Introduction
Leukemia is among the most common malignancies associated with hospital admissions for acute kidney injury (AKI).1, 2 AKI develops in up to one-third of patients affected by hematologic malignancy, particularly in older patients with prior chronic kidney disease (CKD),3 and has been shown to have an adverse effect on long-term prognosis in this patient population.4 Intravascular volume depletion, tumor lysis syndrome, and drug-induced acute tubular injury are among the most common causes of leukemia-associated AKI.4, 5 Radiologic studies may be useful to exclude obstructive uropathy secondary to retroperitoneal lymphadenopathy or tumorous masses. Direct infiltration of the renal parenchyma by leukemic cells, although a common finding at autopsy,5 is only rarely associated with the development of symptomatic AKI.4 Rare cases of AKI secondary to intravascular leukostasis have also been described, particularly in patients with white blood cell counts greater than 100,000/mm3.6 Lysozyme-induced nephropathy (LyN) is a rare and underrecognized complication of chronic myelomonocytic leukemia (CMML) and other forms of monocytic leukemia in which lysozyme, a small cationic protein, is released into the circulation, filtered by the glomerulus and reabsorbed by the proximal tubule, causing toxic tubular injury.7
Case Presentation
Clinical History and Initial Laboratory Data
A 69-year-old white man with a history of CKD (baseline serum creatinine, 2.0 mg/dl; estimated glomerular filtration rate, 35 ml/min/1.73 m2) and well-controlled HIV infection (CD4 count, 500 cells/mm3; viral load, <20 copies/ml) presented to the emergency department after 10 days of watery diarrhea. Two months prior, he had been diagnosed with CMML, but had not commenced treatment. Maintenance antiretroviral therapy included darunavir, emtricitibine, and ritonavir. Physical examination findings were notable for a blood pressure of 119/63 mm Hg, splenomegaly, and the absence of edema.
Initial laboratory evaluation (Table 1) revealed markedly elevated serum creatinine (10.9 mg/dl; estimated glomerular filtration rate, 5 ml/min/1.73 m2) associated with oliguria. Complete blood count revealed leukocytosis, anemia, and thrombocytopenia. Urinalysis revealed 3+ protein by dipstick. Urine protein:creatinine ratio was 6.7 g/g on a spot measurement. Urinary microscopy, performed on a specimen collected after Foley catheter insertion, showed 21 to 30 red blood cells and 31 to 40 white blood cells per high-power field, but no cellular casts or crystals. Serologic workup results were negative (Table 1). The patient showed no improvement in renal function with volume resuscitation. In light of the additional laboratory findings of hyperuricemia with hyperphosphatemia, hypocalcemia, and elevated lactate dehydrogenase, the patient was started on dialysis and allopurinol for suspected tumor lysis syndrome. A kidney biopsy was performed on the 10th hospital day.
Table 1.
Initial laboratory findings
| Parameter | Value (reference range) |
|---|---|
| SCr (mg/dl) | 10.9 (0.6–1.2) |
| eGFR (ml/min/1.73 m2) | 5 (>90) |
| Serum urea nitrogen (mg/dl) | 72 (8–20) |
| Serum potassium (mmol/l) | 4.4 (3.5–5.5) |
| Serum uric acid (mg/dl) | 25 (3.8–8.0) |
| Serum phosphorus (mg/dl) | 9.7 (2.4–4.1) |
| Serum calcium (mg/dl) | 7.1 (8.5–10.2) |
| Serum albumin (g/dl) | 2.5 (3.5–5.1) |
| LDH (IU/l) | 1100 (140–280) |
| Hemoglobin (g/dl) | 9 (12.0–16.0) |
| WBC count (× 103/μl) | 67.4 (4.5–13.5) |
| Differential blood count (%) | |
| Neutrophils | 48 (40–70) |
| Monocytes | 39 (0–10) |
| Lymphocytes | 9 (20–50) |
| Eosinophils | 0 (0–6) |
| Urine dipstick protein | 3+ |
| Urine RBC (/hpf) | 21–30 (none)a |
| Urine WBC (/hpf) | 31–40 (0–2)a |
| Spot urine PCR (g/g) | 6.7 (<0.3) |
| Urine culture | No growth |
| C3 (mg/dl) | 124 (88–165) |
| C4 (mg/dl) | 37 (14–44) |
| ANA | Neg (neg) |
| MPO-ANCA | <1:20 (<1:20) |
| PR3-ANCA | <1:20 (<1:20) |
| Hepatitis C antibody | Neg (neg) |
| Anti-GBM antibody | Neg (neg) |
| Hepatitis B core antigen | Neg (neg) |
| Serum cryoglobulins | Neg (neg) |
| SPEP | No M-spike |
ANA, anti−nuclear antibody; ANCA, anti−neutrophil cytoplasmic antibody; anti-GBM, anti−glomerular basement membrane; eGFR, estimated glomerular filtration rate; hpf, high-power field; LDH, lactate dehydrogenase; MPO, myeloperoxidase; Neg, negative; PCR, protein:creatinine ratio; RBC, red blood cell; SCr, serum creatinine; SPEP, serum protein electrophoresis WBC, white blood cell.
