Abstract
Introduction
The incidence of acute kidney injury (AKI) in hospitalized patients is rising, and survivors are at high risk for cardiovascular events and mortality. Effective strategies that improve long-term outcomes of AKI are unknown.
Methods
A retrospective cohort study was performed between 2008 and 2011. All subjects were followed until 31 March 2013, with a minimum follow-up of 2 years. Participants were adults 18 years of age or older, who developed AKI during a hospitalization and had chronic kidney disease (CKD) following discharge (n = 19,707 mean age 69.9 years, mean postdischarge estimated glomerular filtration rate (eGFR) 43.0 ml/min/1.73 m2). Exposure to statins was examined prior to the index hospitalization as well as within 2 years following hospital discharge. The primary outcome was mortality; secondary outcomes included all-cause re-hospitalization and cardiovascular events.
Results
Within 2 years of discharge, only 38.3% of the participants were prescribed a statin. After adjustment for comorbidities, statin use prior to admission, demographics, baseline kidney function, and a number of other factors, statin use was associated with lower mortality (hazard ratio, 0.74; 95% confidence interval, 0.69, 0.79) in AKI survivors with CKD. Patients who received a statin also had a lower risk of all cause rehospitalization (adjusted hazarad ratio, 0.90; 95% confidence interval, 0.85, 0.94). Statin use was not associated with a reduction in cardiovascular events.
Discussion
Among AKI survivors with CKD, statin use was associated with a lower risk of mortality and rehospitalization rates. This finding suggests that there is an opportunity to improve postdischarge care in AKI survivors.
Keywords: acute kidney injury, cardiovascular, mortality, statins
Acute kidney injury (AKI) is a common complication in hospitalized patients, and has been consistently associated with an increased risk of death, de novo or worsening chronic kidney disease (CKD), and end-stage renal disease (ESRD).1, 2, 3, 4, 5 Patients discharged after an episode of AKI have a 40% increased risk of death in the 2 years following hospitalization,6 and a 50% to 60% increased risk of cardiovascular events, compared to patients who do not develop AKI.7 There are currently no effective therapies targeting established AKI; however, identifying and treating patients with CKD following an episode of AKI may improve health outcomes. Although recently published data suggested that nephrologist follow-up was associated with a 24% reduction in risk of death after hospitalization with severe AKI requiring dialysis,8 little is known about processes of care that modify outcomes after episodes of AKI.
Statins are effective for reducing cardiovascular morbidity and mortality in patients with CKD.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 We sought to evaluate whether the use of statins was associated with better outcomes among patients with CKD after AKI.
Methods
Study Population and Data Sources
We used the Alberta Kidney Disease Network population-based database, which has been described in detail elsewhere.19 The study cohort20 included all adults 18 years or older residing in Alberta who were admitted to the hospital between 1 July 2008 and 31 March 2011 and had an episode of AKI during hospitalization. To be eligible for inclusion, patients had to have at least 1 outpatient serum creatinine (Scr) measurement within 180 days prior to hospitalization to establish baseline kidney function, ≥ 1 measurement during the hospitalization to establish AKI, and ≥ 1 SCr, urine dipstick (udip), urine albumin to serum creatinine ratio (ACR), or urine protein to serum creatinine ratio (PCR) measurement in the follow-up period after hospital discharge to establish CKD. If participants had more than 1 hospitalization during this period, only the first was considered (index hospitalization). Participants who died or progressed to ESRD (estimated GFR [eGFR] < 15 ml/min per 1.73 m2, chronic dialysis, prior kidney transplantation) before or during the index hospitalization were excluded. The cohort was restricted to patients who had CKD after an AKI episode. All subjects were followed up from the discharge date of their index hospitalization until 31 March 2013, with a minimum follow-up of 2 years.
The study was reviewed and approved by the institutional review boards at the Universities of Alberta and Calgary.
Assessment of Baseline Kidney Function
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to determine the estimated glomerular filtration rate (eGFR).21 Baseline kidney function was defined as the mean outpatient Scr in the 180 days prior to the index hospitalization.
