.1006/geno.2000.6499. [DOI] [PubMed] [Google Scholar]
- 20.Pepys M.B., Hawkins P.N., Booth D.R. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature. 1993;362:553–557. doi: 10.1038/362553a0. [DOI] [PubMed] [Google Scholar]
- 21.Gillmore J.D., Booth D.R., Madhoo S. Hereditary renal amyloidosis associated with variant lysozyme in a large English family. Nephrol Dial Transplant. 1999;14:2639–2644. doi: 10.1093/ndt/14.11.2639. [DOI] [PubMed] [Google Scholar]
- 22.Maury C.P.J., Kere J., Tolvanen R., de la Chapelle A. Finnish hereditary amyloidosis is caused by a single nucleotide substitution in the gelsolin gene. FEBS Lett. 1990;276:75–77. doi: 10.1016/0014-5793(90)80510-p. [DOI] [PubMed] [Google Scholar]
- 23.Ghiso J., Haltia M., Prelli F. Gelsolin variant (Asn-187) in familial amyloidosis, Finnish type. Biochem J. 1990;272:827–830. doi: 10.1042/bj2720827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy E., Haltia M., Fernandez-Madrid I. Mutation in gelsolin gene in Finnish hereditary amyloidosis. J Exp Med. 1990;172:1865–1867. doi: 10.1084/jem.172.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efebera Y.A., Sturm A., Baack E.C. Novel gelsolin variant as the cause of nephrotic syndrome and renal amyloidosis in a large kindred. Amyloid. 2014;21:110–112. doi: 10.3109/13506129.2014.891502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasr SH, Dasari S, Hasadsri L, et al. Novel type of renal amyloidosis derived from apolipoprotein-CII [e-pub ahead of print]. J Am Soc Nephrol. pii: ASN.2015111228. Accessed July 2016. [DOI] [PMC free article] [PubMed]
- 27.Valleix S., Verona G., Jourde-Chiche N. D25V apolipoprotein C-III variant causes dominant hereditary systemic amyloidosis and confers cardiovascular protective lipoprotein profile. Nat Commun. 2016;7:10353. doi: 10.1038/ncomms10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillmore J.D., Stangou A.J., Lachmann H.J. Organ transplantation in hereditary apolipoprotein AI amyloidosis. Am J Transplant. 2006;6:2342–2347. doi: 10.1111/j.1600-6143.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- 29.Gilbertson J.A., Hunt T., Hawkins P.N. Amyloid typing: experience from a large referral centre. In: Picken M.M., Dogan A., Herrera G.A., editors. Amyloid and Related Disorders. Humana Press; Totowa, NJ: 2012. pp. 231–238. [Google Scholar]
- 30.Rodriguez F.J., Gamez J.D., Vrana J.A. Immunoglobulin derived depositions in the nervous system: novel mass spectrometry application for protein characterization in formalin-fixed tissues. Lab Invest. 2008;88:1024–1037. doi: 10.1038/labinvest.2008.72. [DOI] [PubMed] [Google Scholar]
- 31.Perkins D.N., Pappin D.J., Creasy D.M., Cottrell J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Talmud P., Tybjaerg-Hansen A., Bhatnagar D. Rapid screening for specific mutations in patients with a clinical diagnosis of familial hypercholesterolaemia. Atherosclerosis. 1991;89:137–141. doi: 10.1016/0021-9150(91)90053-6. [DOI] [PubMed] [Google Scholar]
- 33.Gillmore J.D., Lachmann H.J., Wechalekar A., Hawkins P.N. Hereditary fibrinogen A alpha-chain amyloidosis: clinical phenotype and role of liver transplantation. Blood. 2010;115:4313. doi: 10.1182/blood-2010-01-261750. author reply 4314–4315. [DOI] [PubMed] [Google Scholar]
- 34.Bybee A., Kang H.G., Ha I.S. A novel complex indel mutation in the fibrinogen Aα chain gene in an Asian child with systemic amyloidosis. In: Grateau G., Kyle R.A., Skinner M., editors. Amyloid and Amyloidosis. CRC Press; Boca Raton, FL: 2005. p. 315. [Google Scholar]
- 35.Gillmore J.D., Booth D.R., Rela M. Curative hepatorenal transplantation in systemic amyloidosis caused by the Glu526Val fibrinogen α-chain variant in an English family. QJM. 2000;93:269–275. doi: 10.1093/qjmed/93.5.269. [DOI] [PubMed] [Google Scholar]
- 36.Lachmann H.J., Booth D.R., Booth S.E. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786–1791. doi: 10.1056/NEJMoa013354. [DOI] [PubMed] [Google Scholar]