Conversion factors for units: SCr in mg/dl to μmol/l, ×88.4; SUN in mg/dl to mmol/l, ×0.357.
Results obtained from Foley catheter−collected urine.
Kidney Biopsy Results
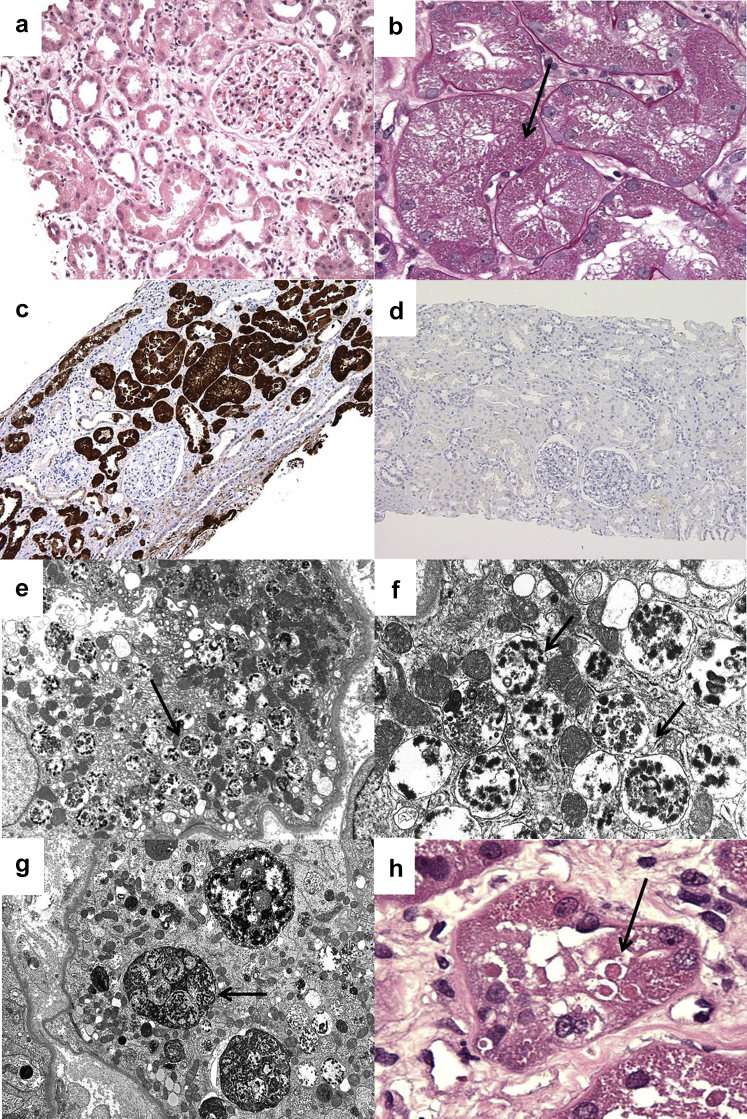
The sampling for light microscopy included 16 glomeruli, 2 of which were globally sclerotic. The remaining 14 glomeruli appeared largely unremarkable, and no lesions of focal segmental glomerulosclerosis were identified. The predominant abnormalities involved proximal tubular epithelial cells, which exhibited widespread degenerative changes including luminal ectasia, cytoplasmic simplification and vacuolization, irregular luminal profiles, loss of brush border, enlarged nuclei with prominent nucleoli, and focal apoptotic figures (Figure 1a). The proximal tubular injury was accompanied by mild interstitial edema and mild interstitial inflammation composed of lymphocytes and monocytes. Many proximal tubular cells had hypereosinophilic granular cytoplasm owing to the presence of abundant intracytoplasmic, PAS-positive granules (Figure 1b). Scattered larger rounded, eosinophilic inclusions that were moderately periodic acid–Schiff positive and nonargyrophilic with the Jones methenamine silver stain were also seen (Figure 1h). Immunohistochemical staining for lysozyme (Lysozyme EP134; RabMAb, Rocklin, CA) revealed strong positivity in the distribution of the proximal tubular cell cytoplasm (Figure 1c, d). No atypical casts or intracytoplasmic crystalline-type inclusions were seen. Mild tubular atrophy and interstitial fibrosis involved 15% of the cortex sampled.