Identification of Acute Kidney Injury
AKI was identified by changes between baseline (pre-hospital) and peak in-hospital Scr. AKI was defined as an increase in serum creatinine by 50% or greater within 7 days or by 26.5 μmol/L within 48 hours and/or requirement for acute dialysis within 7 days of the index hospitalization. AKI severity was determined using the consensus criteria for AKI staging from the recently published Kidney Disease Improving Global Outcomes (KDIGO) AKI guidelines.22 Requirement for acute dialysis for AKI was determined using a validated approach, based on diagnosis and procedural administrative codes.23
Assessment of CKD After AKI
The presence of CKD was assessed using Scr, urine ACR, urine PCR, or udip measured 90 days or more after the AKI episode to allow sufficient time for recovery of renal function (Supplementary Figure S1). A 90-day time frame for recovery was chosen based on the Kidney Disease Outcomes Quality Initiative guidelines,24 which define CKD as a persistent decline in kidney function lasting >90 days. If subjects had more than 1 outpatient SCr measurement, the measurement closest to 90 days was considered. Postdischarge CKD was defined as eGFR < 60 ml/min/1.73 m2, ACR > 30 mg/g, PCR > 150 mg/g, or Udip > trace, consistent with the KDIGO CKD guidelines.25
Assessment of Medication Use After Discharge
Prescription drug information was obtained from the Pharmaceutical Information Network (PIN) database. We classified statin exposure into the following groups: no previous prescription, new prescription (defined as at least 1 prescription within 2 years after discharge from the index hospitalization), stopping a previous prescription, and continuing a prescription. Patients were classified in the continuing prescription group if they had at least 1 prescription in the 6 months prior to admission and at least 1 prescription within 2 years postdischarge. High-dose statin was defined as the highest dosage for each statin drug (Supplementary Table S1).
Assessment of Comorbid Conditions
Relevant demographic characteristics, preexisting comorbid conditions (defined using validated algorithms),20, 26 hospitalizations and outpatient physician visits (general practitioner as well as specialist visits), details of the index hospitalization including primary admission diagnosis, and intensive care unit stay were obtained using hospitalization data, claims files, and ambulatory care classification system files. We obtained primary International Classification of Diseases (10th revision) codes and used these to classify primary admission diagnoses using a previously published approach.26 Resource intensity weight, similar to diagnostic related group weight, was used to categorize acuity and severity of illness.27, 28 Cholesterol level was defined as the mean outpatient total cholesterol in the 1 year prior to the index hospitalization. The cholesterol levels were classified into 5 risk categories according to the Framingham coronary heart disease risk score.29 Patients who did not have a cholesterol measurement during this time period were classified in an unknown group.
Outcomes
The primary outcome was mortality; secondary outcomes included all-cause re-hospitalization and cardiovascular events. All outcomes were assessed after the discharge date of the index hospitalization. All-cause re-hospitalization was defined as any hospitalization occurring after the index hospitalization. All hospitalizations were identified using AH data. All-cause mortality was identified using administrative data sources. Cardiovascular events were defined as myocardial infarction, stroke, or revascularization procedure, as defined by validated algorithms.30, 31, 32
Statistical Analysis
Continuous variables were described using the mean and SD or the median with interquartile range as appropriate. Categorical variables were described as proportions of the cohort.
Multivariable Cox proportional hazard models with time-varying covariates were used to estimate the association between use of statin following index hospitalization and all-cause mortality, re-hospitalization, and cardiovascular events. The adjusted models included terms for age, sex, income quintile, aboriginal race, location of residency, health care use preceding the index hospitalization, Canadian Institute for Health Information resource intensity weight, intensive care unit admission during the index hospitalization, primary diagnostic code for hospitalization, procedures associated with AKI (cardiac catheterization, cardiac and abdominal aortic surgery), comorbid conditions, baseline kidney function (based on eGFR), cholesterol level, statin, angiotensin-converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB), and β-blocker use in the 6 months preceding admission and following discharge (Supplementary Figure S2). Time of origin was the discharge date for the index hospitalization. The proportional hazard assumption was evaluated and satisfied by examining plots of the log-negative-log within-group survivorship functions versus log-time.
First use of statin was used to update the exposure status during the course of the follow-up (i.e., a person on a statin would contribute person time to the “no statin use” before the first statin was prescribed and contribute person time to the “statin use” group after the first statin was prescribed). Patients were censored if they moved out of the province or reached the end of the study date (31 March 2013) for all outcomes. For the secondary outcomes, patients were censored if they died.
Analyses were repeated after further categorizing statin users into the following groups: new prescription postdischarge, continuing a previous prescription within 2 years postdischarge, and stopping use of a pre-hospital admission prescription. In the sensitivity analysis, we excluded patients who required acute dialysis to determine whether associations were similar in patients with less severe AKI. We also performed a sensitivity analysis excluding patients with proteinuria at any time during the study period to assess whether associations were similar in patients with non-proteinuric CKD. We repeated analyses after excluding patients who had any pre-existing cardiovascular disease, to examine findings in patients less likely to have pre-existing established cardiovascular disease. We compared the effect of statin use after stratifying the cohort according to whether patients had pre-existing CKD or de novo CKD (post-AKI) patients. We also compared outcomes in patients on high-dose versus low-dose statins.
Results
Patient Characteristics
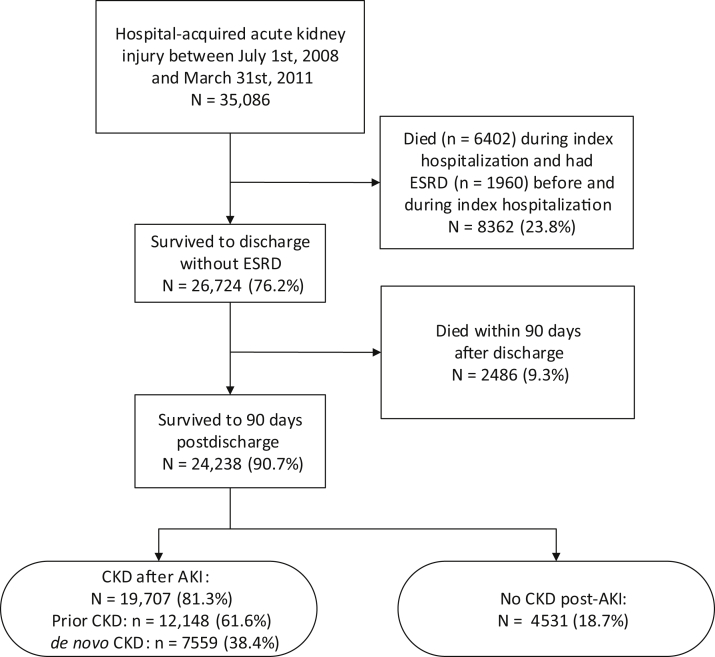
Between 1 July 2008 and 31 March 2011, there were 35,086 patients 18 years of age or older residing in Alberta with hospital-acquired AKI (Figure 1). Of these participants, 26,724 (76.2%) survived to 90 days after their AKI episode. The study cohort included 19,707 patients with CKD after the AKI episode: 12,148 (61.6%) had prior CKD and 7,559 (38.4%) had de novo CKD (Figure 1). The mean patient age was 69.9 years, 52.5% were men, and 83.7% lived in an urban location (Table 1). The mean number of hospitalizations during the 3 years preceding the index hospitalization was 1.7 (interquartile range [IQR] 0−2), and 18.4% of the cohort had a cardiovascular diagnostic code as the diagnosis most responsible for the index hospitalization. The majority of participants (80.9%) had hypertension, and a large number of patients had diabetes (42.6%), chronic heart failure (33.4%), and history of stroke or TIA (23.1%).
Figure 1.
Selection of study population after episode of hospital-acquired acute kidney injury (AKI). CKD, chronic kidney disease; ESRD, end-stage renal disease.
Table 1.
Characteristics of statin users and non−statin users
| All subjects | Statin users | Non−statin users | P value | |
|---|---|---|---|---|
| Number of subjects (%) | 19,707 | 7539 (38.3) | 12,168 (61.7) | |
| Age, yr, mean (SD) | 69.9 (14.9) | 70.7 (12) | 69.3 (16.5) | <0.01 |
| Sex, male (%) | 52.5 | 57.6 | 49.4 | <0.01 |
| Aboriginal (%) | 3.8 | 3.2 | 4.2 | <0.01 |
| Income quintile | 0.59 | |||
| Lowest (level = 1) (%) | 24.9 | 24.6 | 25.1 | |
| Highest (level = 5) (%) | 15.9 | 15.6 | 16.0 | |
| Urban location (%) | 83.7 | 84.3 | 83.2 | 0.04 |
| Healthcare access 3-year preceding hospital admission, mean (median, IQR) | ||||
| Number of hospitalizations | 1.7 (1, 0–2) | 1.7 (1, 0–2) | 1.8 (1, 0–2) | 0.02 |
| Number of GP visits | 50.8 (36, 21–64) | 48.2 (37, 22–61) | 52.4 (36, 20–66) | <0.01 |
| Number of nephrologist visits | 1.1 (0, 0–0) | 1.2 (0, 0–0) | 1 (0, 0–0) | <0.01 |
| Number of cardiology visits | 3.1 (0, 0–2) | 4.6 (0, 0–5) | 2.2 (0, 0–1) | <0.01 |
| Number of internist visits | 7.2 (3, 0–8) | 7.7 (3, 1–9) | 6.9 (2, 0–8) | <0.01 |
| Number of emergency visits | 5.8 (3, 1–6) | 5.5 (3, 1–6) | 6 (3, 1–7) | <0.01 |
| CIHI resource intensity weight, mean (SD) | 3.1 (6.3) | 2.7 (4.4) | 3.4 (7.2) | <0.01 |
| Intensive care unit during hospitalization (%) | 18.9 | 24.8 | 15.3 | <0.01 |
| Primary diagnostic code for hospitalization (%) | ||||
| Cardiovascular | 18.4 | 26.0 | 13.6 | <0.01 |