Figure 1.
A low-power view demonstrates widespread proximal tubular degenerative changes and interstitial edema. (a) A glomerulus appears unremarkable (hematoxylin and eosin, original magnification ×200). (b) At higher magnification, many proximal tubular cells are distended by numerous small intracytoplasmic PAS+ granules (arrow; periodic acid–Schiff, original magnification ×400). (c) Immunohistochemial staining for lysozyme shows intense granular reactivity in the distribution of proximal tubular cell cytoplasm (immunoperoxidase, original magnification ×100). (d) No significant staining is seen in a paired negative control obtained from an allograft postreperfusion biopsy (immunoperoxidase, original magnification ×100). (e) On ultrastructural evaluation, proximal tubular cells contain abundant membrane-bound vacuoles (arrow; original magnification ×8000). (f) On closer inspection, the vacuoles in proximal tubules contain clumped, degenerating organellar debris, consistent with autophagolysosomes (arrow; original magnification ×25,000). (g) In rare cells, the autophagolysomes appear to form larger, membrane-bound aggregates (arrow; original magnification ×6,000), corresponding to the (h) scattered large round eosinophilic inclusions seen in a minority of cells (arrow; hematoxylin and eosin, original magnification ×600).
Immunofluorescence staining for IgG, IgM, IgA, C3, C1, fibrinogen, albumin, and kappa (κ) and lambda (λ) light chains revealed no significant positivity. Immunofluorescence staining for κ and λ light chains repeated on pronase-digested paraffin tissue sections again was negative, providing evidence against light chain proximal tubuloapthy.
On ultrastructural evaluation, glomeruli exhibited no significant abnormalities. Specifically, there was only 10% foot process effacement, and no electron-dense deposits were seen. Proximal tubules displayed diffuse degenerative changes including loss of apical brush border, cytoplasmic simplification, dilatation of the endoplasmic reticulum, and intraluminal cellular debris. The most distinctive finding was abundant membrane-bound cytoplasmic vacuoles containing clumped, electron-dense, degenerating cellular organellar debris, consistent with autophagolysosomes (Figure 1e, f). The autophagolysosomes were diffusely distributed in proximal tubular epithelia and in some instances formed larger, membrane-bound aggregates that approached the size of the nucleus (Figure 1g). No intracellular crystals or dysmorphic mitochondria were identified.
Diagnosis
A diagnosis of lysozyme-induced nephropathy (LyN) was made.
Clinical Follow-up
Additional testing revealed markedly elevated serum lysozyme levels (101 μg/ml; nl range 2.7–9.4) and lysozymuria (>11 μg/ml; nl <3), supporting a diagnosis of lysozyme-induced nephropathy. The patient was started on chemotherapy with azacitidine, resulting in normalization of his white blood cell count. Serum lysozyme levels remained elevated 4 months after initiation of chemotherapy, but ultimately decreased to within reference range. The patient remained dialysis dependent from the time of kidney biopsy, and expired 18 months later in the setting of relapsing leukemia with blast crisis.
Discussion
CMML is a rare, aggressive, malignant, hematopoietic neoplasm that most commonly affects patients over the age of 65 years and is characterized by peripheral blood monocytosis with myelodysplastic features. Lysozyme, also known as muramidase, is a lytic enzyme with bactericidal properties that is synthesized by monocytes and can be produced in large amounts by neoplastic cells of monocyte lineage.7 Increased serum and urine lysozyme levels (lysozymuria) were first described in patients with monocytic or myelomonocytic leukemia, including both acute and chronic forms, in the late 1960s.8 Lysozyme-induced nephropathy (LyN) is a rare and underrecognized cause of AKI in a subset of these patients, particularly those with CMML.3, 7, 9
Lysozyme is a small cationic protein (molecular weight, 15 kDa) that is freely filtered by the glomerulus.8 Lysozyme is reabsorbed in the proximal tubule, where it is taken up by endocytosis and catabolized in phagolysosomes.10 Despite the proximal tubules’ high absorptive capacity for lysozyme,11, 12 marked overproduction of lysozyme in patients with monocytic leukemias may exceed the transport maximum, leading to nephrotic-range nonalbumin proteinuria, as appears to have occurred in this case.13, 14 By protein electrophoresis, lysozyme migrates in the gamma (γ) region. Therefore, the presence of nonalbumin proteinuria with an increased γ-globulin fraction but without detectable monoclonal bands by immunofixation electrophoresis may be a useful indicator of lysozymuria in the appropriate clinical context.15 Unfortunately, these studies were not performed.