| Respiratory | 9.1 | 8.9 | 9.3 | 0.245 |
| Gastrointestinal | 10.3 | 8.4 | 11.4 | <0.01 |
| Infectious disease | 4.6 | 3.8 | 5.1 | <0.01 |
| Cancer | 7.6 | 5.4 | 9.0 | <0.01 |
| Orthopedic | 4.6 | 4.9 | 4.4 | 0.09 |
| Hematologic | 5.8 | 6.2 | 5.5 | 0.04 |
| Genitourinary | 11.1 | 10.4 | 11.5 | 0.02 |
| Injury/poisoning | 5.1 | 4.5 | 5.4 | <0.01 |
| Other disease | 23.5 | 21.4 | 24.7 | <0.01 |
| Procedure or condition during index hospitalization (%) | ||||
| Sepsis | 4.8 | 3.8 | 5.4 | <0.01 |
| Cardiac surgery | 2.7 | 4.7 | 1.5 | <0.01 |
| Cardiac catheterization | 3.7 | 6.5 | 1.9 | <0.01 |
| Abdominal aortic aneurysm repair | 0.5 | 0.8 | 0.3 | <0.01 |
| Pneumonia | 10.7 | 10.0 | 11.2 | 0.01 |
| Liver failure | 0.7 | 0.2 | 1.0 | <0.01 |
| Acute myocardial infraction | 10.1 | 16.3 | 6.3 | <0.01 |
| Noncardiac surgery | 17.0 | 15.3 | 18.1 | <0.01 |
| Comorbid disease (%) | ||||
| Diabetes | 42.6 | 55.7 | 34.5 | <0.01 |
| Hypertension | 80.9 | 90.5 | 74.9 | <0.01 |
| Myocardial infarction | 13.0 | 19.8 | 8.8 | <0.01 |
| Chronic heart failure | 33.4 | 38.3 | 30.4 | <0.01 |
| Stroke or TIA | 23.1 | 25.9 | 21.4 | <0.01 |
| Cancer | 9.0 | 7.8 | 9.6 | <0.01 |
| Liver disease | 2.4 | 1.0 | 3.2 | <0.01 |
| Peripheral vascular disease | 7.2 | 10.0 | 5.4 | <0.01 |
| Kidney function | ||||
| Baseline eGFR, ml/min/1.73 m2, mean (SD) | 62.3 (25.8) | 58.8 (23.1) | 64.4 (27.1) | <0.01 |
| Prior CKD (%) | 61.6 | 66.2 | 58.8 | <0.01 |
| Prior CKD defined by eGFR | 33.3 | 34.0 | 32.9 | <0.01 |
| Prio CKD defined by proteinuria | 11.6 | 11.8 | 11.5 | 0.02 |
| Prior CKD defined by eGFR and proteinuria | 16.7 | 20.3 | 14.5 | <0.01 |
| AKI stage (%) | <0.01 | |||
| AKI stage 1 | 75.9 | 77.9 | 74.6 | <0.01 |
| AKI stage 2 | 14.8 | 13.5 | 15.6 | <0.01 |
| AKI stage 3 (no dialysis) | 7.1 | 6.2 | 7.6 | <0.01 |
| Dialysis | 2.3 | 2.4 | 2.2 | 0.37 |
| Baseline total cholesterol, mmol/l | <0.01 | |||
| <4.1 | 29.7 | 40.6 | 23.0 | <0.01 |
| 4.15−5.17 | 18.7 | 20.6 | 17.5 | <0.01 |
| 5.18−6.21 | 10.2 | 10.1 | 10.3 | 0.67 |
| 6.22−7.24 | 3.3 | 3.6 | 3.1 | 0.05 |
| ≥7.25 | 1.3 | 1.9 | 1.0 | <0.01 |
| Unknown | 37 | 23 | 45 | <0.01 |
AKI, acute kidney injury; CIHI, Canadian Institute for Health Information; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack.
Primary Outcomes
More than one-half (54.4%) of the cohort were never prescribed a statin, 12.0% received a new prescription, and 26.3% were continued on a statin within 2 years after hospital discharge. Only 7.3% of previous statin prescriptions were not restarted after hospital discharge (Table 2).
Table 2.
Number of patients (%) who were using statin before and after index hospitalization
| Within 6 months before admission | 2 yr After discharge | |
|---|---|---|
| Statin | 6620 (33.6%) | 7539 (38.3%) |
| Never prescribed | New prescription | Stopping previous prescription | Continuing prescription | |
|---|---|---|---|---|
| Statin | 10,729 (54.4%) | 2358 (12.0%) | 1439 (7.3%) | 5181 (26.3%) |
Over a total of 53,700 person-years of follow up, the adjusted hazard ratio for mortality associated with statin use after hospital discharge, compared with no statin use, was 0.74 (95% confidence interval [CI], 0.69, 0.79). Statin use after hospitalization was also associated with a lower risk of all cause re-hospitalization (hazard ratio [HR], 0.90; 95% CI, 0.85, 0.94) (Table 3).
Table 3.
Hazard ratios of statin use after hospital discharge
| Outcome | Adjusted hazard ratio (95% CI) | Number of events | Follow-up time in person-yr | Crude hazard ratio (95% CI) |
|---|---|---|---|---|
| Survival | 0.74 (0.69–0.79) | 6758 | 53700.94 | 0.75 (0.71–0.79) |
| All cause re-hospitalization | 0.90 (0.85–0.94) | 15,256 | 25144.77 | 0.94 (0.9–0.97) |
| Cardiovascular event | 0.95 (0.87–1.04) | 3493 | 48764.46 | 1.54 (1.43–1.65) |
CI, confidence interval.
Adjusted factors: Angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB), β-blocker, and statin use within 6 months before admission, ACEI/ARB and β-blocker after discharge, age, sex, income quintile, urban location, health care use 3 years before hospital admission, Canadian Institute for Health Information resource intensity weight, intensive care unit, primary diagnostic code for hospitalization, procedure or condition during index hospitalization, comorbid disease, baseline kidney function (estimated glomerular filtration rate), and total cholesterol risk categories.
Separate Cox proportional hazard models with medication use as time-varying covariates were fit for the outcome of all-cause mortality, all-cause re-hospitalization, and cardiovascular events. All patients were followed up for at least 2 years starting at the date of hospital discharge, with further censoring for death, outmigration from Alberta, and the end of study (31 March 2013) in the model fit of all-cause re-hospitalization and cardiovascular events, and for outmigration from Alberta and end of study in the model fit for mortality.
Both a new statin prescription (HR, 0.73; 95% CI, 0.66, 0.80) and continuing a previous prescription (HR, 0.76; 95% CI, 0.71, 0.81) after hospital discharge was associated with better survival compared to no statin use. There was also lower all-cause re-hospitalization in both patients who were given a new statin prescription or continued on a previous statin prescription after hospital discharge, compared to no statin use (Table 4).
Table 4.
Hazard ratios of statin use for never prescribed, new prescription, stopping previous prescription, or continuing previous prescription
| Outcome | Statin use | Hazard ratio (95% CI) |
|---|---|---|
| Survival | Never prescribed | 1 |
| New prescription | 0.73 (0.66–0.80) | |
| Stopping previous prescription | 1.00 (0.91–1.09) | |
| Continuing prescription | 0.76 (0.71–0.81) | |
| All cause re-hospitalization | Never prescribed | 1 |
| New prescription | 0.85 (0.79–0.91) | |
| Stopping previous prescription | 0.98 (0.93–1.03) | |
| Continuing prescription | 0.91 (0.87–0.95) | |
| Cardiovascular event | Never prescribed | 1 |
| New prescription | 0.92 (0.81–1.05) | |
| Stopping previous prescription | 1.01 (0.90–1.13) | |
| Continuing prescription | 1.00 (0.92–1.10) |
| Prescription | 6 mo Before admission | 2 yr After discharge |
|---|---|---|
| Never prescribed | No prescription | No prescription |
| New prescription | No prescription | At least 1 prescription |
| Stopping previous prescription | At least 1 prescription | No prescription |
| Continuing prescription | At least 1 prescription | At least 1 prescription |
CI, confidence interval.
Adjusted factors: Angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB), β-blocker and statin use within 6 months before admission, ACEI/ARB and β-blocker after discharge, age, sex, income quintile, urban location, health care use 3 years before hospital admission, Canadian Institute for Health Information resource intensity weight, intensive care unit, primary diagnostic code for hospitalization, procedure or condition during index hospitalization, comorbid disease, baseline kidney function (estimated glomerular filtration rate), and total cholesterol risk categories.