In the setting of monocytic and myelomonocytic leukemias, including chronic and acute forms, a steady proportion of the filtered load of lysozyme accumulates in proximal tubular cells, allowing the kidney to act as a reservoir for circulating lysozyme.12 There appears to be a threshold above which the concentration of lysozyme, a lytic enzyme with bactericidal properties,7 becomes toxic to proximal tubular cells, leading to the development of acute tubular injury and AKI. Indeed, lysozymuria can impair proximal tubular cell function,16 leading to renal insufficiency, hypokalemia secondary to renal potassium wasting, and low-level tubular albuminuria, but not Fanconi syndrome.9, 17 However, elevated serum and urine lysozyme levels commonly occur in patients with monocytic and myelomonocytic leukemias and are usually not associated with the development of AKI.16, 18 Therefore, kidney biopsy is needed to establish a definitive diagnosis of LyN.
Descriptions of the pathologic findings in LyN are limited. An early report described tubular degenerative changes accompanied by hyaline droplets in proximal tubules, but also described hyaline droplets in patients with monocytic or myelomonocytic leukemia and intact renal function.16 A more recent report described coarse protein granules in proximal tubules by light microscopy, corresponding with the ultrastructural finding of large and prominent but relatively isomorphic lysosomes.7 In the case reported herein, the direct toxicity of lysozyme was associated with the distinctive ultrastructural finding of abundant autophagolysosomes containing degenerating organellar debris and strong immunostaining of proximal tubules for lysozyme. Similar ultrastructural findings have been described in rats transplanted with chloroleukemia, a myelomonoblastic tumor that also secretes large amounts of lysozyme, leading to lysozymuria.19
Other causes of AKI should be excluded in patients with CMML. Laboratory evaluation of our patient also revealed hyperuricemia, mild hyperphosphatemia, and mild hypocalcemia, raising the possibility of concurrent tumor lysis syndrome. However, no intratubular deposition of uric acid crystals or calcium phosphate salts was seen on kidney biopsy. Nonetheless, elevated serum uric acid levels may still have contributed to AKI in our patient, as hyperuricemia may reduce renal plasma flow and may inhibit proximal tubular cell proliferation.20 Although unlikely in the case reported herein, alternative etiologies of lysozymuria should be considered. Heavy lysozymuria may occur rarely as a form of overflow proteinuria in the setting of other disease states associated with macrophage activation and overproduction of lysozyme (including sarcoidosis) or at very low levels in the setting of tubular proteinuria related to proximal tubular cell dysfunction.7, 9
The treatment of CMML is initially supportive; however, the development of leukemia-associated organ damage may be an indication for more aggressive treatment, including cytoreductive therapy or use of hypomethylating agents.21 Variable improvement in kidney function has been described in patients receiving CMML-specific chemotherapy who have direct leukemic infiltration or overproduction of lysozyme by the malignant cells.22 Although our patient had a good hematologic response to chemotherapy, he remained dialysis dependent.
Conclusion
In conclusion, acute and chronic monocytic and myelomonocytic neoplasms, most notably CMML, are associated with overproduction of lysozyme, resulting in elevated serum and urine levels. Lysozyme is freely filtered by the glomerulus, and can be associated with nephrotic-range lysozymuria. Lysozyme accumulates in proximal tubular cells, and there is a threshold at which this accumulation is associated with renal potassium wasting, toxic proximal tubular injury, and AKI. LyN is a rare and likely underrecognized etiology of AKI that has a distinctive pathologic appearance characterized by acute tubular necrosis, with increased cytoplasmic granules in proximal tubular cells at the light microscopic level, corresponding to abundant phagolysosomes containing partially digested organellar debris at the ultrastructural level, with strong proximal tubular cytoplasmic staining for lysozyme (Table 2). Recognition of this rare etiology of AKI may guide chemotherapeutic intervention in the management of acute and chronic monocytic and myelomonocytic leukemias.
Table 2.
Lysozyme-induced nephropathy (LyN): teaching points
|
|
|
|
Disclosure
All the authors declared no competing interests.