Separate Cox proportional hazard models with medication use as time-varying covariates were fit for the outcomes of all-cause mortality, all-cause re-hospitalization, and cardiovascular events. All patients were followed up for at least 2 years starting at the date of hospital discharge, with further censoring for death, outmigration from Alberta, and the end of study (31 March 2013) in the model fit of all-cause re-hospitalization and cardiovascular events, and for outmigration from Alberta and end of study in the model fit for mortality.
Sensitivity Analysis
In the sensitivity analysis excluding patients with cardiovascular disease (n = 15,116), those who used a statin after hospital discharge had lower mortality (HR, 0.70; 95% CI, 0.64, 0.76), and all-cause re-hospitalization (HR, 0.90; 95% CI, 0.85, 0.95) (Table 5). Exclusion of patients with the most severe AKI requiring dialysis (n = 19,250) and proteinuria before or after the index hospitalization (n = 15,792) did not change results for survival, re-hospitalization rates, or cardiovascular events (Table 5).
Table 5.
Hazard ratios of statin use for patients not requiring dialysis, with no proteinuria and no pre-existing cardiovascular disease
| Hazard ratio (95% CI) |
|||
|---|---|---|---|
| Excluding dialysis patients | Excluding patients with proteinuria | Patients without pre-existing CVDa | |
| Number of subjects | 19,250 | 15,792 | 15,116 |
| Survival | 0.74 (0.69–0.79) | 0.74 (0.69–0.80) | 0.70 (0.64–0.76) |
| All-cause rehospitalization | 0.89 (0.85–0.94) | 0.89 (0.84–0.93) | 0.90 (0.85–0.95) |
| Cardiovascular events | 0.96 (0.87–1.05) | 0.95 (0.86–1.04) | 0.92 (0.81–1.05) |
CI, confidence interval; CVD, cardiovascular disease.
Pre-existing CVD includes acute myocardial infarction, coronary artery bypass grafting, cardiac catherization, congestive heart failure, cerebrovascular accident (including all stroke-related events), and percutaneous coronary interventions in the 3 years before admission and during the index hospitalization.
Statin use was associated with lower mortality and all cause re-hospitalization in both patients with pre-existing CKD and de novo CKD. Statin use was not associated with lower risk of cardiovascular events in patients with pre-existing or de novo CKD (Table 6).
Table 6.
Hazard ratios of statin use after hospital discharge for patients with prior chronic kidney disease (CKD) and de novo CKD
| Outcome | Hazard ratio (95% CI) |
|
|---|---|---|
| Prior CKD | De novo CKD | |
| Number of subjects | 12,148 | 7559 |
| Survival | 0.76 (0.70–0.83) | 0.67 (0.59–0.77) |
| All cause rehospitalization | 0.90 (0.85–0.96) | 0.86 (0.79–0.94) |
| Cardiovascular events | 0.96 (0.87–1.07) | 0.92 (0.77–1.10) |
Low-dose and high-dose statin had similar effects on mortality, all-cause re-hospitalization, and cardiovascular events (Table 7).
Table 7.
Hazard ratios of high- and low-dose statin use
| Outcome | Hazard ratio (95% CI) | Number of subjects |
|---|---|---|
| Survival | ||
| Low-dose | 0.74 (0.69–0.79) | 6651 |
| High-dose | 0.71 (0.62–0.82) | 888 |
| No statin | 1.00 [reference] | 12,168 |
| High-dose versus low-dose | 0.97 (0.84–1.11) | – |
| All-cause rehospitalization | ||
| Low-dose | 0.89 (0.85–0.94) | 4993 |
| High-dose | 0.91 (0.82–1.00) | 679 |
| No statin | 1.00 [reference] | 14,035 |
| High-dose versus low-dose | 1.01 (0.92–1.12) | – |
| Cardiovascular events | ||
| Low-dose | 0.94 (0.85–1.03) | 6310 |
| High-dose | 1.10 (0.94–1.29) | 815 |
| No statin | 1.00 [reference] | 12,582 |
| High-dose versus low-dose | 1.18 (1.02–1.37) | – |
CI, confidence interval.
A total of 888 patients used high-dose statin before death occurred or the end of the follow-up period; 679 patients used high-dose statin before re-hospitalization after discharge from the index hospitalization or the end of the follow-up period; and 815 patients used high-dose statin before a cardiovascular event occurred or the end of the follow-up period.
Discussion
Using a large population-based cohort we characterized statin use in patients with CKD after an episode of AKI in the hospital. Over a follow-up period of at least 2 years after discharge, AKI survivors who were dispensed a statin after the index hospitalization had a lower risk of death compared to those with no statin use. Statin use was also associated with a decreased risk of all-cause re-hospitalization.
The risks of developing CKD following an episode of AKI are well documented in both pediatric33 and adult34 cohort studies. Recent work from our group confirms that failure to recover renal function to within 25% of baseline SCr within 90 days after moderate to severe AKI is associated with an increased risk of long-term mortality (adjusted HR, 1.26; 95% CI, 1.10, 1.43).35 Management of CKD after AKI may mitigate the risks of progressive CKD and increased mortality. A recently published systematic review of randomized controlled trials confirmed the importance of ACEi, ARB, β-blocker, and statin use as effective therapies for the treatment of CKD stages 1 to 3.36 Statins showed reduced mortality and cardiovascular events versus placebo or control in patients with impaired eGFR. To date no studies have examined the effect of statin use in AKI survivors.
Conservative population based estimates of AKI incidence in hospitalized adults are in the range of 3,000 per 100,000 person years,37 and the majority of these patients will survive hospital discharge. Recent KDIGO guidelines recommended that patients be followed up 3 months after an AKI episode to assess for CKD; however, there has been a lack of information to guide the care that these patients should receive. Based on our results, AKI survivors may benefit from statin therapy postdischarge, an intervention that does not require specialized care and could be readily implemented. In our study, only 39.1% of the cohort who had CKD after AKI were dispensed a statin within 2 years of their index hospitalization, despite their high risk profile for cardiovascular disease, and despite recently released international guidelines for lipid management in CKD, which recommends that adults >50 years of age with eGFR < 60 ml/min/1.73 m2 should be treated with a statin.38 Furthermore, 5.8% of statin prescriptions were not restarted postdischarge. We also observed better survival in the subgroup of patients who were given a new statin prescription compared to those who did not receive a statin. These findings suggest that there is an opportunity to improve postdischarge care in AKI survivors with CKD.
Several recent studies have suggested that AKI is a risk factor for cardiovascular disease and future cardiovascular events.39 Our results showed a trend toward a reduction in cardiovascular events in patients who received a statin, although it was not significant. This may be explained by the fact that patients who develop AKI and progress to CKD likely have undetected or early cardiovascular disease.
Despite reporting outcomes adjusted for many important confounders, there are some limitations to our analysis, related primarily to the retrospective use of administrative and laboratory data and observational design. First, entry into the cohort was limited to subjects who had ≥1 outpatient SCr measurement within 180 days prior to hospitalization and >1 inpatient SCr measurement performed as part of hospital care in Alberta. However, repeated measurements of SCr are common in patients hospitalized for acute medical and surgical problems, and based on prior work, patients who do not have SCr drawn after hospitalization have outcomes similar to those who do not have AKI; therefore, it is likely that we were able to capture all AKI patients at high risk for poor outcomes after discharge. Second, we were unable to obtain measures of some potentially important covariates such as blood pressure, urine output, nutritional status, and deconditioning after hospitalization with AKI. Third, as patients were not randomized to different processes of care, there is potential for treatment-by-indication bias, whereby certain patient characteristics prompt differences in prescription of a statin, thereby introducing confounding. However, because we expect that patients at higher cardiovascular risk are more likely to receive a statin, we expect that the direction of bias in our study is toward the null, and thus the strength of any observed associations between medications and outcomes is likely conservative. It is also possible that statin use was a marker of better follow-up care. Finally, our exposure was defined as a dispensed prescription, and the rate of compliance among patients in the statin group is likely not 100%.
Conclusions
The use of a statin in AKI survivors who progress to CKD after hospital discharge is associated with lower mortality and re-hospitalization. This observation requires further evaluation in prospective studies evaluating postdischarge care strategies for AKI survivors. In particular, our results suggest a need for a trial to evaluate treatment with statin in survivors of AKI, to determine whether this intervention improves long-term outcomes in high-risk patients.
Disclosure
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor, Alberta Health or Alberta Health Services express any opinion in relation to this study. All the authors declared no competing interests.
Acknowledgments
This study was funded through an operating grant from the Canadian Institute of Health Research (NP). The ICDC is funded through a CRIO Team Grant from Alberta Innovates–Health Solutions. Phoebe Ye provided additional statistical support.
Footnotes
Table S1. Drug identification numbers for the group of medications known as statins.
Figure S1. Distribution of the first abnormal laboratory test results (SCr/udip/umcr/pcr) after AKI episode.
Figure S2. Cumulative proportion of subjects who used angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, statin, and β-blocker postdischarge.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Drug identification numbers for the group of medications known as statins.