References
- 1.Salahudeen A.K., Doshi S.M., Pawar T. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol. 2013;8:347–354. doi: 10.2215/CJN.03530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen C.F., Johansen M.B., Langeberg W.J. Incidence of acute kidney injury in cancer patients: a Danish population-based cohort study. Euro J Intern Med. 2011;22:399–406. doi: 10.1016/j.ejim.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Ganguli A., Sawinski D., Berns J.S. Kidney diseases associated with haematological cancers. Nat Rev Nephrol. 2015;1:478–490. doi: 10.1038/nrneph.2015.81. [DOI] [PubMed] [Google Scholar]
- 4.Canet E., Zafrani L., Lambert J. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: impact on remission and survival. PLoS One. 2013;8:e55870. doi: 10.1371/journal.pone.0055870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luciano R.L., Brewster U.C. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis. 2014;21:27–35. doi: 10.1053/j.ackd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Perazella M.A., Eisen R.N., Frederick W.G., Brown E. Renal failure and severe hypokalemia associated with acute myelomonocytic leukemia. Am J Kidney Dis. 1993;22:462–467. doi: 10.1016/s0272-6386(12)70154-3. [DOI] [PubMed] [Google Scholar]
- 7.Patel T.V., Rennke H.G., Sloan J.M. A forgotten cause of kidney injury in chronic myelomonocytic leukemia. Am J Kidney Dis. 2009;54:159–164. doi: 10.1053/j.ajkd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osserman E.F., Lawlor D.P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124:921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muggia F.M., Heinemann H.O., Farhangi M., Osserman E.F. Lysozymuria and renal tubular dysfunction in monocytic and myelomonocytic leukemia. Am J Med. 1969;47:351–366. doi: 10.1016/0002-9343(69)90219-8. [DOI] [PubMed] [Google Scholar]
- 10.Ottosen P.D., Bode F., Madsen K.M., Maunsbach A.B. Renal handling of lysozyme in the rat. Kidney Int. 1979;15:246–254. doi: 10.1038/ki.1979.32. [DOI] [PubMed] [Google Scholar]
- 11.Cojocel C., Baumann K. Renal handling of endogenous lysozyme in the rat. Ren Physiol. 1983;6:258–265. doi: 10.1159/000172910. [DOI] [PubMed] [Google Scholar]
- 12.Osserman E.F., Canfield R.E., Beychok S., Columbia University. Institute of Cancer Research . Academic Press; New York: 1974. Lysozyme [proceedings] [Google Scholar]
- 13.Aguado M.J., Garcia de Bustos J., Ojeda E. Proteinuria caused by lysozymuria mimics nephrotic syndrome. Nephron. 2000;86:183. doi: 10.1159/000045738. [DOI] [PubMed] [Google Scholar]
- 14.Mok C.C., Tam S.C., Kwong Y.L. Pseudonephrotic syndrome caused by lysozymuria. Ann Intern Med. 1994;121:818. doi: 10.7326/0003-4819-121-10-199411150-00020. [DOI] [PubMed] [Google Scholar]
- 15.Levinson S.S., Elin R.J., Yam L. Light chain proteinuria and lysozymuria in a patient with acute monocytic leukemia. Clin Chem. 2002;48:1131–1132. [PubMed] [Google Scholar]
- 16.Pruzanski W., Platts M.E. Serum and urinary proteins, lysozyme (muramidase), and renal dysfunction in mono- and myelomonocytic leukemia. J Clin Invest. 1970;49:1694–1708. doi: 10.1172/JCI106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickering T.G., Catovsky D. Hypokalaemia and raised lysozyme levels in acute myeloid leukaemia. Q J Med. 1973;42:677–682. [PubMed] [Google Scholar]
- 18.Wiernik P.H., Serpick A.A. Clinical significance of serum and urinary muramidase activity in leukemia and other hematologic malignancies. Am J Med. 1969;46:330–343. doi: 10.1016/0002-9343(69)90036-9. [DOI] [PubMed] [Google Scholar]
- 19.Klockars M., Azar H.A., Hermida R. The relationship of lysozyme to the nephropathy in chloroleukemic rats and the effects of lysozyme loading on normal rat kidneys. Cancer Res. 1974;34:47–60. [PubMed] [Google Scholar]
- 20.Wilson F.P., Berns J.S. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21:18–26. doi: 10.1053/j.ackd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onida F., Barosi G., Leone G. Management recommendations for chronic myelomonocytic leukemia: consensus statements from the SIE, SIES, GITMO groups. Haematologica. 2013;98:1344–1352. doi: 10.3324/haematol.2013.084020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aribi A., Borthakur G., Ravandi F. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109:713–717. doi: 10.1002/cncr.22457. [DOI] [PubMed] [Google Scholar]