Distribution of the first abnormal laboratory test results (SCr/udip/umcr/pcr) after AKI episode.
Cumulative proportion of subjects who used angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, statin, and β-blocker postdischarge.
References
- 1.Chertow G.M., Burdick E., Honour M. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Liangos O., Wald R., O'Bell J.W. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 3.Wald R., Quinn R.R., Luo J. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 4.Ishani A., Nelson D., Clothier B. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 5.Ishani A., Xue J.L., Himmelfarb J. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafrance J.P., Miller D.R. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coca S.G., Yusuf B., Shlipak M.G. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harel Z., Wald R., Bargman J.M. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83:901–908. doi: 10.1038/ki.2012.451. [DOI] [PubMed] [Google Scholar]
- 9.Asselbergs F.W., Diercks G.F., Hillege H.L. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 10.Ridker P.M., MacFadyen J., Cressman M., Glynn R.J. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention–an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol. 2010;55:1266–1273. doi: 10.1016/j.jacc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Colhoun H.M., Betteridge D.J., Durrington P.N. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54:810–819. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Chonchol M., Cook T., Kjekshus J., Pedersen T.R., Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis. 2007;49:373–382. doi: 10.1053/j.ajkd.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Lemos P.A., Serruys P.W., de Feyter P. Long-term fluvastatin reduces the hazardous effect of renal impairment on four-year atherosclerotic outcomes (a LIPS substudy) Am J Cardiol. 2005;95:445–451. doi: 10.1016/j.amjcard.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Kendrick J., Shlipak M.G., Targher G. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the air Force/Texas coronary atherosclerosis prevention study. Am J Kidney Dis. 2010;55:42–49. doi: 10.1053/j.ajkd.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fassett R.G., Robertson I.K., Ball M.J. Effect of atorvastatin on kidney function in chronic kidney disease: a randomised double-blind placebo-controlled trial. Atherosclerosis. 2010;213:218–224. doi: 10.1016/j.atherosclerosis.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura H., Mizuno K., Ohashi Y. Pravastatin and cardiovascular risk in moderate chronic kidney disease. Atherosclerosis. 2009;206:512–517. doi: 10.1016/j.atherosclerosis.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Koren M.J., Davidson M.H., Wilson D.J. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis. 2009;53:741–750. doi: 10.1053/j.ajkd.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M., Isles C., Curhan G.C. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557–1563. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 19.Hemmelgarn B.R., Clement F., Manns B.J. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30–38. doi: 10.1186/1471-2369-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H., Khan N., Hemmelgarn B.R. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–1428. doi: 10.1161/HYPERTENSIONAHA.109.139279. [DOI] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 23.Waikar S.S., Wald R., Chertow G.M. Validity of International Classification of Diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation KDOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(2 suppl 1) S1–S266. [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 26.Tonelli M., Wiebe N., Fortin M. Methods for identifying 30 chronic conditions: application to administrative data. BMC Medical Inform Decis Mak. 2015;17:31. doi: 10.1186/s12911-015-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarnecki L., Gordon L. Analysis of acuity trends using resource intensity weights via the CIHI portal. Stud Health Technol Inform. 2009;143:42–46. [PubMed] [Google Scholar]
- 28.Jacobs P., Yim R. Canadian Agency for Drugs and Technologies in Health; Ottawa, Ontario, Canada: 2009. Using Canadian administrative databases to derive economic data for health technology assessments. [Google Scholar]
- 29.Wilson P.W.F., D’Agostino R.B., Levy D. Prediction of coronary heart disease using risk factor categories. Am Heart Assoc. 1998;97:1837–1848. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 30.Austin P.C., Daly P.A., Tu J.V. A multicentre study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 31.Lee D.S., Donovan L., Austin P.C. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Kokotailo R.A., Hill M.D. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 33.Mammen C., Al Abbas A., Skippen P. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Siew E.D., Peterson J.F., Eden S.K. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23:305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannu N., James M., Hemmelgarn B., Klarenbach S., Alberta Kidney Disease Network Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink H.A., Ishani A., Taylor B.C. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force for an American College of Physicians clinical practice guideline. Ann Intern Med. 2012;156:570–581. doi: 10.7326/0003-4819-156-8-201204170-00004. [DOI] [PubMed] [Google Scholar]
- 37.Liano F., Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 38.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 39.Wu V.C., Wu C.H., Huang T.M. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drug identification numbers for the group of medications known as statins.
Distribution of the first abnormal laboratory test results (SCr/udip/umcr/pcr) after AKI episode.
Cumulative proportion of subjects who used angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, statin, and β-blocker postdischarge.